Abstract
Subcutaneous insulin glargine/lixisenatide (Suliqua™) is a titratable, fixed-ratio combination of a long-acting basal insulin analogue and a glucagon-like peptide-1 (GLP-1) receptor agonist for the treatment of adult patients with inadequately controlled type 2 diabetes. Once-daily insulin glargine/lixisenatide, in combination with metformin, provided effective glycaemic control and was generally well tolerated in the 30-week, multinational, phase 3 LixiLan-O and LixiLan-L trials in insulin-naive and -experienced adult patients with inadequately controlled type 2 diabetes. Although long-term clinical experience with this fixed-ratio combination is currently lacking, given its convenient once-daily regimen and beneficial effects on glycaemic control and bodyweight loss in the absence of an increase in the incidence of hypoglycaemia, insulin glargine/lixisenatide is an emerging option for the treatment of adult patients with type 2 diabetes to improve glycaemic control when this has not been provided by metformin alone or metformin combined with another OAD or basal insulin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Titratable fixed-ratio combination of a long-acting insulin analogue (insulin glargine) and a GLP-1 receptor agonist (lixisenatide); once-daily regimen |
As add-on therapy to metformin in insulin-naive patients, provides better glycaemic control than add-on insulin glargine or lixisenatide |
In insulin-experienced patients switching from another basal insulin, provides better glycaemic control than insulin glargine (both with metformin) |
Generally well tolerated; safety profile is generally consistent with profiles of its individual components |
1 Introduction
Globally, diabetes imposes a significant and ever increasing burden from a societal and healthcare perspective, with the number of individuals affected with the disease predicted to increase to 642 million by 2040 [1]. Type 2 diabetes, which accounts for ≈90–95% of cases of diabetes, is a chronic progressive disease resulting from dysregulation of glucose homeostasis [2, 3]. Maintenance of good glycaemic control (typically defined as a glycated haemoglobin (HbA1c) level of <7 or ≤6.5% [3, 4]) is important in terms of preventing the long-term micro- and macrovascular complications of diabetes [3, 5]. However, achieving good glycaemic control remains challenging in many patients, despite the availability of several classes of antidiabetic drugs that target different pathogenic pathways associated with type 2 diabetes, including more recent classes such as incretin mimetics [subcutaneous glucagon-like peptide-1 (GLP-1) receptor agonists (e.g. albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide) and oral dipeptidyl peptidase-4 (DPP-4) inhibitors (e.g. alogliptin, linagliptin, sitagliptin)] and oral sodium glucose cotransporter-2 (SGLT2) inhibitors (e.g. canagliflozin, dapagliflozin, empagliflozin) [3, 6,7,8]. In addition, given the progressive nature of the disease, intensification of pharmacotherapy with at least two drugs is often required to achieve and maintain adequate glycaemic control [3, 4].
The combination of a basal insulin with a GLP-1 receptor agonist, through their complementary mechanisms of action, is one strategy for intensification of pharmacotherapy to improve glycaemic control and provide fewer limiting side effects than either agent individually [6, 9]. Insulin glargine/lixisenatide (Suliqua™) is a fixed-ratio combination of a long-acting basal insulin analogue and a GLP-1 receptor agonist that is available in the EU [10] and USA (see supplementary information) [11] for the treatment of adult patients with inadequately controlled type 2 diabetes. This narrative review, which is written from an EU perspective, discusses the clinical use of subcutaneous insulin glargine/lixisenatide in insulin-naive or -experienced patients with inadequately controlled type 2 diabetes and summarizes the pharmacological properties of its individual components.
2 Pharmacodynamic Properties
Insulin glargine and lixisenatide target different pathways in glucose homeostasis and, with their complementary mechanisms of action, when combined as fixed-ratio insulin glargine/lixisenatide provide sustained lowering of fasting plasma glucose (FPG; key target of insulin glargine) and postprandial plasma glucose (PPG; main target of lixisenatide) levels after all meals, thereby improving glycaemic control [10]. As with endogenous insulin and other insulin analogues, insulin glargine reduces blood glucose levels via inhibition of glucose production in the liver and stimulation of peripheral glucose uptake, especially by skeletal muscle and fat (reviewed previously in Drugs [12, 13]). Insulin glargine also enhances protein synthesis and inhibits lipolysis and proteolysis [12, 13]. Lixisenatide is a highly selective, high affinity GLP-1 receptor agonist, with an approximately fourfold higher affinity for the GLP-1 receptor than human GLP-1 (reviewed previously in Drugs [14]). The GLP-1 receptor is the target for native GLP-1, an endogenous incretin hormone that plays a crucial role in glucose homeostasis, including glucose-dependent potentiation of insulin synthesis by pancreatic β-cells and suppression of glucagon secretion from α cells in the pancreas [3, 7]. As reviewed previously, lixisenatide acts to stimulate insulin secretion when blood glucose is increased, but not at normoglycaemia, thereby limiting the risk of hypoglycaemia [14]. Lixisenatide also suppresses glucagon secretion in a glucose-dependent manner; thus, in patients experiencing hypoglycaemia, the rescue mechanism of glucagon secretion is preserved [14].
Once-daily lixisenatide for 28 days delayed gastric emptying compared with placebo in patients with type 2 diabetes, thereby reducing the rate at which meal-derived glucose was absorbed and appeared in the circulation [15]. A significant (p < 0.05) inverse relationship was shown for lixisenatide between delayed gastric emptying and the PPG area under the plasma concentration–time curve [15].
Consistent with the relatively constant concentration–time profile of insulin glargine over a 24-h period, with no pronounced peak when administered alone, the glucose utilization rate-time profile was similar when given as the insulin glargine/lixisenatide combination [10]. As with other insulins, the time course of action of insulin glargine/lixisenatide may vary between individuals and within the same individual. In clinical studies with insulin glargine (100 U/mL), the glucose-lowering effect of intravenous insulin glargine on a molar basis (i.e. when given at the same doses) is approximately the same as that for human insulin [10].
Coadministration of insulin glargine and lixisenatide had no impact on the pharmacodynamic effects of insulin glargine; the effects on the pharmacodynamics of lixisenatide have not been evaluated in phase 1 studies [10]. In a cross-over study (two 4-week treatment periods) in 28 patients with type 2 diabetes receiving stable dosages of metformin, add-on lixisenatide and insulin glargine produced significant (p < 0.05) additive effects on first-phase insulin secretion compared with its individual components (abstract) [16]. In the pivotal phase 3 LixiLan-O trial (see Sect. 4.1 for discussion of trial design and efficacy outcomes), each of the individual components of insulin glargine/lixisenatide provided similar contributions to the reduction in PPG levels (2.62 mmol/L for insulin glargine vs. 2.26 mmol/L for lixisenatide); however, as might be predicted given their differing mechanisms of action, insulin glargine was associated with a significantly greater improvement in FPG (3.24 vs. 1.43 mmol/L; p < 0.001) [abstract] [17]. Similarly, in animal studies, the glucose-lowering effects of lixisenatide and insulin glargine were additive when the two drugs were administered concomitantly compared with either drug alone [14].
3 Pharmacokinetic Properties
The insulin glargine/lixisenatide ratio has no clinically relevant impact on the pharmacokinetics of insulin glargine and lixisenatide [10]. The rate of absorption of lixisenatide is not affected by the site of administration (i.e. abdomen, thigh or deltoid). In patients with type 1 diabetes, insulin glargine exhibited no pronounced peak after administration of insulin glargine/lixisenatide combinations. The median time to attain maximum plasma concentrations of lixisenatide after insulin glargine/lixisenatide combinations was 2.5–3 h. There were no clinically relevant differences in exposure to insulin glargine or lixisenatide after insulin glargine/lixisenatide administration compared with separate simultaneous injections of insulin glargine and lixisenatide in patients with type 1 diabetes [10].
Lixisenatide is 55% bound to plasma proteins [10]. After subcutaneous insulin glargine/lixisenatide, the apparent volume of distribution of lixisenatide was 100 L and for insulin glargine was 1700 L [10].
As a peptide, elimination of lixisenatide occurs via glomerular filtration, followed by tubular reabsorption and subsequent metabolic degradation [10]. The resultant smaller peptides and amino acids are reintroduced in the protein metabolism. After multiple-dose administration in patients with type 2 diabetes, the mean terminal half-life of lixisenatide was ≈3 h and the mean apparent clearance was ≈35 L/h. Insulin glargine is rapidly metabolized to two active metabolites (M1 and M2) in diabetic patients. The M1 metabolite is the principal circulating metabolite, with pharmacological studies indicating that the effect of subcutaneous insulin glargine is principally based on exposure to the M1 metabolite [10].
Gender, age, bodyweight and ethnicity had no clinically relevant effect on the pharmacokinetics of lixisenatide [10]. Since lixisenatide is primarily eliminated by the kidneys, hepatic dysfunction is not expected to affect the pharmacokinetics of lixisenatide; no pharmacokinetic studies have been conducted in this population. In subjects with mild [creatinine clearance (CLCR) 60–90 mL/min], moderate (CLCR 30–60 mL/min) or severe (CLCR 15–30 mL/min) renal impairment, exposure to lixisenatide was increased by 46, 51 and 87%, respectively (see Sect. 6). Insulin glargine has not been studied in patients with renal or hepatic impairment (see Sect. 6) [10].
No interaction studies with insulin glargine/lixisenatide have been conducted, with the following interactions based on studies with its individual components [10]. No pharmacokinetic interactions are known for insulin glargine. Lixisenatide is not metabolized by CYP450 enzymes. In vitro, lixisenatide did not alter the activity of CYP450 enzymes or human transporters evaluated [10]. Consult local prescribing information for details of potential drug–drug interactions.
4 Therapeutic Efficacy
4.1 In Insulin-Naive Patients
The efficacy of subcutaneous insulin glargine/lixisenatide, as add-on therapy to metformin in adult patients with inadequately controlled type 2 diabetes was evaluated in the 30-week, randomized, open-label, multinational, phase 3 LixiLan-O trial, which forms the focus of discussion [18]. Results from a randomized, open-label, multinational, phase 2 trial [n = 323 in the modified intent to treat population (mITT)] support the efficacy of add-on insulin glargine/lixisenatide in adult patients with inadequately controlled type 2 diabetes on metformin monotherapy [19]; this trial is not discussed further.
In LixiLan-O, eligible patients (aged ≥18 years) had inadequate glycaemic control (i.e. HbA1c level of ≥7.5 to ≤10% if on metformin alone or ≥7 to ≤9% if on two oral antidiabetic drugs (OADs)] despite ≥3 months’ treatment with metformin with or without a second OAD and had a disease duration of ≥1 year prior to screening (mean duration 8.8 years) [18]. Key exclusion criteria were the use of an OAD other than a sulfonylurea, glinide, SGLT-2 inhibitor or a DPP-4 inhibitor during the 3 months prior to screening, prior use of insulin (except short-term treatment due to intercurrent illness such as gestational diabetes) and previous discontinuation of a GLP-1 receptor agonist due to safety, tolerability or a lack of efficacy. After a 4-week run-in period (OADs other than metformin were discontinued at the start of this period), patients were randomized to add-on treatment with once-daily insulin glargine/lixisenatide (n = 468 mITT), insulin glargine (n = 466) or lixisenatide (n = 233); see Table 1 for dosage regimens [18].
The primary endpoint was the least-square mean (LSM) change from baseline to week 30 in HbA1c in the mITT population [18]. The coprimary hypothesis of superiority of insulin glargine/lixisenatide to lixisenatide alone and noninferiority of insulin glargine/lixisenatide to insulin glargine alone had to be established for the primary endpoint before hierarchical testing of secondary efficacy endpoints and the superiority of insulin glargine/lixisenatide versus insulin glargine alone for the primary endpoint [18].
At 30 weeks, add-on insulin glargine/lixisenatide provided superior improvements from baseline in HbA1c levels than add-on insulin glargine or lixisenatide, based on pre-specified hierarchical testing of the primary endpoint (Table 1) [18].
Significantly (p < 0.0001) more patients in the insulin glargine/lixisenatide than in the insulin glargine group also achieved the predefined composite endpoints of HbA1c of <7% without bodyweight gain (43. vs. 25%) and HbA1c of <7% without bodyweight gain and no documented symptomatic hypoglycaemia (i.e. plasma glucose ≤3.9 mmol/L) (32 vs. 19%). Rates for each of these respective composite endpoints in the lixisenatide group were 28% [between-group difference (BGD) vs. insulin glargine/lixisenatide 15.2%; 95% CI 8.1–22.4] and 26% (BGD 5.6%; 95% CI −1.3 to 12.6) [these comparisons was not included in pre-specified hierarchical testing procedure] [18]. A significantly (p < 0.0001) higher proportion of patients in the insulin glargine/lixisenatide group than in the add-on insulin glargine or lixisenatide groups achieved a target HbA1c of <7 and ≤6.5% at week 30 (Table 1; these endpoints were not included in pre-specified hierarchical testing procedure) [18].
There was no difference in the LSM change from baseline to week 30 in FPG levels between the insulin glargine/lixisenatide and insulin glargine group, reflecting the similar basal insulin titration to target FPG levels in both groups (Table 1) [18]. LSM changes in FPG levels favoured add-on insulin glargine/lixisenatide over add-on lixisenatide (Table 1). Add-on insulin glargine/lixisenatide also significantly improved 2-h PPG excursion during a standardized meal test at 30 weeks compared with add-on insulin glargine (LSM change −2.3 vs. −0.2 mmol/L; p < 0.0001), with marked improvements from baseline to 30 weeks in 2-h PPG levels in all groups (Table 1). After 30 weeks’ treatment, insulin glargine/lixisenatide recipients also experienced a greater improvement in the average 7-point self-monitored plasma glucose (SMPG) value than insulin glargine recipients (LSM BGD −0.69 mmol/L; 95% CI −1.65 to −1.16; p < 0.0001). Mean 7-point SMPG values were numerically lower in the insulin glargine/lixisenatide group than in the insulin glargine or lixisenatide groups at all assessed timepoints during the day, except for similar pre-breakfast values between the insulin glargine/lixisenatide and insulin glargine groups [18].
At study end, LSM changes in bodyweight significantly favoured add-on insulin glargine/lixisenatide over add-on insulin glargine, with a slight decrease in bodyweight in the insulin glargine/lixisenatide group versus a slight increase in the insulin glargine group (Table 1) [18]. Lixisenatide recipients also experienced a reduction in bodyweight from baseline to 30 weeks (Table 1; not included in pre-specified hierarchical testing procedure) [18].
At week 30, there was no difference in the final mean basal insulin daily dose between the insulin glargine/lixisenatide and insulin glargine group (mean daily dose 39.8 vs. 40.3 U), with both treatments titrated to the same target FPG level [18]. In both groups, ≈70% of patients were receiving final daily insulin doses of ≥30 to ≤60 U and ≈44% doses of >40 to ≤60 U, with 16% of patients in the insulin glargine/lixisenatide group and 20% in the insulin glargine group receiving the maximum permitted dose of 60 U [18].
Exploratory mITT analyses supported the glycaemic efficacy of add-on insulin glargine/lixisenatide in a subgroup of patients with a baseline HbA1c of ≥9%, with these results consistent with those in the overall population (abstract) [20]. In this subgroup analysis, LSM changes in HbA1c from baseline to week 30 were significantly greater in the insulin glargine/lixisenatide group (−2.9%; n = 49) than in the insulin glargine (−2.5%; p = 0.03; n = 55) or lixisenatide (−1.7%; p < 0.0001; n = 29) groups.
In a post hoc subgroup analysis, improvements in glycaemic control with add-on insulin glargine/lixisenatide in elderly patients (aged ≥65 years) were consistent with those in younger patients (aged <65 years) [21].
In post hoc analyses, the glycaemic efficacy of add-on insulin glargine/lixisenatide across all evaluated subgroups of patients stratified by baseline HbA1c (<8 vs. ≥8%), duration of type 2 diabetes (<7 vs. ≥7 years) and body mass index (BMI; <30 vs. ≥30 kg/m2) was consistent with that in the overall population (abstract) [22].
4.2 In Insulin-Experienced Patients
The efficacy of switching from a basal insulin to insulin glargine/lixisenatide was evaluated in the randomized, open-label, multinational, phase 3 LixiLan-L trial in adult patients (aged ≥18 years) with inadequately controlled type 2 diabetes on basal insulin with or without up to two OADs [23]. Eligible patients had to have been treated with basal insulin for ≥6 months prior to screening (on a stable regimen for ≥3 months and a stable insulin dose of 15–40 U/day for ≥2 months); the dose of any OAD had to have been stable for the 3 months prior to screening. Key exclusion criteria were the use of an OAD other than metformin, a sulfonylurea, glinide, SGLT2 inhibitor or a DPP-4 inhibitor, prior use of a nonbasal insulin (except short-term treatment due to intercurrent illness) in the year prior to screening and previous discontinuation of a GLP-1 receptor agonist due to safety, tolerability or a lack of efficacy. The mean duration of type 2 diabetes was 12 years and patients had a mean BMI of 31 kg/m2. After a 6-week run-in period during which only metformin if previously taken was continued, patients were randomized to add-on once-daily insulin glargine/lixisenatide (n = 366 mITT) or insulin glargine (n = 365); see Table 1 for dosage regimens. The primary endpoint was the LSM change from baseline to week 30 in HbA1c in the mITT population [23]. Secondary endpoints were tested using a prespecified hierarchical order [23].
After 30 weeks, treatment with insulin glargine/lixisenatide with or without metformin was associated with significantly (p < 0.0001) greater improvements in HbA1c levels than insulin glargine with or without metformin therapy (Table 1) [23]. The percentage of patients attaining the composite endpoint of HbA1c of <7% with no bodyweight gain (34 vs. 13%) was significantly (p < 0.0001) higher in the insulin glargine/lixisenatide than in the insulin glargine group. Rates of achieving HbA1c targets of <7 and ≤6.5% were also higher in the insulin glargine/lixisenatide group (Table 1; these endpoints were not included in pre-specified hierarchical testing procedure) [23].
There was no BGD for LSM changes in FPG levels from baseline to week 30 (Table 1), reflecting the titration to target of the basal insulin [23]. However, add-on insulin glargine/lixisenatide improved postprandial glycaemic control to a significantly greater extent than add-on insulin glargine, based on 2-h PPG excursion values (LSM change −3.9 vs. −0.5 mmol/L; p < 0.0001) and 2-h PPG (Table 1). The 7-point SMPG profile also improved to a significantly greater extent in the insulin glargine/lixisenatide than in the insulin glargine group (LSM change −1.5 vs. −0.6 mmol/L; p < 0.0001), with numerically lower 7-point SMPG values in the insulin glargine/lixisenatide group at all assessed timepoints except for pre-breakfast values [23].
At study end, LSM changes from baseline in bodyweight significantly favoured add-on insulin glargine/lixisenatide over add-on insulin glargine (BGD −1.4 kg; 95 CI −1.8 to −0.9; p < 0.0001) [Table 1] [23].
There was no difference in the final mean basal insulin daily dose (46.7 U in both groups) between the insulin glargine/lixisenatide and insulin glargine group at 30 weeks, with 27 and 31% of patients receiving the maximum permitted dose of 60 U at study end [23].
In a post hoc subgroup analysis, improvements in glycaemic control with add-on insulin glargine/lixisenatide in elderly patients (aged ≥65 years) were consistent with those in younger patients (aged <65 years) [21].
In exploratory analyses, relative to add-on insulin glargine, add-on insulin glargine/lixisenatide improved glycaemic control across all evaluated subgroups of patients stratified by baseline HbA1c (<8 vs. ≥8%), duration of type 2 diabetes (<10 vs. ≥10 years) and BMI (<30 vs. ≥30 kg/m2), with these improvements consistent with those in the overall population [24]. The glycaemic efficacy of insulin glargine/lixisenatide was also generally consistent across all final dose categories of its individual components (insulin glargine and lixisenatide) in post hoc analyses, with these results consistent with those in the overall mITT population (abstract) [25].
5 Tolerability
Add-on insulin glargine/lixisenatide was generally well tolerated during 30 weeks of therapy in the pivotal LixiLan-O [18] and LixiLan-L [23] trials, with the safety profile of insulin glargine/lixisenatide generally reflecting the established safety profiles of its individual components [18, 23]. Approximately half of the patients in the insulin glargine/lixisenatide and insulin glargine groups experienced at least one treatment-emergent adverse event (TEAE; 56.9 vs. 48.6% [18]; 53 vs. 52% [23]), with 67.4% of lixisenatide recipients experiencing these events [18]. Most TEAEs were of mild to moderate severity in all treatment groups [18, 23], with relatively few patients discontinuing treatment because of these events in the insulin glargine/lixisenatide (2.6% [18]; 2.7% [23]) and insulin glargine (1.9% [18]; 0.8% [23]) groups (vs. 9.0% in the lixisenatide group [18]). As per the EU summary of product characteristics, hypoglycaemia is the only very common (i.e. incidence ≥10%) adverse reaction occurring during insulin glargine/lixisenatide treatment, with common (i.e. incidence ≥1 to <10%) adverse reactions being dizziness, nausea, diarrhoea and vomiting [10].
In individual trials, infections and infestations [23], and/or gastrointestinal (GI) disorders [18, 23] were the most common adverse events by organ class occurring in the LixiLan-O [18] and LixiLan-L [23] trials. In LixiLan-O, GI disorders occurred in numerically fewer patients in the insulin-glargine/lixisenatide and insulin glargine groups than in the lixisenatide group (21.7 and 12.6 vs. 36.9% of patients), reflecting the numerically lower rates of nausea (9.6 and 3.6 vs. 24.0%) and vomiting (3.2 and 1.5 vs. 6.4%) [18]. Respective rates of diarrhoea in these three groups were 9.0, 4.3 and 9.0%. Relatively few patients discontinued study treatment because of these GI adverse events (≤0.4, 0 and ≤2.6% of patients in the insulin glargine/lixisenatide, insulin glargine and lixisenatide groups, respectively) [18]. In LixiLan-L, infections and infestations occurred in 26.8% of insulin glargine/lixisenatide recipients and 30.7% of insulin glargine recipients, with corresponding rates of GI disorders of 17.0 and 7.9% [23]. The frequency of nausea (10.4 vs. 0.5% of patients), vomiting (3.6 vs. 0.5%) and diarrhoea (4.4 vs. 2.7%) were all numerically higher in the insulin glargine/lixisenatide than in the insulin glargine group. Very few patients in the insulin glargine/lixisenatide group and no patients in the insulin glargine group discontinued study treatment because of nausea (1.1 vs. 0%), diarrhoea (0% in both groups) or vomiting (0% in both groups) [23].
The incidence of documented symptomatic hypoglycaemia was similar in the insulin glargine/lixisenatide and insulin glargine groups in LixiLan-O (25.6 and 23.6%; vs. 6.4% in the lixisenatide group) [18] and LixiLan-L (40.0 and 42.5%) [23]. The respective event rates of documented symptomatic hypoglycaemia in the insulin glargine/lixisenatide, insulin glargine and lixisenatide groups in LixiLan-O were 1.4, 1.2 and 0.3 events per patient-year (PY) of exposure [18]. In LixiLan-L, the event rates of documented symptomatic hypoglycaemia in the insulin glargine/lixisenatide and insulin glargine groups were 3.03 and 4.22 events per PY of exposure [23]. Very few patients (0–1.1%) in any treatment group experienced severe symptomatic hypoglycaemia (defined as requiring another person’s assistance to administer carbohydrate, glucagon or other resuscitative actions) in the LixiLan-O and LixiLan-L trials [18, 23]. For example, in the insulin glargine/lixisenatide, insulin glargine and lixisenatide groups, severe symptomatic hypoglycaemia occurred in 0, 0.2 and 0% of patients, respectively, in the LixiLan-O trial; corresponding event rates were 0, <0.01 and 0 events per PY of exposure [18].
Serious TEAEs occurred in a similar proportion of patients in the insulin glargine/lixisenatide (3.8% [18]; 5.5% [23]), insulin glargine (4.1% [18]; 4.9% [23]) and lixisenatide (3.9% [18]) groups. None of the deaths (0.4–0.6% of patients in each group) that occurred in the LixiLan-O trial were considered to be treatment-related [18]. In the LixiLan-L trial, severe TEAEs leading to death occurred in one patient in the insulin glargine/lixisenatide group (pneumonia) and two patients in the insulin glargine group (one case each of gallbladder cancer and cardiopulmonary failure) [23]. There were no pancreatic adverse events or pancreatic neoplasms reported in the LixiLan-L trial [23], with no adjudicated pancreatitis events occurring in any treatment group in the LixiLan-O trial [18].
Across both trials, allergic reactions (urticaria) that were considered to be possibly related to insulin glargine/lixisenatide treatment occurred in 0.3% of patients [10]. Cases of generalized allergic reaction, including anaphylactic reactions and angioedema, have occurred during insulin glargine and lixisenatide use. Some patients (1.7%) taking insulin-containing therapy, including insulin glargine/lixisenatide, have experienced erythema, local oedema and pruritus at the site of injection [10].
No clinical safety concerns were identified in terms of laboratory parameters (including amylase and lipase), vital signs, physical examination or ECG parameters [18, 23].
After 30 weeks’ treatment, anti-insulin glargine antibodies developed in 21.0 and 26.2% of patients receiving insulin glargine/lixisenatide in the two phase 3 trials, with anti-lixisenatide antibodies occurring in ≈43% of patients [10]. Anti-insulin glargine antibodies showed cross-reactivity to human insulin in ≈93% of patients. The presence of these antibodies had no clinically relevant impact on safety or efficacy [10].
5.1 Cardiovascular Safety
The cardiovascular (CV) safety profiles of insulin glargine and lixisenatide were evaluated in the ORIGIN (n = 12,537) [26] and ELIXA (n = 6068) [27] clinical trials, respectively. No dedicated CV outcome trial has been conducted for insulin glargine/lixisenatide [10].
The randomized, open-label, multinational ORIGIN trial compared insulin glargine to standard care in adult patients (mean age 63.5 years) with CV risk factors plus impaired fasting glucose, impaired glucose tolerance or type 2 diabetes [26]. The incidence of major adverse CV events (MACE) did not differ between the insulin glargine and standard care group after a median follow-up of 6.2 years [2.94 vs. 2.85 events per 100 patient-years’ exposure; hazard ratio (HR) 1.02; 95% CI 0.94–1.11] (primary outcome), with MACE defined as the composite of the first occurrence of CV death, nonfatal MI and nonfatal stroke [26].
The randomized, double-blind, multinational ELIXA trial in patients with type 2 diabetes (mean age ≈60 years) who had experienced a recent acute coronary event compared lixisenatide with placebo (both in combination with standard care), with a primary composite MACE outcome of the time to first occurrence of CV death, nonfatal myocardial infarction (MI), nonfatal stroke or hospitalization for unstable angina [27]. After a median follow-up of 25 months, lixisenatide was noninferior (p < 0.0001), but not superior, to placebo in terms of the primary composite 4-point MACE outcome (primary endpoint event occurred in 406 vs. 399 patients; HR 1.02; 95% CI 0.89–1.17) [27].
6 Dosage and Administration
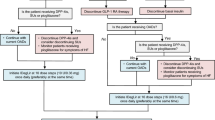
In the EU, insulin glargine/lixisenatide in combination with metformin is recommended for the treatment of adult patients with type 2 diabetes to improve glycaemic control when this has not been provided by metformin alone or metformin combined with another OAD or basal insulin [10]. Insulin glargine/lixisenatide is injected subcutaneously in the abdomen, deltoid or thigh once daily within an hour before a meal; preferably prior to the same meal each day [10].
Insulin glargine/lixisenatide is available in two pens that provide different dosing options based on the dose range of the pen [10]. The insulin glargine/lixisenatide 100 U/mL plus 50 μg/mL pen delivers dose steps of 10–40 units of insulin glargine in combination with 5–20 μg of lixisenatide; the insulin glargine/lixisenatide 100 U/mL plus 33 μg/mL pen delivers dose steps of 30–60 units of insulin glargine in combination with 10–20 μg of lixisenatide. The dose of insulin must be individualized based on clinical response and is titrated based on the patient’s need for insulin. The lixisenatide dose is increased or decreased along with the insulin glargine dose and also depends on which pen is used. Therapy with basal insulin or OADs other than metformin should be discontinued prior to initiation of insulin glargine/lixisenatide. The starting dose of insulin glargine/lixisenatide is based on previous antidiabetic treatment and should not exceed a lixisenatide starting dose of 10 μg. The maximum daily dose is insulin glargine 60 U and lixisenatide 20 μg, corresponding to 60 dose steps. [10].
Insulin glargine/lixisenatide should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis [10]. No dosage adjustments are required based on age, mild to moderate renal impairment or hepatic impairment; in patients with mild to moderate renal impairment and in those with hepatic impairment insulin requirements may be diminished and thus, frequent glucose monitoring and dose adjustment may be necessary. Insulin glargine/lixisenatide is not recommended in patients with severe renal impairment or end-stage renal disease as there is insufficient therapeutic experience relating to the use of lixisenatide in these patients [10].
Local prescribing information should be consulted for detailed information, including its use in special patient populations, contraindications, warnings and drug interactions.
7 Place of Insulin Glargine/Lixisenatide in the Management of Type 2 Diabetes
The major focus of the multifactorial approach recommended in current treatment guidelines for type 2 diabetes is attainment of good glycaemic control to prevent the onset and/or progression of microvascular and macrovascular complications of the disease, including MI, stroke, kidney failure, blindness and lower limb amputation [3, 4, 28]. Treatment guidelines emphasize the need for an individualized stepwise approach to pharmacotherapy, based on the clinical manifestations of the disease, existing comorbidities [e.g. obesity, renal disease or CV disease (CVD)] and drug characteristics (e.g. route of administration, propensity for drug–drug interactions, safety, costs) [3, 4, 28]. The availability of several classes of antidiabetic drugs has facilitated optimization of treatment regimens, with each class of drug having different but complementary mechanisms of action, different safety profiles, propensities for drug interactions and routes of administration. Several drugs are also now available as fixed-dose (typically consisting of metformin and a drug from another class of OAD) or titratable, fixed-ratio combinations that target multiple sites of tissue, organ and cellular dysfunction and provide a more convenient dosage regimen, thereby potentially improving compliance [29].
In the EU, fixed-ratio combinations consisting of a basal insulin and a GLP-1 receptor agonist include insulin glargine/lixisenatide [10] and insulin degludec/liraglutide (reviewed in Drugs [30]) [31]. In the absence of head-to-trials, the relative positioning of these fixed-ratio combinations remains to be determined.
Current UK [4] and ADA/EASD [3] guidelines recommend that, along with dietary and lifestyle modifications, metformin is given as first-line therapy unless contraindicated, based on its low costs and well established efficacy and safety profile (associated with slight bodyweight reduction, no propensity to cause hypoglycaemia and a decreased risk of CVD events [3]). Given the typically progressive course of the disease, maintaining good glycaemic control becomes increasingly difficult, with most patients requiring combination therapy with two or more antidiabetic drugs and, eventually, most will require insulin therapy [3, 4]. Minimizing the risk of adverse events such as bodyweight gain and hypoglycaemia is also important when deciding on the choice of add-on therapy. In terms of bodyweight, metformin, GLP-1 receptor agonists and SGLT2 inhibitors are associated with beneficial effects and DPP-4 inhibitors and α-glucosidase inhibitors with neutral effects, whereas sulfonylureas, meglitinides, thiazolidinediones and insulin therapies all increase the risk of bodyweight gain [3, 32,33,34,]. Sulfonylureas, insulin and, to a lesser extent, meglitinides, also increase the risk of hypoglycaemia, with other classes having neutral effects on this risk [3]. In terms of other adverse events, metformin, GLP-1 receptor agonists and α-glucosidase inhibitors are associated with GI adverse events, with metformin also associated with a rare risk of lactic acidosis. Thiazolidinediones have been associated with an increased risk of fracture and oedema/heart failure, and SGLT2 inhibitors with genitourinary infections, polyuria, volume depletion/hypotension/dizziness and increased low-density lipoprotein levels [3].
In the pivotal 30-week LixiLan-O and LixiLan-L trials, add-on once-daily subcutaneous insulin glargine/lixisenatide improved glycaemic control (Sect. 4), with slight reductions in bodyweight (Sect. 4) and no increase in the incidence of documented symptomatic hypoglycaemia versus insulin glargine (Sect. 5). As might be expected given the similar basal insulin titration to target FPG levels, there were no differences between insulin glargine/lixisenatide and insulin glargine groups in terms of changes in FPG levels at study end (Sect. 4). In insulin-naive patients, adding insulin glargine/lixisenatide to metformin provided better glycaemic efficacy in terms of HbA1c reductions and improvements in PPG after each meal than adding either of the individual components, with significantly more insulin glargine/lixisenatide recipients achieving target HbA1c levels and predefined composite target endpoints (Sect. 4.1). In insulin-experienced patients, switching from a basal insulin to insulin glargine/lixisenatide (+metformin) resulted in greater improvements in HbA1c levels than switching to insulin glargine (+metformin), with significantly more insulin glargine/lixisenatide recipients achieving target HbA1c levels and predefined composite target endpoints (Sect. 4.2). These trials were of relatively short duration, with long-term data required to determine the duration of the response of this titratable, fixed-ratio combination. Support for the efficacy of combining insulin glargine or another basal insulin with lixisenatide (administered separately) in treatment-experienced patients with inadequately controlled type 2 diabetes comes from the extensive lixisenatide GetGoal clinical trial programme [33–36], with most of these trials having been reviewed previously [14, 37].
Insulin glargine/lixisenatide in combination with metformin was generally well tolerated during 30 weeks’ treatment in insulin-naive and -experienced patients, with a tolerability profile that was generally consistent with those of its individual components (Sect. 5). Most TEAEs were of mild to moderate intensity, the most common of which were of a GI nature (both trials) and, in insulin-experienced patients, infections and infestations, and relatively few patients discontinued treatment because of these events. In insulin-naive patients, GI adverse events occurred in numerically fewer patients in the insulin glargine/lixisenatide and insulin glargine groups than in the lixisenatide groups, reflecting the lower rates of nausea and vomiting in these basal insulin groups. This potentially reflects the slower initial titration of lixisenatide as a component of fixed-ratio insulin glargine/lixisenatide than when lixisenatide was administered separately, thereby minimizing the risk of GI events [18]. In both trials, add-on insulin glargine/lixisenatide was associated with numerically higher rates of GI adverse events than add-on insulin glargine (Sect. 5).
Given the risk of microvascular and macrovascular complications in patients with type 2 diabetes, establishing the CV safety of antihyperglycaemic drugs is an important issue [38], with the European Medicines Agency’s guidance recommending that a long-term, controlled outcome study of 18–24 months’ follow-up is expected for drugs when an adverse CV effect is suspected [39]. In separate dedicated CV outcome trials of insulin glargine plus standard care (vs. standard care) and lixisenatide plus standard care (vs. placebo plus standard care), no specific safety concerns were identified for either of these drugs after a median follow-up of 6.2 years and 25 months, respectively (Sect. 5.1).
In the absence of head-to-head trials, the relative position of add-on insulin glargine/lixisenatide in the management of type 2 diabetes remains to be determined, with long-term efficacy and tolerability data for this recently approved titratable, fixed-ratio combination currently lacking. Combining a GLP-1 receptor agonist plus basal insulin as add-on therapy generally improved glycaemic control and reduced bodyweight compared with other antidiabetic medications and mitigated the risk of basal insulin-induced hypoglycaemia, based on a meta-analysis of randomized trials in patients with type 2 diabetes [9].
Globally, type 2 diabetes is associated with significant costs from a societal and healthpayer perspective, with these costs being an important consideration in determining the choice of treatment. Given the recent approval of insulin glargine/lixisenatide, it is not unexpected that robust pharmacoeconomic studies for this fixed-ratio combination are currently lacking.
In conclusion, once-daily insulin glargine/lixisenatide, in combination with metformin, provided effective glycaemic control and was generally well tolerated in the 30-week, multinational, phase 3 LixiLan-O and LixiLan-L trials in insulin-naive and -experienced adult patients with inadequately controlled type 2 diabetes. Although long-term clinical experience with this fixed-ratio combination is currently lacking, given its convenient once-daily regimen and beneficial effects on glycaemic control and bodyweight in the absence of an increase in the incidence of hypoglycaemia, insulin glargine/lixisenatide is an emerging option for the treatment of adult patients with type 2 diabetes to improve glycaemic control when this has not been provided by metformin alone or metformin combined with another OAD or basal insulin.
Data Selection Insulin glargine/lixisenatide: 115 records
Database(s): EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data [searches last updated 17 June 2017]. Records were limited to those in English language. |
Search terms: iGlarLixi, LixiLan, Suliqua, Soliqua, glargine, Lantus, Iglar, lixisenatide, AVE0010, Lyxumia, Lixi. |
References
International Diabetes Federation. Diabetes and cardiovascular disease: executive summary. 2016. http://www.idf.org/cvd. Accessed 9 Mar 2017.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centered approach. Update to position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9.
National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. 2016. http://www.nice.org.uk/guidance/ng28. Accessed 9 Mar 2017.
Milligan S. Combination therapy for improvement of long-term macrovascular and microvascular outcomes in type 2 diabetes: rationale and evidence for early initiation. J Diabetes Complicat. 2016;30:1177–85.
Wilding JPH, Bain SC. Role of incretin-based therapies and sodium-glucose co-transporter-2 inhibitors as adjuncts to insulin therapy in type 2 diabetes, with special reference to IDegLira. Diabet Med. 2016;33(7):864–76.
Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabet Obes Metabol. 2016;18(3):203–16.
Anderson SL, Trujillo JM. Lixisenatide in type 2 diabetes: latest evidence and clinical usefulness. Ther Adv Chronic Dis. 2016;7(1):4–17.
Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228–34.
European Medicines Agency. Suliqua 100 units/mL + 50 micrograms/mL solution in a prefilled pen, Suliqua 100 units/mL + 33 micrograms/mL solution in a prefilled pen: summary of product characteristics. 2017. http://www.ema.europa.eu. Accessed 8 Mar 2017.
Sanofi-aventis US LLC. Soliqua™ 100/33 (insulin glargine and lixisenatide injection), for subcutaneous use: US prescribing information. 2016. http://www.fda.gov/. Accessed 14 Mar 2017.
Blair H, Keating GM. Insulin glargine 300 U/mL: a review in diabetes mellitus. Drugs. 2016;76:363–74.
Dunn CJ, Plosker GL, Keating GM, et al. Insulin glargine: an updated review of its use in the management of diabetes mellitus. Drugs. 2003;63(16):1743–78.
Scott LJ. Lixisenatide: a review of its use in patients with type 2 diabetes mellitus. BioDrugs. 2013;27(5):509–23.
Lorenz M, Pfeffer C, Steinsträßer A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes: relationship to postprandial glycemia. Regul Pept. 2013;185:1–8.
Meier JJ, Menge B, Schenker N, et al. Mechanisms of action of the glucose-lowering effect of lixisenatide in combination with insulin glargine [abstract no. 281-OR]. Diabetes. 2015;64(Suppl 1):A74.
Kovatchev B, Umpierrez G, Renard E. The differential and combined action of insulin glargine and lixisenatide on the fasting and post-prandial components of glucose control [abstract no. 3]. Diabetologia. 2016;59(Suppl 1):S2.
Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39(11):2026–35.
Rosenstock J, Diamant M, Aroda VR, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof-of-concept randomized trial. Diabetes Care. 2016;39(9):1579–86.
Davies M, Russell-Jones D, Barber TM, et al. iGlarLixi fixed-ratio combination in patients with HbA1c ≥9%: LixiLan-O subgroup analysis [abstract]. 2017. In: 77th ADA Meeting; 2017.
Handelsman Y, Chovanes C, Dex T, et al. Efficacy and safety of insulin glargine/lixisenatide fixed-ratio combination in elderly patients with T2D [abstract no. 954-P]. Diabetes. 2016;65(Suppl 1):A246.
Davis M, Leiter LA, Grunberger G, et al. Impact of baseline HbA1c, BMI, and diabetes duration on the efficacy and safety of LixiLan (insulin glargine/lixisenatide titratable fixed-ratio combination) vs. insulin glargine and lixisenatide in the LixiLan-O trial [abstract no. 1028-P]. Diabetes. 2016;65(Suppl 1):A268.
Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39(11):1972–80.
Wysham C, Bonadonna RC, Aroda V, et al. Consistent findings in glycaemic control, body weight and hypoglycaemia with iGlarLixi (insulin glargine/lixisenatide titratable fixed-ratio combination) vs insulin glargine across baseline HbA1c, BMI and diabetes duration categories in the LixiLan-L trial. Diabetes Obes Metab. 2017. doi:10.1111/dom.12961.
Ritzel R, Vidal J, Aroda VR, et al. Efficacy and safety across the final dose ranges in patients with T2DM receiving insulin glargine/lixisenatide fixed-ratio combination in the LixiLan-L trial [abstract no. 1024-P]. Diabetes. 2016;65(Suppl 1):A266–7.
ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28.
Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57.
International Diabetes Federation. Global diabetes plan 2011-2021. 2011. http://www.idf.org. Accessed 21 Mar 2017.
Levin PA. Practical combination therapy based on pathophysiology of type 2 diabetes. Diabetes Metabol Syndr Obesity Targets Ther. 2016;9:355–69.
Greig SL, Scott LJ. Insulin degludec/liraglutide: a review in type 2 diabetes. Drugs. 2015;75(13):1523–34.
European Medicines Agency. Xultophy 100 units/mL + 3.6 mg/mL liraglutide solution for injection: summary of product characteristics. 2014. http://www.ema.europa.eu. Accessed 21 March 2017.
Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care. 2015;38:1161–72.
Riddle MC, Forst T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine. Diabetes Care. 2013;36(9):2497–503.
Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin. Diabetes Care. 2013;36(9):2489–96.
Seino Y, Min KW, Niemoeller E, et al. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910–7.
Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 trial. Diabetes Care. 2016;39(8):1318–28.
Eto K, Naito Y, Seino Y. Evaluation of efficacy and safety of lixisentide add-on treatment to basal insulin therapy among T2DM patients with different body mass indices from GetGoal trials. Diabetol Metab Syndr. 2015;7:106.
Zannad F, Stough WG, Lipicky RJ, et al. Assessment of cardiovascular risk of new drugs for the treatment of diabetes mellitus: risk asessment vs. risk aversion. Eur Heart J. 2016;2:200–5.
European Medicines Agency. Guidelines on clinical investiagtion of medicinal products in the treatment or prevention of diabetes mellitus. 2012. http://www.ema.europa.eu. Accessed 22 Mar 2017.
Acknowledgements
During the peer review process, the manufacturer of insulin glargine/lixisenatide was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Lesley Scott is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/1D48F060445E4DE0.
Additional information
The manuscript was reviewed by: S.C. Bain, Swansea University, Institute of Life Science, Swansea, United Kingdom; J.C. Eriksson, Department of General Practice and Primary Health Care, University of Helsinki, and Unit of General Practice, Finland and Helsinki University Central Hospital, Helsinki, Finland; N. Papanas, Democritus University of Thrace, Diabetes Care, Second Department of Internal Medicine, Alexandroupolis, Greece; J. Perez-Castrillon, Universidad de Valladolid, Institute of Endocrinology and Nutrition, Valladolid, Spain; A.J. Scheen, Division of Diabetes, Nutrition and Metabolic Disorders, Department of Medicine, CHU Liège, and Division of Clinical Pharmacology, CIRM, University of Liège, Liège, Belgium; T. Tzotzas, Agios Pavlos General Hospital, Thessaloniki, Greece.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scott, L.J. Insulin Glargine/Lixisenatide: A Review in Type 2 Diabetes. Drugs 77, 1353–1362 (2017). https://doi.org/10.1007/s40265-017-0783-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0783-4