Abstract
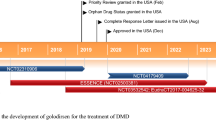
Casimersen (Amondys 45™) is an antisense oligonucleotide of the phosphorodiamidate morpholino oligomer subclass developed by Sarepta Therapeutics for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a mutation in the DMD gene that is amenable to exon 45 skipping. Administered by intravenous infusion, casimersen is designed to bind to exon 45 of the DMD gene pre-mRNA, resulting in skipping of this exon during mRNA processing, intended to allow for production of an internally truncated but functional dystrophin protein in patients with DMD. Casimersen received its first approval on 25 February 2021, in the USA, for the treatment of DMD in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping. The approval, granted under the US FDA Accelerated Approval Program, was based on an observed increase in dystrophin production in skeletal muscle in patients treated with casimersen. Casimersen is continuing in phase III development for the treatment of DMD in several other countries worldwide. This article summarises the milestones in the development of casimersen leading to this first approval for DMD. As with other approvals under the Accelerated Approval Program, continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.14312786. |
An antisense oligonucleotide developed by Sarepta Therapeutics for the treatment of Duchenne muscular dystrophy (DMD) |
Received its first approval on 25 February 2021 in the USA |
Approved for use in the treatment of DMD in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping |
1 Introduction
Duchenne muscular dystrophy (DMD), an X-linked recessive disorder occurring almost exclusively in males, is a severe, degenerative neuromuscular disease caused by mutations in the gene which encodes dystrophin (i.e. the DMD gene) [1]. In DMD, frameshift or nonsense mutations in the DMD gene prevent the production of functional dystrophin, resulting in progressive, life-shortening disease. Management of DMD has involved a multidisciplinary approach to treat symptoms and modify disease progression; however, there is no cure for the disease [1]. Standard-of-care therapy for DMD includes the long-term use of glucocorticosteroids [2].
One approach that has been investigated in the development of therapies for DMD has been to target restoration of the production of functional dystrophin, including through the use of exon skipping [1, 3,4,5]. In DMD, exon skipping involves the use of antisense oligonucleotides, which bind to the target exon in the pre-mRNA dystrophin transcript, to induce the skipping of the exon during mRNA processing, restoring the reading frame and leading to the production of an internally truncated but functional dystrophin protein [1, 3,4,5].

Casimersen (Amondys 45™) is an antisense oligonucleotide, designed to induce DMD exon 45 skipping, which has been developed by Sarepta Therapeutics using the company’s proprietary phosphorodiamidate morpholino oligomer (PMO) chemistry technology [6, 7]. Casimersen received its first approval, in the USA, in February 2021 for the treatment of DMD in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping [6, 8]. The approval, granted under the US FDA’s Accelerated Approval Program, was based on an observed increase in dystrophin production in skeletal muscle in patients treated with casimersen [6]. An increase in dystrophin production is considered reasonably likely to predict clinical benefit; however, clinical benefit, including improved motor function, is yet to be established [8]. Continued approval of casimersen in the treatment of DMD may be contingent on verification of a clinical benefit in confirmatory trials [6].
Casimersen is available in the USA in single-dose vials containing the drug as a 100 mg/2 mL (i.e. 50 mg/mL) solution [6]. The recommended dosage of casimersen is 30 mg/kg once weekly, administered by intravenous (IV) infusion. For the infusion, casimersen should be diluted in 0.9% sodium chloride injection to make a total volume of 100–150 mL and the diluted solution should be infused over 35–60 min via an in-line 0.2-micron filter. Before administration, application of a topical anaesthetic cream to the infusion site can be considered. Prior to initiating casimersen, serum cystatin C, urine dipstick and urine protein-to-creatinine ratio (UPCR) should be measured; measurement of glomerular filtration rate can also be considered but may not be a reliable measure of kidney function in patients with DMD. During treatment, urine dipstick should be monitored every month, and serum cystatin C and UPCR every three months [6].
Casimersen is continuing in phase III development for the treatment of DMD in several countries worldwide.
1.1 Company Agreements
Sarepta Therapeutics has an agreement with the University of Western Australia (UWA), first signed in 2008, to support the development of several exon-skipping drugs [9]. In April 2013, Sarepta and the UWA expanded the original deal, entering into an exclusive, worldwide licensing agreement for intellectual property rights to support the development of exon-skipping drug candidates for the treatment of DMD. Under the agreement, Sarepta was granted rights to the UWA's extensive patent portfolio in DMD [9]. In June 2016, under an amendment to the agreement, the UWA waived rights to certain royalties and amended the timing of certain other royalty payments [10].
Sarepta holds patents covering casimersen in the USA and Europe [10].
2 Scientific Summary
Casimersen is an antisense oligonucleotide of the PMO subclass and is composed of 22 subunits [6]. PMOs are synthetic molecules in which the five-membered ribofuranosyl rings found in natural DNA and RNA are replaced by a six-membered morpholino ring.
2.1 Pharmacodynamics
Casimersen is designed to bind to exon 45 of the DMD gene pre-mRNA resulting in exclusion (or skipping) of this exon during mRNA processing [6]. In patients with genetic mutations that are amenable to exon 45 skipping, the action of casimersen is intended to allow for production of an internally truncated but functional dystrophin protein that can compensate for the lack of functional dystrophin in patients with DMD [6].
2.2 Pharmacokinetics
Casimersen exposure is approximately dose proportional over the tested dose range of 4–30 mg/kg [11]. Following a single IV dose, peak plasma concentrations are reached at the end of the infusion [6]. Little to no accumulation of casimersen is observed with once-weekly dosing [6, 11].
Binding of casimersen to human plasma proteins is low (8–32%) and concentration-independent [6]. Following an IV dose of casimersen 30 mg/kg, the mean apparent volume of distribution at steady state was 367 mL/kg. Casimersen plasma clearance was 180 mL/h/kg, and its elimination half-life was 3.5 h. Excretion is mostly (> 90%) via the urine as unchanged drug. As casimersen does not undergo hepatic metabolism, casimersen systemic clearance is not expected to be affected by hepatic impairment. Furthermore, casimersen has a low potential for clinically relevant drug-drug interactions [6].
Features and properties of casimersen
Alternative names | Amondys 45; SRP-4045 |
Class | Antisense oligonucleotides; phosphorodiamidate morpholino oligomers |
Mechanism of action | Binds to exon 45 of dystrophin pre-mRNA; restores the open-reading frame (by skipping exon 45) resulting in the production of an internally truncated but functional dystrophin protein |
Route of administration | Intravenous infusion |
Pharmacodynamics | Increases dystrophin levels in muscle tissues of patients with Duchenne muscular dystrophy |
Pharmacokinetics | Exposure is approximately dose proportional over dose range of 4–30 mg/kg; little to no accumulation with once-weekly dosing; elimination half-life = 3.5 h; excretion is mostly (> 90%) via the urine as unchanged drug |
Most common adverse events | Upper respiratory tract infection, cough, pyrexia, headache, arthralgia and oropharyngeal pain |
ATC codes | |
WHO ATC code | M09A-X (Other drugs for disorders of the musculo-skeletal system) |
EphMRA ATC code | M5X (All other musculoskeletal products) |
2.3 Therapeutic Trials
Casimersen increases dystrophin production in patients with DMD that is amenable to exon 45 skipping, based on interim results from the ongoing, 96-week, randomised, double-blind, placebo-controlled phase III ESSENCE trial (NCT02500381) [11]. Although yet to be established, increased dystrophin production is considered reasonably likely to predict clinical benefits in patients with DMD [8].
According to interim results of the ESSENCE trial, mean dystrophin levels (measured as a percentage of normal i.e. in healthy subjects) increased from 0.93 at baseline to 1.74 at week 48 among patients treated with IV casimersen 30 mg/kg once weekly (mean change from baseline, 0.81; p < 0.001) compared with a change from 0.54 to 0.76 among placebo recipients (mean change from baseline, 0.22; p = 0.09). These data give a mean change from baseline between-group-difference of 0.59 (p = 0.004) [6]. Among evaluable patients at the interim analysis, all casimersen recipients displayed an increase in exon 45 skipping (100% response rate) [11]. Further, there was a positive correlation between exon 45 skipping and dystrophin production (Spearman rank correlation, 0.627; p < 0.001).
Patients in the trial are males aged 7–13 years with DMD and a confirmed genetic mutation amenable to exon 45 skipping [11]. Other key inclusion criteria include having a mean 6-min walk test (6MWT) distance of ≥ 300 m and ≤ 450 m, stable pulmonary function (forced vital capacity ≥ 50% of predicted), and being on a stable dose of oral corticosteroids for ≥ 24 weeks prior to entering the trial. Patients were randomised 2:1 to receive IV casimersen 30 mg/kg or placebo once weekly. The interim results are drawn from data from 43 evaluable patients who had a muscle biopsy at week 48 (27 casimersen recipients, 16 placebo recipients; median age of 9 years) [6].
Key clinical trials of casimersen in Duchenne muscular dystrophy
Drug(s) | Phase | Status | Location(s) | Sponsor (Collaborator) | Identifier(s) |
|---|---|---|---|---|---|
Casimersen, golodirsen, placebo | III | Ongoing/recruiting | Multinational | Sarepta Therapeutics | NCT02500381; ESSENCE |
Casimersen, golodirsen | III | Enrolling by invitation | Multinational | Sarepta Therapeutics | NCT03532542 |
Casimersen, eteplirsen, golodirsen | II | Enrolling by invitation | USA | Kevin Flanigan (Sarepta Therapeutics) | NCT04179409; SRPT-Dup-US-001 |
Casimersen, placebo | I/II | Completed | USA | Sarepta Therapeutics | NCT02530905 |
2.4 Adverse Events
IV casimersen appears to be generally well tolerated, based on available evidence. In the safety population of the ongoing ESSENCE trial (Sect. 2.3), adverse reactions that were observed in ≥ 20% of casimersen recipients (n = 57) and with an incidence > 5 percentage points higher than in placebo recipients (n = 31) were upper respiratory tract infection (65% vs 55%), cough (33% vs 26%), pyrexia (33% vs 23%), headache (32% vs 19%), arthralgia (21% vs 10%) and oropharyngeal pain (21% vs 7%) [6].
Further safety and tolerability data for casimersen are available from a phase I/II trial in 12 non-ambulatory (6MWT distance < 300 m) males aged 7–21 years with DMD and a mutation amenable to exon 45 skipping (NCT02530905) [11, 12]. After a 12-week, double-blind, placebo-controlled dose-titration period, all patients in the trial received open-label IV casimersen 30 mg/kg once weekly for up to an additional 132 weeks. During casimersen treatment in the combined double-blind and open-label periods, all patients experienced ≥ 1 treatment-emergent adverse event (TEAE); however, most events (91%) were of mild severity, and no patient discontinued the study drug or had a dose reduction due to a TEAE. Two patients experienced TEAEs considered to be possibly or probably related to casimersen treatment (one case of mild flushing and one case of moderate iron deficiency); both events resolved during the study. Three patients receiving casimersen experienced a total of five serious TEAEs, none of which were considered to be related to casimersen treatment and all of which resolved during the study.
Based on animal data, casimersen may cause kidney toxicity [6]. Although kidney toxicity was not observed in casimersen clinical trials, kidney toxicity (including life-threatening events) has been observed in patients receiving other antisense oligonucleotides. Kidney function should be monitored during treatment with casimersen. However, it should be noted that creatinine may not be a reliable measure of kidney function in patients with DMD because of the effect of reduced skeletal muscle mass on creatinine measurements. Patients with known kidney function impairment should be closely monitored during treatment with casimersen [6]. Local prescribing information should be consulted for kidney function monitoring recommendations.
2.5 Ongoing Clinical Trials
The ongoing, randomised, double-blind, placebo-controlled phase III ESSENCE trial (Sect. 2.3) has an expected completion in 2024. In addition to patients with DMD mutations amenable to exon 45 skipping, the trial includes patients with DMD mutations amenable to exon 53 skipping randomised (2:1) to receive double-blind IV golodirsen 30 mg/kg or placebo once weekly. The primary endpoint of the trial is the change from baseline in the total distance walked in a 6MWT at week 96. Following the 96-week double-blind period, participants enter a 48-week open-label period during which all patients receive casimersen 30 mg/kg or golodirsen 30 mg/kg once weekly [11].
NCT03532542 is an ongoing, long-term, open-label, phase III extension study for patients with DMD enrolled in clinical trials evaluating casimersen or golodirsen. The main objective of the study is to evaluate the safety and tolerability of long-term treatment with casimersen or golodirsen in patients with DMD. Patients with DMD and mutations amenable to exon 45 or exon 53 skipping, respectively, who have completed a clinical trial evaluating casimersen or golodirsen will receive open-label IV infusions of casimersen 30 mg/kg or golodirsen 30 mg/kg once weekly for up to 144 weeks. The primary endpoint of the trial is the number of patients with serious adverse events.
NCT04179409 is an ongoing, 48-week, open-label phase II trial to determine the efficacy and safety of casimersen, eteplirsen, or golodirsen for the treatment of patients with DMD who have a single exon duplication of either exon 45, 51 or 53, respectively. The primary endpoint of the trial is the change in dystrophin expression from baseline at week 48.
3 Current Status
Casimersen received its first approval on 25 February 2021, in the USA (under the US FDA Accelerated Approval Program), for the treatment of DMD in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping [8]. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
References
Duan D, Goemans N, Takeda S, et al. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021;7(1):13.
Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17(3):251–67.
Datta N, Ghosh PS. Update on muscular dystrophies with focus on novel treatments and biomarkers. Curr Neurol Neurosci Rep. 2020;20(14):1–12.
Rodrigues M, Yokota T. An overview of recent advances and clinical applications of exon skipping and splice modulation for muscular dystrophy and various genetic diseases. In: Yokota T, Maruyama R, editors. Exon skipping and inclusion therapies. Methods in molecular biology, vol. 1828. New York: Humana Press; 2018.
Verhaart IEC, Aartsma-Rus A. Therapeutic developments for Duchenne muscular dystrophy. Nat Rev Neurol. 2019;15(7):373–86.
US FDA. Amondys 45 (casimersen) injection, for intravenous use: US prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213026lbl.pdf. Accessed 18 Mar 2021.
Sarepta Therapeutics Inc. Sarepta Therapeutics announces FDA approval of Amondys 45™ (casimersen) injection for the treatment of Duchenne muscular dystrophy (DMD) in patients amenable to skipping exon 45 [media release]. 2021. https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-fda-approval-amondys-45tm. Accessed 18 Mar 2021.
US FDA. FDA approves targeted treatment for rare Duchenne muscular dystrophy mutation [media release]. 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-targeted-treatment-rare-duchenne-muscular-dystrophy-mutation-0. Accessed 18 Mar 2021.
Sarepta Therapeutics. Sarepta Therapeutics and University of Western Australia announce exclusive worldwide licensing agreement for exon-skipping program in Duchenne muscular dystrophy [media release]. 2013. https://investorrelations.sarepta.com/static-files/e20a099e-f99d-4237-8651-fa9c6d479090. Accessed 18 Mar 2021.
United States Securities and Exhange Commission. Sarepta Therapeutics, Inc.—Form 10-K. 2018. https://www.sec.gov/Archives/edgar/data/873303/000156459019005170/srpt-10k_20181231.htm. Accessed 18 Mar 2021.
Wagner K, Kuntz N, Koenig E, et al. Casimersen treatment in patients with Duchenne muscular dystrophy: safety, tolerability, and pharmacokinetics over 144 weeks of treatment [poster P.288]. In: World Muscle Society Virtual Congress. 2020.
Kuntz N, Wagner K, East L, et al. Casimersen treatment in eligible patients with Duchenne muscular dystrophy: safety, tolerability, and pharmacokinetics over 144 weeks of treatment [abstract no. P.288]. Neuromuscul Disord. 2020;30(Suppl 1):S130–1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. M. Shirley is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Shirley, M. Casimersen: First Approval. Drugs 81, 875–879 (2021). https://doi.org/10.1007/s40265-021-01512-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01512-2