Abstract
Plecanatide (TrulanceTM) is an oral guanylate cyclase-C agonist that is being developed by Synergy Pharmaceuticals for the treatment of gastrointestinal disorders, such as chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C). It is a synthetic analogue of human uroguanylin, a 16 amino acid peptide that regulates ion and fluid transport in the gastrointestinal tract. In January 2017, plecanatide received its first global approval in the USA for the treatment of adult patients with CIC. Plecanatide is undergoing phase III investigation in IBS-C. This article summarizes the milestones in the development of plecanatide leading to this first approval in CIC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plecanatide (TrulanceTM) is a guanylate cyclase-C (GC-C) agonist being developed by Synergy Pharmaceuticals for the treatment of gastrointestinal disorders, such as chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C) [1]. Plecanatide is a synthetic analogue of uroguanylin, a 16 amino acid peptide that regulates ion and fluid transport in the gastrointestinal tract by acting as a GC-C agonist. [2]. Activation of GC-C in the intestinal lumen by plecanatide and its active metabolite leads to a cascade of events (increased production of cyclic guanosine monophosphate, activation of cystic fibrosis transmembrane conductance regulator, and stimulation of chloride and bicarbonate secretion), which result in increased intestinal fluid and accelerated transit [2, 3]. Although several agents are available for the treatment of CIC, including the first in class GC-C agonist linaclotide (indicated for the treatment of CIC and IBS-C), challenges associated with inter-individual efficacy, tolerability and patient satisfaction necessitate the development of new treatment options [2].
An oral formulation of plecanatide has been approved in the USA for the treatment of adults with CIC [3]. The recommended dosage is 3 mg once daily, taken with or without food. The US label of plecanatide carries a boxed warning regarding the risk of serious dehydration in paediatric (<6 years of age) patients based on nonclinical studies and is contraindicated in these patients [3]. While plecanatide should be avoided in patients aged 6–18 years, the efficacy and safety of the drug have not been evaluated in patients aged <18 years. Plecanatide is also contraindicated in patients with known or suspected mechanical gastrointestinal obstruction [3].
Plecanatide is undergoing phase III investigation in IBS-C. It had also undergone preclinical investigations in inflammatory bowel disease (IBD) and ulcerative colitis; however, development in these indications has been discontinued.
1.1 Company Agreements
Callisto Pharmaceuticals was merged into Synergy Pharmaceuticals in January 2013 [4]. Synergy Pharmaceuticals

Clinical development of plecanatide. CIC chronic idiopathic constipation, Est estimated, IBS-C irritable bowel syndrome with constipation
entered into a service agreement with Capebio LLC in September 2007; Capebio was to provide research and development services regarding plecanatide for a minimum period of 11 months beginning 1 October 2007. In May 2006, Callisto Pharmaceuticals (now Synergy Pharmaceuticals) selected EpiStem Ltd. to provide specialised preclinical efficacy testing in the area of IBD. EpiStem was to use its IBDQUANT® services to provide quantitative and mechanistic data to assess the efficacy of plecanatide and other drug candidates being developed by Callisto. However, the development focus has since shifted away from IBD [5].
1.2 Patent Information
As of December 2016, Synergy Pharmaceuticals has 21 issued patents (expiration date range 2022–2034) related to GC agonists in the US covering plecanatide and dolcanatide. Also, there are three allowed US applications related to methods of manufacture of plecanatide and two related to formulations and method of using plecanatide with expiry in 2032 and 2031, respectively. Synergy entered into a binding letter of intent with Ironwood Pharmaceuticals in September 2012, which will give Synergy an exclusive worldwide license to its patent for method of use for plecanatide [6]. In January 2012, the European Patent Office upheld Synergy’s claims to plecanatide (EP 1 379 224), which was previously opposed by Ironwood Pharmaceuticals and another party [7]. A key patent, number 7 041 786, provides composition of matter protection for plecanatide. This patent was issued to Synergy Pharmaceuticals in May 2006, and has an expiry date in March 2023 (not including any patent term extension). Earlier, Synergy had received a Notice of Allowance for its patent on plecanatide from the US Patent and Trademark Office, titled “Guanylate Cyclase Receptor Agonists for the Treatment of Tissue Inflammation and Carcinogenesis” [8, 9].
Additionally, Synergy holds eight foreign patents which cover composition-of-matter of plecanatide, all of which expire in 2022 (not including any supplemental patent certificate extension of term). These foreign patents cover Austria, Belgium, Switzerland, Cyprus, Germany, Denmark, Spain, Finland, France, UK, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Monaco, The Netherlands, Portugal, Sweden, Turkey, Hong Kong, Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyz Republic, Moldova, Russian Federation, Tajikistan, Turkmenistan, Canada, China and Japan. Synergy also has 17 pending US patent applications and 82 pending foreign patent applications covering plecanatide and dolcanatide and various derivatives and analogues of plecanatide and dolcanatide and their uses and manufacture.
2 Scientific Summary
2.1 Pharmacodynamics
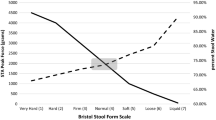
Plecanatide, differs from uroguanylin only in the substitution of Asp3 with Glu3 [10]. It activates GC-C receptors in a dose-dependent manner with an EC50 value of 1.9 × 10−7 mol/L. Following oral administration of a single dose of plecanatide (dose range 0.1–48.6 mg) to healthy volunteers (n = 71) in a placebo-controlled phase I trial, there was a general trend of higher doses of plecanatide producing greater stool consistency [as measured by the 7-point Bristol Stool Form scale (BSFS)] [2].
Plecanatide shows anti-nociceptive activity in animal models, which may be attributable to inhibition of mucosal inflammation and suppression of sensory afferent activity [11]. In rodent models of inflammatory [trinitrobenzene sulfonic (TNBS) acid-induced] and non-inflammatory (partial restraint stress-induced) visceral hypersensitivity to colorectal distension, oral plecanatide reduced the number of abdominal wall contractions in response to colorectal distension without significant alterations of colonic wall elasticity; reductions in colonic volume adaptation to increasing pressure after TNBS instillation were also evident [11]. Furthermore, once-daily plecanatide (administered by oral gavage) significantly (p < 0.05) ameliorated spontaneous or TNBS-induced colitis, and gastrointestinal inflammation in dextran sulphate sodium- or TNBS-induced colitis [10].
According to an in vitro study, pH-dependent activation of GC-C receptors by plecanatide may promote bowel movements without causing severe diarrhoea [12]. Furthermore, in molecular dynamics simulations, plecanatide showed optimal activity at pH 5, indicating that the proximal intestine (pH 5–6) would be the ideal site of action [13].
2.2 Pharmacokinetics
After an oral dose of 3 mg, plasma concentrations of plecanatide and its active metabolite are below the limit of quantification, indicating minimal absorption and negligible systemic availability [3]. Therefore, the standard pharmacokinetic parameters such as the area under the plasma concentration–time curve, maximum plasma concentration and half-life concentrations could not be established for plecanatide. Furthermore, oral plecanatide exerts its effects locally in the gastrointestinal tract and is expected to be minimally distributed to the tissues; plecanatide shows little or no binding to human serum albumin or human α-1-acid glycoprotein [3]. In the gastrointestinal tract, plecanatide is metabolized to its active metabolite and both are then proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids [3]. Excretion of plecanatide has not been studied in humans. In vitro, plecanatide and its active metabolite did not inhibit CYP2C9 and CYP3A4 or induce CYP3A4, and were neither substrates nor inhibitors of transporters P-glycoprotein or breast cancer resistance protein [3].
Features and properties of plecanatide
Alternative names | GCRA; Guanilib; SP-304; TrulanceTM |
Class | Anti-inflammatories; peptides |
Mechanism of action | Guanylate cyclase C agonists |
Route of administration | Oral |
Pharmacodynamics | Activates guanylate cyclase C in the intestinal lumen; increases extra- and intracellular cGMP; activates CFTR; stimulates chloride and bicarbonate secretion resulting in increased intestinal fluid and accelerated transit |
Pharmacokinetics | Minimally absorbed with negligible systemic availability; minimally distributed in tissues |
Adverse events | |
Most frequent | Diarrhoea |
Occasional | Sinusitis, upper respiratory tract infection, abdominal distension, flatulence, abdominal tenderness, increased liver biochemical tests (ALT, AST) |
ATC codes | |
WHO ATC code | A03 (drugs for functional gastrointestinal disorders); A07E (intestinal antiinflammatory agents); L01 (antineoplastic agents) |
EphMRA ATC code | A3 (functional gastro-intestinal disorder drugs); A7E (intestinal anti-inflammatory agents); L1 (antineoplastics) |
Chemical name | l-Leucine, l-asparaginyl-l-α-aspartyl-l-α-glutamyl-l-cysteinyl-l-α-glutamyl-l-leucyl-l-cysteinyl-l-valyl-l-asparaginyl-l-valyl-l-alanyl-l-cysteinyl-l-threonylglycyl-l-cysteinyl-, cyclic (4 → 12),(7 → 15)-bis(disulphide) |
2.3 Therapeutic Trials
2.3.1 Patients with CIC
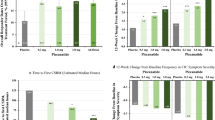
Plecanatide improved complete spontaneous bowel movements (CSBM) in two phase III clinical trials in patients with CIC, the CIC3 [NCT01982240; intention-to-treat (ITT) population 1346] [14] and the National CIC3 (NCT02122471; ITT population 1337) [abstract presentation] [15] trials. The primary efficacy measure of durable overall CSBM response rates (defined as the proportion of patients who had ≥3 CSBMs in a given week and an increase of ≥1 CSBM from baseline in the same week for ≥9 weeks out of the 12-week treatment period including ≥3 of the last 4 weeks of the study) were significantly (p < 0.005) higher with the approved dosage of plecanatide 3 mg once daily than with placebo [(21.0 vs. 10.2%) [14] and (20.1 vs. 12.8%) [15]]. CIC3 and National CIC3 were double-blind trials in which patients who met the modified Rome III criteria for CIC were randomized to plecanatide 3 or 6 mg, or placebo once daily for 12 weeks [14, 15]. Recipients of plecanatide 6 mg did not show any additional treatment benefit but had a higher incidence of some adverse events, and hence, this dosage is not recommended [3].
In the CIC3 and National CIC3 trials, plecanatide improved (p < 0.001) stool frequency [14, 15] and stool consistency [16] over the 12 week treatment period. The change from baseline in CSBM frequency at 12 weeks was significantly greater with plecanatide 3 mg than with placebo (2.5 vs. 1.2/week [14]; 2.3 vs. 1.4/week [15]). Similarly, plecanatide 3 mg significantly improved the frequency of SBMs versus placebo at 12 weeks (3.2 vs. 1.3/week [14]; 3.2 vs. 1.8/week [15]). Improvements in stool frequency were seen as early as week 1 and were maintained through 12 weeks. At 2 weeks post treatment (i.e. week 14), plecanatide recipients returned to baseline for these outcomes [3]. In both trials, plecanatide 3 mg was associated with significant (p < 0.05) improvements in Patient Global Assessment (PGA) of constipation severity, treatment satisfaction and patient’s desire to continue treatment, compared with placebo [17].
Results from a pooled analysis of the CIC3 and National CIC3 trials were consistent with those of the individual trials with respect to stool frequency, stool consistency, straining and bloating [18]. Consistent results for PGAs were reported in an open-label study (n = 2370), where patients treated with plecanatide 3 mg for 52 weeks were quite satisfied and quite likely to continue treatment [19].
2.3.2 Patients with IBS-C
In patients with IBS-C, 12 weeks’ treatment with plecanatide (3 or 6 mg) in two pivotal, randomized, double-blind, placebo-controlled, phase III trials [SP304203-04 (NCT02387359); SP304203-05 (NCT02493452)] significantly improved overall responder rates (primary endpoint) compared with placebo (p < 0.01 vs. placebo for both treatment groups) [1, 20]. In the larger of the two trials (n = 1135) [1], the overall responder rates were 21.5, 24.0 and 14.2% in plecanatide 3 mg, 6 mg and placebo groups; in the other trial (n = 1054), overall responder rates were 30.2 and 29.5% in plecanatide 3 and 6 mg treatment groups versus 17.8% in the placebo group [20].
Key clinical trials of plecanatide (Synergy Pharmaceuticals)
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Plecanatide, placebo | Chronic idiopathic constipation | III | Completed | USA | National CIC3; NCT02122471 |
Plecanatide, placebo | Chronic idiopathic constipation | III | Completed | Canada, North America, USA | CIC3; NCT01982240 |
Plecanatide | Chronic idiopathic constipation | III | Completed | Canada, USA | NCT01919697 |
Plecanatide | Chronic idiopathic constipation | II/III | Completed | USA | NCT01429987 |
Plecanatide, placebo | Chronic idiopathic constipation | II | Completed | USA | NCT01053962 |
Plecanatide, placebo | Irritable bowel syndrome with constipation | III | Completed | USA | SP304203-05; NCT02493452 |
Plecanatide, placebo | Irritable bowel syndrome with constipation | III | Completed | North America, USA | SP304203-04; NCT02387359 |
Plecanatide | Irritable bowel syndrome with constipation | III | Recruiting | USA | NCT02706483 |
Plecanatide, placebo | Irritable bowel syndrome with constipation | II | Completed | USA | CIBS; NCT01722318 |
2.4 Adverse Events
Available clinical data indicate that plecanatide 3 mg once daily (approved dosage) is generally well tolerated in adults with CIC, with diarrhoea being the most commonly reported adverse event [14,15,16, 18].
In the CIC3 and National CIC3 trials combined, the most common adverse reaction that occurred in ≥2% of plecanatide recipients (n = 863) and numerically higher incidence than in the placebo group (n = 870) was diarrhoea (5 vs. 1%) [3]. It was also the most common adverse reaction leading to treatment discontinuation (2 vs. 0.5%). The majority of diarrhoea cases were reported within 4 weeks of initiating treatment. Severe cases of diarrhoea were reported within 3 days of treatment initiation and occurred in 0.6 and 0.3% of plecanatide- and placebo-treated patients; dose suspension and patient rehydration are recommended if severe diarrhoea occurs [3]. Other adverse events reported in more plecanatide- than placebo-treated patients and with an incidence of <2% include sinusitis, upper respiratory tract infection, abdominal distension, flatulence, abdominal tenderness and increased liver biochemical tests (0.2% with alanine aminotransferase >5–15× the upper limit of normal (ULN) and 0.3% of patients with aspartate aminotransferase >5× ULN) [3].
The incidence of adverse events in the plecanatide and placebo groups was 35.4 versus 32.8% in the CIC3 [14] and 25.7 versus 24.7% in the National CIC3 [15] trials. However, the majority of these were of mild to moderate severity and led to discontinuation in 5.1 versus 1.3% [14], and 3.2 versus 3.0% [15] of patients. In an open-label label study (n = 2370), the incidence of diarrhoea over up to 52 or 72 weeks of plecanatide 3 or 6 mg treatment was 7.1%, with 5.3% of patients discontinuing treatment because of adverse events; the majority of patients in this study received treatment with the 6 mg dose (90.5%) [19].
In patients with IBS-C treated with plecanatide in the SP304203-04 and SP304203-05 studies, the most common adverse event was diarrhoea which occurred in 3.2–5.4% of patients in the plecanatide 3 mg group, 3.7–4.3% of patients in the plecanatide 6 mg group and 0.6–1.3% of patients in the placebo group [1, 20]. Diarrhoea led to discontinuations in ≤1.7% of patients in these groups; <1% of patients across both trials experienced a serious adverse events, with no imbalances across treatment groups in either incidences or individual serious adverse events [1, 20].
2.5 Ongoing Clinical Trials
An open-label, phase III study (NCT02706483) is evaluating the safety and tolerability of plecanatide 6 mg over up to 53 weeks in patients with IBS-C who participated in the SP304203-04 or SP304203-05 studies.
3 Current Status
Plecanatide received its first global approval on 19 January 2017, in the USA, for the treatment of CIC in adults [21].
References
Synergy Pharmaceuticals Inc. Synergy Pharmaceuticals announces positive results in first phase 3 trial of plecanatide in patients with irritable bowel syndrome with constipation (IBS-C) [media release]. 2016. http://www.businesswire.com/news/home/20161209005270/en/Synergy-Pharmaceuticals-Announces-Positive-Results-Phase-3. Accessed 24 Feb 2017.
Shailubhai K, Comiskey S, Foss JA, et al. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig Dis Sci. 2013;58(9):2580–6.
US FDA. Trulance (plecanatide) tablets: US prescribing information. 2017. http://content.stockpr.com/synergypharma/files/pages/synergypharma/db/147/description/03+Plecanatide+label_clean_2017-01-19.pdf. Accessed 24 Feb 2017.
Synergy Pharmaceuticals Inc. Synergy Pharmaceuticals announces closing of merger with Callisto Pharmaceuticals [media release]. 2013. http://ir.synergypharma.com/press-releases/detail/1203/synergy-pharmaceuticals-announces-closing-of-merger-with. Accessed 24 Feb 2017.
EpiStem Ltd. Callisto Pharmaceuticals selects EpiStem to provide specialised preclinical efficacy testing for guanilib, its lead drug candidate for IBD [media release]. 2006. http://www.prnewswire.com/news-releases/callisto-pharmaceuticals-selects-epistem-to-provide-specialised-preclinical-efficacy-testing-for-guanilib-its-lead-drug-candidate-for-ibd-69973687.html. Accessed 24 Feb 2017.
Synergy Pharmaceuticals Inc. Synergy Pharmaceuticals reports third quarter 2012 financial results [media release]. 2012. https://globenewswire.com/news-release/2012/11/13/504875/10012415/en/Synergy-Pharmaceuticals-Reports-Third-Quarter-2012-Financial-Results.html. Accessed 24 Feb 2017.
Synergy Pharmaceuticals Inc. European patent office upholds Synergy Pharmaceuticals’ grant of claims to plecanatide [media release]. 2012. http://www.businesswire.com/news/home/20120104005235/en/European-Patent-Office-Upholds-Synergy-Pharmaceuticals%E2%80%99-Grant. Accessed 24 Feb 2017.
Callisto Pharmaceuticals Inc. Callisto Pharmaceuticals announces $5.14 million private placement financing of common stock and warrants; proceeds to fund acceleration of clinical trials of drug candidates and expansion of key strategic initiatives [media release]. 2006. http://www.businesswire.com/news/home/20060207006138/en/Callisto-Pharmaceuticals-Announces-5.14-Million-Private-Placement. Accessed 24 Feb 2017.
Callisto Pharmaceuticals Inc. Callisto announces newly allowed patent on first-in-class drug for colon cancer and other diseases; research data supports drug’s potential as revolutionary, safe, non-toxic preventative for cancer and treatment for gastro-intestinal diseases [media release]. 2005. http://www.businesswire.com/news/home/20051128005738/en/Callisto-Announces-Newly-Allowed-Patent-First-in-Class-Drug. Accessed 24 Feb 2017.
Shailubhai K, Palejwala V, Arjunan KP, et al. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther. 2015;6(4):213–22.
Shailubhai K, Beaufrand C, Palejwala V, et al. Oral treatment with plecanatide and SP-333, agonists of guanylate cyclase-C, attenuates visceral hypersensitivity in rat models [abstract no. PP095]. Neurogastroenterol Motil. 2014;26(Suppl 1):41.
Patwa V, Joshi A, Thadi A, et al. Plecanatide, like uroguanylin, activates guanylate cyclase-C signaling in a pH-dependent manner in T84 cells, and in murine intestinal epithelial cells and tissues [abstract no. 967]. Gastroenterology. 2016;150(4 Suppl 1):S193–4.
Brancale A, Shailubhai K, Ferla S, et al. Structural and dynamic features of plecanatide: insights from molecular dynamics simulations [abstract no. Mo1316]. Gastroenterology. 2016;150(4 Suppl 1):S695.
Miner PB, Jr., Koltun WD, Wiener GJ, et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017. doi:10.1038/ajg.2016.611.
Miner PB, Lentz JD, Berenguer R, et al. Efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation (CIC): results from a multicenter phase III study (study-03) [abstract no. Su1214]. Gastroenterology. 2016;150(4 Suppl 1):S497–8.
Krause R, Foehl H, Koltun W, et al. Effect of plecanatide on stool consistency in the treatment of chronic idiopathic constipation (CIC): results from two phase III studies [abstract no. Sa1444]. Gastroenterology. 2016;150(4 Suppl 1):S317–8.
Nualart M, Morgan W, Berenguer R, et al. Effect of plecanatide on patient assessments in chronic idiopathic constipation (CIC): results from two phase III studies [abstract no. Sa1443]. Gastroenterology. 2016;150(4 Suppl 1):S317.
Rao S, Barrow L, Layton MB. Efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation (CIC): pooled results from two phase 3 studies [abstract no. 523]. Am J Gastroenterol. 2016;111(Suppl 1):S237.
De La Portilla M, Barrow L, Hickey B. Safety and tolerability of plecanatide in patients with chronic idiopathic constipation: long-term evidence from an open-label study [abstract no. 544]. Am J Gastroenterol. 2016;111(Suppl 1):S248–9.
Synergy Pharmaceuticals Inc. Synergy Pharmaceuticals announces positive results in second phase 3 trial of plecanatide in patients with irritable bowel syndrome with constipation (IBS-C) [media release]. 2016. http://ir.synergypharma.com/press-releases/detail/1830/synergy-pharmaceuticals-announces-positive-results-in. Accessed 24 Feb 2017.
US FDA. FDA approves Trulance for chronic idiopathic constipation [media release]. 2017. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm537725.htm. Accessed 24 Feb 2017.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Z. T. Al-Salama and Y. Y. Syed are salaried employees of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Al-Salama, Z.T., Syed, Y.Y. Plecanatide: First Global Approval. Drugs 77, 593–598 (2017). https://doi.org/10.1007/s40265-017-0718-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0718-0