Abstract
Apalutamide (Erleada®) is an oral selective androgen receptor (AR) inhibitor that binds directly to the ligand-binding domain of the AR. It is approved in the EU and the USA for the treatment of adult men with metastatic castration-sensitive prostate cancer (mCSPC). In a multinational, phase III study (TITAN) in this patient population, the addition of apalutamide (240 mg once daily) to androgen deprivation therapy (ADT) significantly improved median radiographic progression-free survival (rPFS), median overall survival (OS) and the median time to cytotoxic chemotherapy, while maintaining health-related quality of life (HR-QOL) and not substantially differing from placebo plus ADT in safety. Although mature OS data are awaited with interest, the addition of apalutamide to ADT extends the treatment options available for standard of care in adult men with mCSPC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Selective AR inhibitor |
Significantly prolongs median rPFS, median OS and the median time to cytotoxic chemotherapy |
Maintains HR-QOL and the safety profile, with rash being the most common grade ≥ 3 adverse event |
1 Introduction
Androgen derivation therapy (ADT; typically achieved through suppressing testicular androgen secretion) has long been the standard of care for patients with metastatic castration-sensitive prostate cancer (mCSPC) [1, 2]. However, despite an initial response to ADT, castration resistance invariably develops [2, 3]. In such patients, intracellular androgen levels are increased and the androgen receptor (AR) is overexpressed, suggesting an adaptive mechanism [2]. Such understanding has led to the development of androgen axis inhibitors [i.e. androgen synthesis inhibitors (e.g. abiraterone acetate plus prednisone) and AR inhibitors (e.g. apalutamide, enzalutamide)] [2,3,4], with recent studies in patients with mCSPC demonstrating improved survival benefits with additional androgen receptor suppression (i.e. ADT plus an androgen axis inhibitor) [4].
Oral apalutamide (Erleada®) has recently been approved in the EU [5] and the USA [6] for the treatment of adult men with mCSPC, with this article reviewing pharmacological (summarized in Table 1), therapeutic efficacy and tolerability data relevant to its use in this patient population. Apalutamide is also approved for the treatment of non-metastatic castration-resistant prostate cancer (nmCRPC) in various countries/regions, including the EU [5] and the USA [6], although its use in this patient population (which has been discussed previously [7, 8]) is beyond the scope of this review.
2 Therapeutic Efficacy of Apalutamide
A randomized, double-blind, placebo-controlled, multinational, phase III study (TITAN) evaluated the therapeutic efficacy of adding oral apalutamide to ADT for the treatment of adult men with mCSPC [9]. TITAN enrolled patients with confirmed adenocarcinoma of the prostate and documented distant metastatic disease (i.e. ≥ 1 lesion on bone scanning, with or without visceral or lymph node involvement) who were castration sensitive (i.e. were not receiving ADT at the time of disease progression). Patients had an Eastern Cooperative Oncology Group performance status score of 0 or 1; their previous therapy for prostate cancer was limited to docetaxel (≤ 6 cycles, with no evidence of progression during treatment or before randomization), ADT (for ≤ 6 months for mCSPC or ≤ 3 years for localized prostate cancer), one course of radiation or surgical therapy for metastatic disease-associated symptoms, and localized treatments (e.g. prostatectomy or radiation therapy) completed ≥ 1 year before randomization. Those who had received a gonadotropin-releasing hormone agonist within 28 days before randomization were required (prior to randomization) to take a first-generation antiandrogen (bicalutamide, flutamide or nilutamide) for ≥ 14 days (discontinued before randomization) [9]. Patients with known brain metastases; metastases limited to either the lymph nodes or viscera (e.g. liver or lung); a history of seizure or a condition that may predispose to seizure; or arterial or venous thromboembolic events, congestive heart failure, myocardial infarction, severe angina or a recent history of ventricular arrhythmias, and those who had previously received therapy with another next generation antiandrogen (e.g. enzalutamide), a cytochrome P450 17 inhibitor (e.g. abiraterone acetate), immunotherapy (e.g. sipuleucel-T), a radiopharmaceutical agent or another non-permitted treatment for prostate cancer, were among those excluded [5, 9, 10].
The assignment of patients to randomized treatment arms (apalutamide 240 mg or placebo, both administered orally once daily) was stratified by the Gleason score at diagnosis (≤ 7 vs > 7), prior docetaxel use (yes vs no), and geographic region (EU and North America vs all other countries) [9]. Patients in both treatment arms received concomitant ADT therapy, with treatment continuing until disease progression or the occurrence of unacceptable treatment-related toxicity [9, 10].
The co-primary efficacy endpoints were radiographic progression-free survival (rPFS; defined as the time from randomization to the first imaging-based documentation of progressive disease, or death, whichever occurred first) and overall survival (OS; defined as the time from randomization to death from any cause), with OS assessed only if the difference between apalutamide plus ADT and placebo plus ADT for rPFS was statistically significant [9]. Radiographic progressive disease was defined as either new bone lesions on bone scanning or a progression of soft-tissue lesions (as measured by computed tomography or magnetic resonance imaging), and was assessed by the investigator. OS was to be evaluated in two prespecified interim analyses and a final analysis; the first interim OS analysis was conducted at the time of the primary (i.e. final) rPFS analysis (data cut-off date of 23 November 2018; median follow-up duration of 22.7 months). At this timepoint, 365 events (134 and 231 in the apalutamide plus ADT and placebo plus ADT groups, respectively) of radiographic disease progression (corresponding to 99% of the prespecified total of 368 events) and 200 deaths (83 and 117 in the apalutamide plus ADT and placebo plus ADT groups, respectively) [corresponding to 49% of the prespecified total of 410 events] had occurred; the median trial intervention duration was 20.5 and 18.3 months and the median treatment cycle number was 23 and 19 in the apalutamide plus ADT and placebo plus ADT groups, respectively. The secondary endpoints (time to cytotoxic chemotherapy; time to pain progression; time to chronic opioid use; time to skeletal-related event) were assessed in a hierarchical manner, and evaluated only if the between-group differences for both co-primary endpoints were statistically significant [9].
Baseline patient demographics and clinical characteristics were well balanced between the treatment groups [9]. Overall, patients had a median age of 68 years; 16.4% had undergone prostatectomy or received radiotherapy for localized disease, and 10.7% had received previous docetaxel therapy. High volume disease [adapted from CHAARTED (Chemohormonal Therapy versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer); defined as visceral metastases and ≥ 1 bone lesion, or ≥ 4 bone lesions with ≥ 1 outside the axial skeleton] was described in 62.7% of patients and low volume disease (defined as the presence of bone lesions not meeting the definition of high-volume disease) in 37.3% [9]. Of note, most patients in TITAN were asymptomatic at baseline, with 76% experiencing mild or no pain and 75% experiencing mild or no fatigue [11].
The addition of apalutamide to ADT significantly prolonged median rPFS, corresponding to a 52% reduction in the risk of disease progression or death relative to placebo plus ADT, at the time of the final rPFS analysis (Table 2) [9]. Hazard ratios (HRs) for rPFS favoured apalutamide plus ADT over placebo plus ADT in all prespecified subgroups (based on stratification factors and other baseline characteristics), including both high and low volume disease. Of note, a blinded independent central imaging review confirmed 84.5% concordance to investigator assessments of radiographic progression. The 24-month rPFS rates were 68.2% in apalutamide plus ADT recipients and 47.5% in placebo plus ADT recipients [9].
Apalutamide plus ADT was also associated with a significant reduction in the risk of death relative to placebo plus ADT (33%) at the time of the first prespecified interim OS analysis (Table 2) [9]. In prespecified subgroup analyses, OS outcomes favoured (indicated by a HR < 1) apalutamide plus ADT over placebo plus ADT across all patient subgroups apart from previous docetaxel use. The 24-month OS rates were 82.4% in apalutamide plus ADT recipients and 73.5% in placebo plus ADT recipients [9].
The improvements in rPFS seen with apalutamide plus ADT appear to be achieved regardless of baseline prognostic risk [12] or mCSPC molecular subtype [13], according to post hoc analyses of TITAN data (available as abstracts). In one analysis [12], apalutamide plus ADT significantly (p ≤ 0.001) reduced the risk of disease progression or death relative to placebo plus ADT in both high-risk [HR 0.44 (95% CI 0.34–0.57)] (n = 289 and 286) and low-risk [HR 0.54 (95% CI 0.38–0.78)] (n = 236 and 241) patients. Risk factors included a Gleason score of ≥ 8, ≥ 3 bone lesions, and visceral metastasis; high risk was defined as ≥ 2 risk factors and low risk as ≤ 1 risk factor [12]. In the other analysis [13], the addition of apalutamide to ADT significantly (p = 0.002) reduced the risk of disease progression or death relative to placebo plus ADT across various mCSPC molecular subtypes in the biomarker population [HR 0.49 (95% CI 0.31–0.78)] (n = 110 and 112). Indeed, the apalutamide plus ADT regimen appeared to overcome the poor prognosis associated with the high metastatic risk molecular subtype (seen in 75% of the 222 patients analysed) and the ADT resistance associated with the basal and low AR activity molecular subtypes (seen in 50% and 43% of patients) [13].
In terms of the secondary endpoints, apalutamide plus ADT significantly prolonged the median time to cytotoxic chemotherapy relative to placebo plus ADT (Table 2) [9]. However, there was no significant between-group difference in the median time to pain progression [as assessed by the Brief Pain Inventory–Short Form (BPI–SF) item 3] (Table 2); thus, statistical significance was not tested for the subsequent endpoints (time to chronic opioid use and time to skeletal-related event). With respect to other (exploratory) endpoints, apalutamide plus ADT demonstrated an advantage over placebo plus ADT in the median time to prostate-specific antigen (PSA) progression [not estimable vs 12.9 months; HR 0.26 (95% CI 0.21–0.32)], with PSA levels undetectable (< 0.2 ng/mL) in 68.4% of apalutamide plus ADT recipients and 28.7% of placebo plus ADT recipients. An advantage with apalutamide plus ADT over placebo plus ADT was also seen in the median time to second PFS [defined as the time from randomization to the first occurrence of disease progression (i.e. clinical progression, PSA progression or progression on imaging) while the patient was receiving their first subsequent therapy for prostate cancer, or death due to any cause, whichever occurred first] (HR 0.66; 95% CI 0.50–0.87), although at the time of the final rPFS and prespecified first interim OS analyses the median time to second PFS had not yet been reached in either treatment group. Overall, 87 and 190 patients in the apalutamide plus ADT and placebo plus ADT groups received subsequent mCSPC treatment. Few symptomatic local progression events had occurred at the time of the final rPFS and prespecified first interim OS analyses; no substantial difference between the two groups in the median time to symptomatic local progression was seen (HR 1.20; 95% CI 0.71–2.02) [9].
Health-related quality of life (HR-QOL) did not worsen with the addition of apalutamide to ADT in adult men with mCSPC participating in TITAN [11]. Patient-reported exploratory endpoints of pain and fatigue (intensity and interference), prostate cancer symptoms and overall HR-QOL [as assessed by the BPI-SF, the Brief Fatigue Inventory (BFI), the Functional Assessment of Cancer Therapy–Prostate (FACT-P) version 4, and the EuroQoL five dimensions, five-levels questionnaire (EQ-5D-5L), respectively] did not significantly differ between the apalutamide plus ADT and placebo plus ADT groups at a data cut-off date of 23 November 2018 (median follow-up duration for pain-related endpoints of 19.4–22.1 months) [11].
Following the final rPFS and first prespecified interim OS analyses, the independent data monitoring committee recommended that TITAN be unblinded to permit placebo recipients to switch to apalutamide therapy [9].
3 Tolerability of Apalutamide
In adult men with mCSPC participating in TITAN, the addition of apalutamide to ADT resulted in a safety profile that did not substantially differ from that seen with placebo plus ADT [9]. Moreover, the safety profile of apalutamide plus ADT appears to be consistent with that of placebo plus ADT regardless of baseline prognostic risk (abstract data) [12].
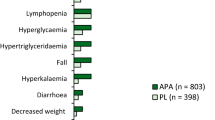
In TITAN, almost all (96.8% and 96.6%) of the patients receiving apalutamide plus ADT or placebo plus ADT (n = 524 and 527) experienced adverse events (AEs), with the most common (occurring in > 15% of patients in either treatment group and with a numerically higher incidence in the apalutamide plus ADT group than the placebo plus ADT group) being rash (27.1% vs 8.5%), hot flush (22.7% vs 16.3%), fatigue (19.7% vs 16.7%), hypertension (17.7% vs 15.6%) and arthralgia (17.4% vs 14.8%) [9]. The rash seen in the apalutamide plus ADT group was commonly generalized or maculopapular and had a median time to onset of 81 days (vs 141 days in the placebo plus ADT group). Apart from rash, other AEs of special interest, namely fall, fracture, hypothyroidism and seizure, respectively, occurred in 7.4%, 6.3%, 6.5% and 0.6% of the patients receiving apalutamide plus ADT and 7.0%, 4.6%, 1.1% and 0.4% of those receiving placebo plus ADT [9]. It is worth noting that patients with a history of seizure or a condition that may predispose to seizure were among those excluded [5, 9].
Grade 3 or 4 AEs occurred in 42.2% of apalutamide plus ADT recipients and 40.8% of placebo plus ADT recipients, with rash (6.3% vs 0.6%) the most frequently reported grade ≥ 3 AE occurring with a numerically higher incidence in the apalutamide plus ADT group than the placebo plus ADT group [9]. In the respective groups, grade ≥ 3 fractures were reported in 1.3% and 0.8% of patients, grade ≥ 3 falls in 0.8% and 0.8%, grade ≥ 3 seizures in 0.2% and 0%, and grade ≥ 3 hypothyroidism in 0% and 0% [9].
AEs resulting in dose reduction or interruption of the study medication [most commonly (> 1%) rash, fatigue and hypertension] were reported in 23% of patients [6]. Serious AEs occurred in 19.8% of apalutamide plus ADT recipients and 20.3% of placebo plus ADT recipients; ischaemic heart disease (a grouped term that included various events [5]) was reported in 4.4% and 1.5% of patients [9]. Treatment discontinuation because of AEs [most commonly rash in apalutamide plus ADT recipients (2.3%)] occurred in 8.0% and 5.3% of patients in the respective groups. AEs leading to death were reported in 1.9% and 3.0% of patients in the apalutamide plus ADT and placebo plus ADT groups, with ischaemic events resulting in death in two patients in each group [9]. It is worth noting that patients with clinically significant cardiovascular disease in the past 6 months were excluded from TITAN [14] (see Sect. 2).
4 Dosage and Administration of Apalutamide
Oral apalutamide is approved in the EU for the treatment of adult men with mCSPC in combination with ADT [5]. It is also approved in the USA for the treatment of patients with mCSPC [6]. Those patients who have not undergone a bilateral orchiectomy should receive concurrent gonadotropin releasing hormone analogue therapy [5, 6]. The recommended dosage of apalutamide is 240 mg (i.e. four 60 mg tablets) once daily; the tablets should be swallowed whole and may be taken with or without food [5, 6]. In the USA [6], the recommended dose of apalutamide may be dispersed in applesauce for patients who have difficulty swallowing the tablets whole.
Therapy with apalutamide should be discontinued in patients who develop a seizure during treatment [5, 6]; in the EU, apalutamide is not recommended in patients with a history of seizures or other predisposing factors (e.g. known brain metastases) [5]. As ADT may prolong the QT interval, the EU summary of product characteristics (SPC) advises clinicians to consider the benefit–risk ratio (including the potential for Torsade de pointes) prior to initiating apalutamide in patients with a history of or risk factors for QT prolongation, and in those receiving concomitant medicinal products that might prolong the QT interval or induce Torsade de pointes (e.g. antipsychotics, class IA or class III antiarrhythmic medicinal products, methadone, moxifloxacin) [5]. Local prescribing information should be consulted for detailed information regarding dosage modifications, missed doses, contraindications, potential drug interactions, use in special patient populations, and other warnings and precautions.
5 Place of Apalutamide in the Management of Metastatic Castration-Sensitive Prostate Cancer
The improved survival benefits observed with adding an androgen axis inhibitor to ADT for the treatment of mCSPC have changed the treatment landscape for this patient population [3, 4]. Indeed, current European Association of Urology (EAU) [2] and National Comprehensive Cancer Network (NCCN) [1] guidelines recommend (strongly [2]; as category 1 [1]) the use of the androgen signalling inhibitors apalutamide, abiraterone acetate (plus prednisone) or enzalutamide, or docetaxel chemotherapy, in combination with ADT for the treatment of patients with mCSPC. Neither guideline specifically recommends one therapy over another and to date no head-to-head studies have been undertaken, although such studies would be of interest in determining the roles of these agents in mCSPC. However, in a network meta-analysis of 13 studies in patients with mCSPC, treatment with apalutamide, abiraterone acetate plus prednisone or enzalutamide, in combination with ADT, was associated with a significant (p < 0.05) reduction in the risk of disease progression or death (secondary outcome), but not death (primary outcome), relative to docetaxel in combination with ADT [15]. Moreover, the odds of developing a grade 3–5 AE were significantly (p < 0.05) lower with apalutamide or enzalutamide plus ADT, but not abiraterone plus ADT, than with docetaxel plus ADT [15]. Of note, these results should be interpreted with caution given the inherent limitations of such analyses. Given the apparently similar efficacy between the different agents, the choice of therapy may be driven by other factors, including duration of therapy, cost, patient and physician preferences, patient comorbidities and safety profile [4, 16].
The guideline recommendations for the androgen receptor inhibitor apalutamide are consistent with the findings of the multinational, phase III TITAN study, in which apalutamide plus ADT was effective in patients with mCSPC (Sect. 2). Specifically, apalutamide plus ADT significantly prolonged median rPFS, median OS and the median time to cytotoxic chemotherapy relative to placebo plus ADT, with beneficial effects on median rPFS and median OS consistent across various subgroups, including disease volume (which is a key predictive factor in the selection criteria for treatment [16]). While mature OS data are awaited with interest, ascertaining the effect of apalutamide on OS will have been hampered by the 91% of placebo recipients who crossed over to receive apalutamide following early unblinding of the study [9, 14].
The clinical benefits seen in TITAN were obtained without compromising patient HR-QOL, with no significant differences between apalutamide plus ADT and placebo plus ADT observed in patient-reported exploratory endpoints of pain and fatigue (intensity and interference), prostate cancer symptoms and overall HR-QOL (Sect. 2). It is worth noting, however, that most patients were asymptomatic in terms of pain and fatigue at baseline and all study participants were required to commence ADT prior to randomization; thus, ongoing ADT benefits, and ADT-associated AEs may have reduced the between-group differences in patient-reported endpoints [11].
Treatment with apalutamide plus ADT resulted in a safety profile that did not substantially differ from that seen with placebo plus ADT in patients with mCSPC (Sect. 3). Rash of any grade was more frequently observed in apalutamide plus ADT recipients than placebo plus ADT recipients and was the most common grade ≥ 3 AE, while grade ≥ 3 falls, fractures, hypothyroidism or seizures (i.e. other AEs of special interest) each occurred in only a few patients (≤ 1.3% and ≤ 0.8% of patients in the respective groups). Ischaemic events resulting in death occurred in two patients in each treatment group (Sect. 3). It is worth noting that TITAN excluded patients with a history of seizure or a condition that may predispose to seizure (Sect. 2). While preclinical data suggest that apalutamide may be associated with lower seizure-inducing potential than enzalutamide (Table 1), more data are needed. TITAN also excluded patients with clinically significant cardiovascular disease in the past 6 months (Sects. 2, 3). Both the EU SPC [5] and the US prescribing information [6] recommend monitoring patients for the signs and symptoms of ischaemic heart disease.
In conclusion, apalutamide plus ADT was associated with significant improvements in median rPFS, median OS and the median time to cytotoxic chemotherapy relative to placebo plus ADT in patients with mCSPC, while maintaining HR-QOL and not substantially differing in safety. Although mature OS data are awaited with interest, the addition of apalutamide to ADT extends the treatment options available for standard of care in this patient population.
Data Selection Apalutamide: 110 Records Identified
Duplicates removed | 20 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 62 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 8 |
Cited efficacy/tolerability articles | 4 |
Cited articles not efficacy/tolerability | 16 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were apalutamide, Erleada, ARN509, metastatic hormone-sensitive prostate cancer. Records were limited to those in English language. Searches last updated 24 August 2020 | |
References
National Comprehensive Cancer Network. NCCN guidelines version 2.2020 prostate cancer. 2020. https://www.nccn.org. Accessed 21 Aug 2020.
European Association of Urology. Prostate cancer. 2020. https://www.uroweb.org/guideline/prostate-cancer/. Accessed 21 Aug 2020.
Katzenwadel A, Wolf P. Androgen deprivation of prostate cancer: leading to a therapeutic dead end. Cancer Lett. 2015;367(1):12–7.
Barata P, Swami U, Agarwal N. The addition of apalutamide to ADT in the treatment of metastatic castration-sensitive prostate cancer: safety and efficacy. Expert Rev Anticancer Ther. 2020;20(3):147–50.
Janssen-Cilag International NV. Erleada (apalutamide): EU summary of product characteristics. 2020. https://www.ema.europa.eu/. Accessed 21 Aug 2020.
Janssen Products LP. ERLEADA® (apalutamide) tablets, for oral use: US prescribing information. 2020. https://www.fda.gov/. Accessed 21 Aug 2020.
Al-Salama ZT. Apalutamide: a review in non-metastatic castration-resistant prostate cancer. Drugs. 2019;79(14):1591–8.
Scott LJ. Apalutamide in non-metastatic castration-resistant prostate cancer: a profile of its use. Drugs Ther Perspect. 2020;36(3):97–105.
Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24.
US National Institutes of Health. ClinicalTrials.gov identifier NCT02489318. 2020. https://www.clinicaltrials.gov/. Accessed 21 Aug 2020.
Agarwal N, McQuarrie K, Bjartell A, et al. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019;20(11):1518–30.
Ozguroglu M, Chowdhury S, Bjartell A, et al. Apalutamide (APA) for metastatic castration-sensitive prostate cancer (mCSPC) in TITAN: outcomes in patients (pts) with low- and high-risk disease [abstract no. 87]. J Clin Oncol. 2020;38(Suppl 6).
Feng FY, Thomas S, Aguilar-Bonavide C, et al. Molecular determinants of outcome for metastatic castration-sensitive prostate cancer (mCSPC) with addition of apalutamide (APA) or placebo (PBO) to androgen deprivation therapy (ADT) in TITAN [abstract no. 5535]. J Clin Oncol. 2020;38(Suppl).
European Medicines Agency. Erleada (apalutamide): assessment report. 2019. https://www.ema.europa.eu/. Accessed 21 Aug 2020.
Marchioni M, Di Nicola M, Primiceri G, et al. New antiandrogen compounds compared to docetaxel in metastatic hormone sensitive prostate cancer: results from a network meta-analysis. J Urol. 2019;203(4):751–9.
Ng K, Smith S, Shamash J. Metastatic hormone-sensitive prostate cancer (mHSPC): advances and treatment strategies in the first-line setting. Oncol Ther. 2020. https://doi.org/10.1007/s40487-020-00119-z.
Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72(6):1494–503.
Rathkopf DE, Morris MJ, Fox JJ, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31(28):3525–30.
Chi KN, Thomas S, Agarwal N, et al. Androgen receptor (AR) aberrations in patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) treated with apalutamide (APA) plus androgen deprivation therapy (ADT) in TITAN [abstract no. 883P]. Ann Oncol. 2019;30(Suppl 5):v347–8.
Belderbos B, de Wit R, Chien C, et al. An open-label, multicenter, phase Ib study investigating the effect of apalutamide on ventricular repolarization in men with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2018;82(3):457–68.
Acknowledgements
During the peer review process, the manufacturer of the agent under review was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Sheridan Hoy is a salaried employee of Adis International Ltd/Springer Nature and declares no conflict of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
Enhanced material for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.12838184.
The manuscript was reviewed by: P. Barata, Tulane University School of Medicine, New Orleans, LA, USA; A. Jang, Tulane University School of Medicine, New Orleans, LA, USA; M. Marchioni, Urology Unit, Department of Medical, Oral and Biotechnological Sciences, G. D’Annunzio University of Chieti, Chieti, Italy.
Rights and permissions
About this article
Cite this article
Hoy, S.M. Apalutamide: A Review in Metastatic Castration-Sensitive Prostate Cancer. Drugs 80, 1579–1585 (2020). https://doi.org/10.1007/s40265-020-01401-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01401-0