Abstract
Non-infectious uveitis mainly affects the working-age population and can contribute to significant social and economic burden. It comprises a heterogeneous group of conditions with varied aetiology. Precise and early diagnosis, excluding masquerade syndromes, is the key to early therapeutic intervention. Treatment should be appropriately selected according to the anatomical sites of inflammation, the diagnosis and known prognosis, and whether there is a systemic inflammatory drive. Corticosteroids in the form of local or systemic therapy form the mainstay of treatment; however, due to unacceptable side effects, the need for long-term use or suboptimal response, corticosteroid-sparing medications may need to be considered early on in the management of non-infectious uveitis. With newer insights into the immunopathology of uveitis and the availability of biologic agents, treatment can be tailored according to individual needs. Many patients have systemic involvement, and hence a multidisciplinary approach is often required to achieve the best outcome in an individual. Patient involvement in the management of non-infectious uveitis, ensuring compliance, and continual monitoring of both the treatment and therapeutic response are the key to achieving optimal outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Heterogeneous aetiology, exclusion of infectious causes and masquerades, multi‐disciplinary approach and long term treatment make non–infectious uveitis a challenge |
A personalised approach to treatment with corticosteroids and/or corticosteroid sparing medications based on disease aetiology and prognosis is the key to optimal therapy. |
1 Introduction
Uveitis is inflammation of the uveal tract (iris, ciliary body and choroid); however, it is not uncommon for adjacent structures to become involved, such as the retinal vasculature, optic nerve and retina. The fact that uveitis accounts for 15 % of all causes of blindness among people of working age in the developed world, highlights the potential severity of this ocular disease [1]. The majority of patients experiencing uveitis require frequent hospital attendances, and therefore it impacts significantly on both medical and social services.
Although uveitis is present in the paediatric population, it is beyond the scope of this article to include the management of children. The aims of the article were therefore twofold: first, to provide an overview of the classification and aetiology of uveitis; and second, to highlight treatments available to the clinician relating to the treatment of non-infectious uveitis.
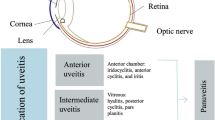
2 The Classification of Uveitis
Uveitis may be classified anatomically, pathologically (granulomatous vs. non-granulomatous) and aetiologically. In addition, strict definitions relating to chronicity are described. A grading system is utilised clinically at each anatomical site in order to assess the severity of the disease. Although some practitioners define uveitis using pathological criteria in the clinical setting, this criteria is strictly a histological one. It is noteworthy that some patients experiencing a granulomatous disease, such as sarcoidosis, may harbour ‘non-granulomatous’ features in their eyes. As such, it is recommended that, in the clinical setting, practitioners classify uveitis based on the presumed aetiology and anatomical location. Table 1 highlights the anatomical, chronological and aetiological classifications of uveitis.
3 Pathophysiology of Non-Infectious Uveitis
Immune mechanisms are believed to play an important role in the pathogenesis of non-infectious uveitis. Genetic predisposition, circumventing the immune privileged status of the eye, molecular mimicry and environmental factors all have a role to play in the activation and interaction between the T lymphocyte subsets and the inflammatory cytokines, which ultimately determines the clinical course of the disease and hence the treatment of the condition.
Regulatory T lymphocytes produce the anti-inflammatory cytokines interleukin (IL)-10, transforming growth factor β (TGF-β) and IL-35 [4]. It is noteworthy that the anti- inflammatory cytokine IL-27 is produced within the retina, and cells in the retinal pigment epithelium induce the production of regulatory T cells which contribute to the state of immune privilege in the eye.
Within the context of environmental and polygenic influences, the pathogenesis of non-infectious uveitis is proposed to be due to an imbalance between the regulatory and inflammatory mechanisms within the immune system.
The CD-4 T-helper lymphocyte is the predominant cell presumed to be responsible for inducing immune-mediated uveitis, such as Behcet’s disease and Vogt Koyanagi Harada (VKH) syndrome. Exogenous stimuli, such as an infectious agent, or endogenous stimuli, such as complement, are presented to the T-helper cell with the human leucocyte antigen (HLA) molecule, leading to activation of the T lymphocyte (Fig. 1). Conditions such as Behcet’s, birdshot chorioretinopathy and VKH have strong HLA associations. These activated T-helper cells produce cytokines, which determines the subsequent path of the inflammatory cascade. A predominantly T-helper cell 1 response is characterised by interferon-γ and IL-2, which results in damage to the targeted tissues. T-helper cell 1 lymphocytes also produce the pro-inflammatory cytokines IL-6 and tumour necrosis factor (TNF)-α. A T-helper cell 2 response subsequently leads to production of IL-4, -5, -10, and -13, leading to stimulation of the B lymphocytes and antibody production. T-helper 17 lymphocyte activation produces IL-17 and IL-23 [4–6].
It is hypothesized that the different clinical manifestations of non-infectious uveitis may be due to the differences in antigens that act as the trigger for the inflammatory cascade. In addition, molecular mimicry between a self-antigen and an antigen on an invading microorganism may incite the inflammatory cascade [6].
4 Treatment of Uveitis
4.1 The Challenges
There are potentially several clinical challenges relating to the management of uveitis.
It is important that infectious or neoplastic causes are ruled out before corticosteroid or immunosuppressive therapy is initiated in patients with ocular inflammation. The indiscriminate use of topical or systemic immunosuppression alone may have a deleterious effect on the treatment outcome in infective cases and, second, may mask the signs in a hitherto undiagnosed intraocular malignancy, leading to a delay in diagnosis and correct management.
The practitioner must be aware of co-existing ocular pathologies when prescribing immunosuppressive treatment for patients with uveitis. For example, in patients with glaucoma who subsequently develop uveitis, topical corticosteroids may increase the risk of raised intraocular pressure. Continual monitoring of disease activity and treatment response is essential to tailor therapy as per individual needs.
To minimise generalised toxicity relating to systemic immunosuppression, the treating practitioner must be aware of the patient’s medical co-morbidities and may need to liaise with other medical colleagues before embarking on such therapy. For example, there is often a need to adjust hyperglycaemic treatment in patients with diabetes who are prescribed oral corticosteroids.
Since a significant number of female patients requiring treatment will be in the reproductive age group, practitioners should be familiar with the potential side effects of systemic and topical therapeutic agents during pregnancy. As such, practitioners will either need to counsel both female and male patients on the use of contraception before embarking on certain systemic immunosuppressive agents (e.g. mycophenolate) or prescribe an agent that is not teratogenic or is detrimental to their fertility (e.g. azathioprine).
4.2 Principles of Treatment
Treatment of non-infectious uveitis may be delivered locally to the eye, systemically, or a combination of the two. The decision and type of treatment is dictated by the underlying diagnosis, aggressiveness of the disease, laterality (unilateral vs. bilateral) and the patient’s co-morbidities, as alluded to earlier. Although the presence of an underlying systemic disease associated with uveitis often warrants the implementation of systemic immunosuppression (e.g. retinal vasculitis secondary to Behcet’s disease), there are also several ocular entities in which systemic immunomodulation is required at the onset (e.g. birdshot chorioretinopathy).
Following the initiation of a treatment plan, it is important that the patient is monitored regularly. A comprehensive ocular examination, including measurement of the visual acuities, intraocular pressures, grading of haze and cells in the anterior chamber and vitreous cavities, and assessment of the patients’ ocular fundi, allows the practitioner to grade the level of disease activity at each visit and hence assess the effectiveness of the treatment prescribed [7]. Ocular fundus fluorescein and indocyanine green angiography, optical coherence tomography and automated visual field examination are also useful tools in the practitioner’s disease—monitoring armamentarium, particularly in the treatment of posterior and pan uveitis. They help in establishing a diagnosis, and determining the disease extent and sight-threatening features such as macular oedema, retinal and choroidal neovascularisation, and peripheral ischaemia, amongst others. Electrodiagnostic tests help in the management of birdshot chorioretinopathy, while fundus autofluorescence (FAF) can be useful in the follow-up of white dot syndromes.
5 Treatment of Non-Infectious Uveitis
5.1 Basic Principles
The choice of treatment depends on a number of factors as described above, including the specific disease entity. Highlighted below is an outline of the basic principles in treating patients with non-infectious uveitis. A more detailed description pertaining to the topical treatment modalities is described in Sect. 5.2, whereas descriptors of more invasive local and systemic treatments are elucidated in Sect. 5.3. A uveitis treatment algorithm is illustrated in Fig. 2.
Step 1: Corticosteroids (topical, periocular or systemic) are often the initial treatment of choice due to their rapid effect. For those requiring oral corticosteroids, the aim is to taper the dose and assess the response with each dose reduction (see Fig. 3).
Guidance for the use of immunosuppressive agents in non-infectious uveitis (adapted from Jabs et al. [24])
Step 2: Failure to achieve the desired response and/or achieve disease control with an acceptable corticosteroid dose necessitates the introduction of second-line immunomodulatory agents, which can be used alone or in combination with other classes of immunomodulatory agents. The choice of corticosteroid-sparing treatment is disease-dependent. Certain inflammatory eye conditions necessitate the early introduction of second-line immunomodulatory agents (e.g. birdshot chorioretinopathy). Control is assessed clinically and through the ability to reduce a patient’s oral prednisolone dose to 10 mg/day or less. Certain sight-threatening diseases, such as retinal vasculitis secondary to Behcet’s Disease, are a clear indication to administer biologic agents early in the disease course in combination with high-dose intravenous and oral corticosteroids, thus circumventing steps 1 and 2 [8].
Step 3: Failure to achieve disease control despite the use of at least two immunomodulatory agents in combination with oral prednisolone >7.5 mg, or an inability to tolerate corticosteroids or immunomodulatory agents, necessitates the incorporation of biologic agents.
5.2 Anterior Uveitis
Anterior uveitis is the most common anatomical form of uveitis encountered in practice. The aims of treatment are to suppress the inflammation and to prevent the formation of posterior synechiae (adhesions between the posterior iris and the crystalline lens). The latter complication may result in the impedance of aqueous outflow, leading to the development of secondary glaucoma. The delivery of treatment is often achieved locally in the majority of cases through the use of topical corticosteroids and cycloplegic agents.
Topical corticosteroids remain the topical immunosuppressant par excellence in the treatment of both acute and chronic anterior uveitis. High-potency agents such as prednisolone acetate and dexamethasone exist as either an acetate or alcohol formulation. The former formulation possesses greater anti-inflammatory properties than the latter [9]. As cataract formation is dose-dependent, one needs to establish an acceptable regimen in the long-term topical treatment of patients experiencing anterior uveitis. This was addressed in the retrospective review of Thorne et al. in which it was noted that there was an 87 % reduced risk of cataract formation in their paediatric cohort who were administering topical corticosteroids three drops daily or less for chronic anterior uveitis [10].
Topical corticosteroids are prescribed for severe acute anterior uveitis (AAU), either half-hourly or hourly depending on the degree of inflammation in the first instance. After a week of intensive treatment, the frequency of drop installation is reduced, tapering slowly over a number of weeks. Patients harbouring severe inflammation may also benefit from a short (2-week) course of oral corticosteroids in order to alleviate the signs and symptoms of their ocular disease. For patients whose disease becomes chronic and persistent, and in those who require more than three drops of topical corticosteroid to control their inflammation, systemic corticosteroid-sparing disease-modifying agents maybe required. Other topical corticosteroids such as loteprednol and rimexolone are found to be useful by some uveitis experts for low-grade chronic inflammation in those who may have a raised intraocular pressure response to stronger corticosteroids.
The SITE (Systemic Immunosuppressive Therapy for Eye Diseases) study cohort showed that periocular (mainly sub-tenons and orbital floor injections) corticosteroids can also be effective in reducing intraocular inflammation and improving visual acuity in cases of anterior uveitis [11].
Topical cycloplegics are often utilised in tandem with topical corticosteroids for several reasons: first, to break existing synechiae; second, to prevent the formation of synechiae; and third, to increase the integrity of the blood aqueous barrier and reduce pain. The agents typically employed include atropine 1 % twice daily, cyclopentolate 1 % three times daily and tropicamide 1 % four times daily. In cases of severe inflammation demonstrating a lack of response of acutely formed synechiae to topical mydriasis, a subconjunctival injection of mydricaine (atropine, epinephrine and procaine) is often administered.
5.3 Intermediate Uveitis and Posterior Uveitis
Intermediate uveitis is a common diagnosis seen in tertiary uveitis centres accounting for up to 12 % of cases [12]. Investigations employed to ascertain the underlying diagnosis are often unrewarding, although occasionally one may uncover an underlying systemic inflammatory disease such as sarcoidosis and multiple sclerosis. The aims of treatment are twofold: first, to treat any sight-threatening component of the disease (e.g. macular oedema); and second, to prevent the development of adverse sequelae such as uveitic glaucoma. In the absence of these risk factors, it is reasonable to merely observe such patients.
Unlike its intermediate counterpart, infective causes are more common in posterior uveitis and, as such, patients harbouring this form of the disease are subjected to more investigations. In addition, once an infective or malignant cause has been excluded, the majority of patients with active posterior uveitis will be offered urgent treatment.
In contrast to the anterior form of the disease, topical corticosteroids alone are inadequate in ameliorating the signs and symptoms relating to the intermediate and posterior uveitides. The practitioner’s therapeutic arsenal includes local treatments, systemic corticosteroids, systemic non-corticosteroid disease-modifying agents and biologic disease-modifying agents.
6 Therapeutic Options
6.1 Local Treatments
In general, local treatments may be offered to the following patients:
-
unilateral disease (typically intermediate uveitis);
-
intolerance to systemic treatment;
-
persistent macular oedema despite adequate control of uveitis with systemic therapy.
6.1.1 Corticosteroids: Periocular
Periocular injections of corticosteroids delivered either through the sub-tenon’s space or within the orbital floor enable the practitioner to target the drug mainly to the ocular structures in an attempt to mitigate systemic side effects. They allow practitioners to obtain better control of ocular inflammation and often enable reduction in systemic immunosuppressant therapy to be made. Triamcinolone 40 mg in 1 mL is commonly administered via this route.
Side effects include ptosis, cataract and raised intraocular pressure [13]. The latter may necessitate the introduction of topical hypotensive agents for a limited period of time in order to minimise the risk of optic nerve damage. It therefore remains incumbent on practitioners to regularly monitor the intraocular pressure regularly in patients receiving such corticosteroid therapy.
6.1.2 Corticosteroids: Intravitreal
In keeping with its periocular counterpart, intravitreal corticosteroids are associated with a greater risk of raised intraocular pressure and are more cataractogenic, but appear to be more effective in severe or refractory cases. These factors should always be borne in mind when administering these agents.
Intravitreal administration of triamcinolone acetonide (2 mg/0.05 mL or 4 mg/0.1 mL) enables a higher concentration within the vitreous compared with periocular delivery [14]. In addition, there is an increased risk of corticosteroid-induced raised intraocular pressure, cataract and endophthalmitis. Fortunately, the risk of the latter complication is low, with the incidence rates of infective and non-infective endophthalmitis with intravitreal triamcinolone lying between 0.09 and 0.87 % and 0.5 and 2 %, respectively [15].
The MUST (Multicenter Uveitis Steroid Treatment) trial was a randomised controlled trial comparing fluocinolone acetonide implants 0.59 mg with systemic immunosuppressive therapy in intermediate, posterior and pan uveitis [16]. This corticoid-releasing implant, designed to last for up to 30 months, consists of fluocinolone acetonide in a non-biodegradable polymer encased in a silicon elastomer cup. To insert this, the eye is opened and the implant is placed in the vitreous sutured to the sclera and the eye is closed, therefore it is a more invasive procedure than intravitreal injections. The corticosteroid is released in the vitreous via a hydrolysed polyvinyl alcohol membrane that permits a slow diffusion. It was approved by the US FDA in 2005 for the treatment of severe, non-infectious, posterior uveitis. The trial showed that there was no difference in the visual acuity at 2 years between the two arms of the trial, and the implant resulted in better control of macular oedema. Nearly all phakic patients receiving an implant underwent cataract surgery and 25 % of all patients receiving these implants underwent glaucoma surgery, but this did not result in any difference in visual acuity at 2 years.
The sustained-release dexamethasone 700 μg vitreous implant (Ozurdex®) is licensed for patients with chronic intermediate or posterior non-infectious uveitis. As a result of its hydrophilic nature, high concentrations can be achieved in the vitreous, allowing the drug to be more potent than its triamcinolone counterpart [17]. The HURON (cHronic Uveitis evaluation of the intRravitreal dexamethasONe) trial, a 26-week, prospective, multicentre, double-masked study in which patients with non-infectious, intermediate or posterior uveitis were randomised to receive either a 0.7 or 0.35 mg dexamethasone implant or sham procedure, demonstrated improvement in visual acuities and a concomitant reduction in inflammation in those who received the aforementioned corticosteroid implant [18].
Moreover, the same drug has been demonstrated to be particularly useful in patients with persistent macular oedema secondary to intermediate or posterior uveitis in whom their disease is otherwise quiescent. Of note, this is the only licensed medication in uveitis [19].
6.1.3 Antivascular Endothelial Growth Factor Therapy
Antivascular endothelial growth factor agents have a role in the management of secondary complications of uveitis, namely cystoid macular oedema, and retinal and choroidal neovascularisation. They are often employed as adjunctive therapy in the management of the aforementioned complications. The agent most commonly utilised in this setting is bevacizumab 1.25 mg in 0.05 mL [20].
6.1.4 Intravitreal Methotrexate
Intravitreal methotrexate has been investigated as a potential treatment for patients with non-infectious posterior uveitis in whom their disease is inadequately controlled with systemic immunosuppressive therapy. A single dose of 400 μg in 0.1 mL resulted in improvement of visual acuity in 79 % (30 of 38 eyes that were treated) of patients in the study by Taylor et al. [21]. In addition, of those who relapsed after one injection, an extended period of remission (up to 18 months) was noted after a second injection. Moreover, over 50 % of patients receiving systemic therapy at the beginning of the study were able to reduce this following a single injection of methotrexate. This, coupled with the fact that the drug has an excellent safety profile when injected into the eye, makes this type of treatment particularly attractive to the uveitis specialist.
6.1.5 Sirolimus
Sirolimus, a mammalian target of rapamycin (mTOR) inhibitor downregulating the T cell-mediated inflammatory cascade, was studied in a randomised, open-labelled study of non-infectious uveitis. Both subconjunctival and intravitreal administration were found to be safe and well tolerated, with improved visual acuity, decreased inflammation and reduced need for systemic corticosteroids [22]. Results of the phase III SAKURA (Study Assessing Double–masked Uveitis Treatment) study revealed a statistically significant benefit in achieving the primary outcome (reduction of vitreous haze) in patients receiving 2 monthly injections of 440 μg of sirolimus [23].
6.2 Systemic Treatments
6.2.1 Systemic Corticosteroids
Systemic corticosteroids remain the drug par excellence in ameliorating the signs and symptoms of sight-threatening ocular inflammation and are often the first-line agents employed in the treatment of non-infectious uveitis. Their potent anti-inflammatory effects notwithstanding, their prolific side-effect profile limits the use of prednisolone in doses >7.5 mg/day for more than 3 months [24].
Prednisolone is initially commenced at a high dose of 1 mg/kg/day orally. Alternatively, pulsed high-dose intravenous methylprednisolone 1 g/day on 3 consecutive days may be initiated for control of severe sight-threatening disease. The SITE cohort study data revealed that pulsed high-dose corticosteroids led to complete control of inflammation in 57 % of patients within 1 month of initiating therapy. No ocular side effects were observed, with one case of colon perforation during treatment [25].
A reducing corticosteroid regimen is implemented with the aim of achieving a dose of <7.5 mg/day within 3 months of treatment initiation. Failure to achieve this and/or failure to observe an adequate response within 4 weeks of high-dose corticosteroid treatment warrants the addition of further disease-modifying agents (see Fig. 4) [24].
6.2.2 Corticosteroid-Sparing Disease-Modifying Agents
6.2.2.1 Antimetabolites
Methotrexate was noted to be moderately effective in the treatment of non-infectious uveitis in the SITE cohort study, with 66 and 58.4 % of patients achieving control of inflammation within 1 year and corticosteroid withdrawal, respectively [26]. The benefits were greatest in those experiencing anterior uveitis (see Table 2).
Mycophenolate is an effective treatment for non-infectious uveitis and is frequently employed as the initial corticosteroid-sparing agent of choice by the authors. Long-term follow-up data relating to the drug in the treatment of all forms of non-infectious uveitis have shown it to be effective in 72 % of patients at the end of year 1 and 82 % after 2 years [27]. Moreover, corticosteroids were stopped completely in 40 % of the same cohort, and 33 % were able to discontinue mycophenolate at the end of 5 years. The probability of discontinuing mycophenolate due to efficacy was 33 % at the end of 5 years [28].
Azathioprine, a purine nucleoside analogue, is moderately effective in the treatment of non-infective uveitis, particularly the intermediate form of the disease. In the SITE study, 69 % of patients experiencing intermediate uveitis gained control of their disease after 6 months with azathioprine compared with 44 % and 24 % in patients treated for posterior or pan uveitis and anterior uveitis respectively.
Galor et al. compared methotrexate, azathioprine and mycophenolate for non-infectious ocular inflammation and found that the time to control inflammation was faster with mycophenolate than with methotrexate [29]. In addition, azathioprine was associated with a higher rate of treatment-related side effects compared with the other two agents.
6.2.2.2 Calcineurin Inhibitors
Cyclosporin was noted to be effective in controlling ocular inflammation at 6 months in 39.3 and 29.2 % of patients receiving this drug for intermediate uveitis and posterior/panuveitis, respectively [30]. However, corticosteroid-sparing effects were only observed in 24.1 % of those receiving the drug for intermediate uveitis and 16.2 % receiving the drug for posterior/pan uveitis. Doses in the range of 151–250 mg were less commonly associated with side effects, and toxicity was more common in the older age group [30].
In a randomised controlled trial comparing tacrolimus with tacrolimus in combination with prednisolone for the maintenance of remission in non-infectious uveitis, the investigators noted that in those patients achieving control of inflammation with tacrolimus, corticosteroids could be withdrawn completely in two-thirds of this cohort [31].
6.2.2.3 Alkylating Agents
In view of the risk of its relatively high side effect profile, cyclophosphamide is considered either when other forms of immunosuppression have failed or there is a systemic indication to utilise this drug (e.g. small vessel vasculitis). Pulsed intravenous administration of cyclophosphamide (15 mg/kg) in combination with pulsed intravenous methylprednisolone (10 mg/kg) at two weekly intervals for the first three pulses followed by 3-weekly pulse intervals for the following three weeks is particularly effective for scleritis and non-infectious uveitis refractory to other types of immunomodulatory therapy [32]. Moreover, the pulsed regimen is associated with less side effects than its oral counterpart.
6.2.2.4 Biologic Disease-Modifying Treatments
Biologic agents are recombinant proteins or antibodies that are targeted against specific molecules or cytokines involved in the inflammatory cascade. Monoclonal antibodies which bind to the TNFα molecule, thus inhibiting its pro-inflammatory effects, are the biologic agents most studied in the treatment of ocular inflammatory disease. Their benefits notwithstanding, the cost of such agents prohibit their use as a first-line agent in the majority of cases.
In 2014, an expert panel of uveitis specialists convened and produced recommendations for the use of anti TNFα biologic agents in patients with ocular inflammatory disorders [8]. The two agents that are commonly used are infliximab (which necessitates regular intravenous infusions) and adalimumab (delivered subcutaneously). Both these agents may be considered as first-line immunomodulatory agents for the treatment of ocular manifestations of Behcet’s disease. In addition, the panel recommended that they should be considered as second-line immunomodulatory agents for the treatment of uveitis secondary to juvenile idiopathic arthritis, and potential second-line agents for the treatment of severe uveitis associated with seronegative spondyloarthropathy and severe posterior/pan uveitis refractory to oral immunomodulation treatment [8].
In the UK, it is proposed that access to the use of adalimumab and infliximab would be provided through specialised uveitis networks, and that the following patients would be eligible [33]:
-
Patients whose condition has proved to be refractory to treatment despite more than 10 mg/day of prednisolone and at least two immunomodulatory drugs.
-
Patients who are clinically unable to continue the above treatments because of severe intolerance or toxicity.
-
Patients who manifest aggressive disease with risk of rapid, permanent and profound visual loss (e.g. retinal vasculitis secondary to Behcet’s disease).
Anti-TNF therapy has numerous contraindications/side effects, including reactivation of latent TB infection, chronic hepatitis B and C, and the presence of demyelinating disorders. Since there is an association with intermediate uveitis and multiple sclerosis, all patients harbouring this disease, in whom consideration of anti-TNF treatment is being made, advocate neuroimaging before a decision is made to prescribe the drug [8]; however, practice may vary in some countries based on local guidelines or practice patterns. For example, in the UK, patients with intermediate uveitis are not routinely investigated for multiple sclerosis, and those with multiple sclerosis are not prescribed infliximab.
Paradoxically, uveitis has been observed as a side effect of anti-TNF agents in patients treated for non-ocular manifestations of rheumatological diseases. Of these, the recombinant fusion protein etanercept appears to be associated with the greatest number of drug-induced uveitis cases [8].
In randomised controlled trials, secukinumab, a human monoclonal antibody blocking IL-17A, did not meet the primary endpoint of reducing uveitis recurrence or inflammation but did reduce the need for concomitant immunosuppressive medications [34].
Rituximab, an anti CD20 chimeric antibody administered as intravenous infusions, results in peripheral depletion of mature B lymphocytes. Various case reports have shown its efficacy in controlling inflammation related to juvenile idiopathic arthritis-related uveitis and Behcet’s disease [35].
Finally, tocilizumab, a fully humanised antibody that binds to both soluble and membrane-bound IL-6 receptors, has been shown to be effective in controlling inflammatory macular oedema that was hitherto refractory to anti-TNF agents and oral immunomodulatory treatment [36].
7 Comparison of Immunosuppressive Agents
A randomised controlled trial comparing methotrexate with mycophenolate for non-infectious uveitis found both the drugs to be comparable in terms of efficacy, tolerability and adverse events. However, patients receiving methotrexate showed a better resolution of macular oedema and were more likely to achieve treatment success [37].
In a comparison of methotrexate, azathioprine and mycophenolate, it was found that mycophenolate controlled inflammation quicker that methotrexate, and azathioprine was more likely to be associated with side effects [29].
8 Treatment Failure and Refractory Uveitis
Success of immunomodulatory therapy is usually defined as the ability to taper down the corticosteroids to ≤7.5 mg/day (or approximately 0.1 mg/kg of body weight) along with control of inflammation. In cases of sub-optimal response, increasing the dose of the existing corticosteroid-sparing medication or adding another agent belonging to a different class of drugs, i.e. combination therapy, may be tried.
In cases of non-response, switching of corticosteroid-sparing medication to another class with a different mechanism of action is advisable. For breakthrough episodes of inflammation, systemic corticosteroid ‘rescue’ therapy is needed to tide over the acute crisis.
A recent study revealed that switching, or combinations of immunosuppressive agents, can achieve control of inflammation. Fifty to 100 % of patients achieved ‘success’ after switching to a new agent, whilst 50–71 % benefited from a combination therapy. Infliximab was the most commonly used biologic agent, with success in 80 % of patients [38].
Adalimumab was found to be effective in refractory non-infectious uveitis, with a 10-week success rate of 68 % and 39 % at week 50 without significant drug-related adverse events [39].
Cordero-Coma et al. report two case of refractory uveitis (juvenile idiopathic arthritis and idiopathic retinal vasculitis) with uncontrolled inflammation on combination therapy with adalimumab and methotrexate, and adalimiumab and cyclosporine A, respectively. Both patients achieved control of inflammation 6–7 months after initiation of golimumab without any significant side effects [40].
9 Health-Related Quality of Life in Uveitis
Ultimately, the patients’ quality of life is the most important outcome of any therapeutic response. Visual acuity, clinical examination and imaging all aid in evaluating and achieve this outcome. Binocular high-contrast visual acuity was found to be a good indicator of how patients with uveitis performed in real-life situations [41].
The MUST trial found no significant difference in health utility analysis scores between patients with intermediate uveitis and posterior uveitis. The majority of patients had health-related quality of life scores comparable to the normal population, and vision in the better-seeing eye seemed to be predictive of the visual function scores, with colour vision being the least affected [42].
10 Conclusions
In order to optimise the therapeutic response to treatment, a number of different facets of care need to be addressed. Precise and early diagnosis, excluding masquerade syndromes, is the key to early therapeutic intervention. Treatment should be appropriately selected according to the anatomical sites of inflammation, the diagnosis and known prognosis, and whether there is systemic inflammatory drive. Additional considerations are coexistent ocular and non-ocular pathology, counselling about potential side effects and, not least, patient wishes. Treatment response needs to be accurate, and preferably determined in an objective manner. Although corticosteroid treatment is the mainstay of most treatment strategies, timely escalation of therapeutic options to include corticosteroid-sparing medication biologic therapy and/or local therapy is required in a significant portion of patients.
References
Rothova A, Suttorp-van Schulten MS, Frits Treffers W, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–6.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature (SUN) for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16.
Deschenes J, Murray PI, Rao NA, et al. International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1):1–2.
Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218:223–36.
Boyd SR, Young S, Lightman S. Immunopathology of the non infectious posterior and intermediate uveitides. Surv Ophthalmol. 2011;46:209–33.
Lin P, Suhler EB, Rosenbaum JT. The future of uveitis treatment. Ophthalmology. 2014;121:365–76.
Nussenblatt RB. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–71.
Levy-Clarke G, Jabs DA, Read RW, et al. Expert panel recommendations for the use of anti-tumour necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121:785–96.
Simonini G, Cantarini L, Bresci C, et al. Current therapeutic approaches to autoimmune chronic uveitis in children. Autoimmun Rev. 2010;9:674–83.
Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2010;117(7):1436–41.
Sen HN, Vitale S, Gangaputra SS, et al. Periocular corticosteroid injections in uveitis. Effects and complications. Ophthalmology. 2014;121:2275–86.
McCannel CA, Holland GN, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology. Am J Ophthalmol. 1996;121:35–46.
Lyon F, Gale RP, Lightman S. Recent developments in the treatment of uveitis: an update. Expert Opin Investig Drugs. 2009;18:609–16.
Inoue M, Takeda K, Morita, et al. Vitreous concentrations of triamcinolone acetonide in human eyes after vitreal or subtenon injection. Am J Ophthalmol. 2004;138:1046–8.
Roth DB, Flynn HW Jr. Distinguishing between infectious and non-infectious endophthalmitis after intravitreal triamcinolone injection. Am J Ophthalmol. 2008;146:346–7.
Kempen JH, Altaweel AA, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior and panuveitis. The Multicentre Uveitis Steroid Treatment Trial. Ophthalmology. 2011;118(10):1916–26.
Lobo AM. Dexamethasone intravitreal implant for the treatment of non-infectious uveitis. Clin Ophthalmol. 2011;5:1613–21.
Lowder C, Belfort R, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–53.
Cao JH, Mulvahill M, Zhang L, et al. Dexamethasone intravitreal implant in the treatment of persistent uveitic macular edema in the absence of active inflammation. Ophthalmology. 2014;121:1871–6.
Gulati N, Forooghian F, Lieberman R, et al. Vascular endothelial growth factor inhibition in uveitis: a systematic review. Br J Ophthalmol. 2011;95(2):162–5.
Taylor SRJ, Banker A, Schalen A, et al. Intraocular methotrexate can induce extended remission in some patients in non-infectious uveitis. Retina. 2013;33(10):2149–54.
Nguyen QD, Ibrahim MA, Watters A, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6 month results of the SAVE study. J Ophthalmic Inflamm Infect. 2013;3(1):3–32.
American Academy of Ophthalmology. Retina 2014 reaching new heights. San Francisco: American Academy of Ophthalmology; 2014. p. 119–21.
Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513.
Charkoudian LD, Ying G, Pujari SS, et al. High-dose intravenous corticosteroids for ocular inflammatory diseases. Ocul Immunol Inflamm. 2012;20(2):91–9.
Gangapurra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116:2188–98.
Doycheva D, Zierhut M, Blumenstock G, et al. Long-term effects of mycophenolate mofetil in chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2011;249:1235–43.
Gangapurra S, Newcomb CW, Liesegang TL, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. 2009;148(4):500–9.
Galor A, Jabs DA, Leder HA, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for non-infectious ocular inflammation. Ophthalmology. 2008;115(10):1826–32.
Kacmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117:576–84.
Lee R, Greenwood R, Taylor H, et al. A randomized trial of tacrolimus versus tacrolimus and prednisolone for the maintenance of disease remission in non-infectious uveitis. Ophthalmology. 2012;119:1223–30.
Khan IJ, Barry RJ, Amissah-Arthur KN, et al. Ten year experience of pulsed intravenous cyclophosphamide and methylprednisolone (PICM protocol) in severe ocular inflammatory disease. Br J Ophthalmol. 2013;97:1118–22.
NHS England. Clinical commissioning policy: infliximab and adalimumab as anti-TNF treatment options for adult patients with severe refractory uveitis. NHS England Specialised Services Clinical Reference Group Specialised Ophthalmology. https://www.engage.england.nhs.uk/consultation/specialised-services-consultation/user_uploads/uveitis-adults-policy.pdf. Accessed 19 Nov 2015.
Dick AD, Tugal-Tutkun I, Foster CS, et al. Secukinumab in the treatment of non-infectious uveitis: results of three randomized, controlled trials. Ophthalmology. 2013;120:777–87.
Tomkins-Netzer O, Taylor SRJ, Lightman S. Can rituximab induce long-term disease remission in patients in patients with intra-ocular non-infectious inflammation? Ophthalmologica. 2013;230:109–15.
Mesquida M, Molins B, Lorenc V, et al. Long- term effects of tocilizumab therapy for refractory uveitis-related macular oedema. Ophthalmology. 2014;121:2380–6.
Rathinam SR, Babu M, Thundikandy R, et al. A randomized clinical trial comparing methotrexate and mycophenolate mofetil for non infectious uveitis. Ophthalmology. 2014;121:1863–70.
Joshi L, Talat L, Yaganti S, et al. Outcomes of changing immunosuppressive therapy after treatment failure in patients with non infectious uveitis. Ophthalmology. 2014;121:1119–24.
Suhler EB, Lowder CY, Goldstein DA, et al. Adalimumab therapy for refractory uveitis: results of a multicentre, open label, prospective trial. Br J Ophthalmol. 2013;97:481–6.
Cordero-Coma M, Salom D, Diaz-Llopis M, et al. Golimumab for uveitis. Ophthalmology. 2011;118:1892.e3–4.
Gardiner AM, Armstrong RA, Dunne MCM, Murray PI. Correlation between visual function and visual ability in patient with uveitis. Br J Ophthalmol. 2002;86:993–6.
Frick KD, Drye LT, Kempen JH, et al. Associations among visual acuity and vision- and health-related quality of life among patients in the multicenter uveitis steroid treatment trial. Invest Ophthalmol Vis Sci. 2012;53:1169–76.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Richard Gale and Susan Lightman have received grants, consulting fees, lecture fees, or fees for participation in review activities from Allergan. Archana Airody and Gregory Heath have no conflict of interests to declare.
Funding
No funding was used to assist in the preparation of this article.
Rights and permissions
About this article
Cite this article
Airody, A., Heath, G., Lightman, S. et al. Non-Infectious Uveitis: Optimising the Therapeutic Response. Drugs 76, 27–39 (2016). https://doi.org/10.1007/s40265-015-0502-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0502-y