Abstract
Introduction
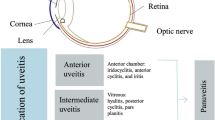
Uveitis encompasses a wide variety of sight-threatening diseases characterized by intraocular inflammation. It is often classified as infectious and non-infectious uveitis. Unlike infectious uveitis, a distinct infectious agent cannot be identified in non-infectious uveitis and disease origin is usually autoimmune, drug related, or idiopathic.
The Issue at Hand
Non-infectious uveitis can often have a relapsing-remitting course, making it difficult to treat, and poses a significant challenge to ophthalmologists. The autoimmune nature of non-infectious uveitis warrants the use of anti-inflammatory and immunomodulatory agents for disease control. However, a subset of patients has persistent or recurrent ocular inflammation despite appropriate treatment, stressing the need for newer therapies aimed at more specific inflammatory targets such as tumour necrosis factor (TNF) alpha agents, anti-interleukin agents, and anti-interleukin receptor agents.
Objectives
This article discusses the various medical options available for the treatment of non-infectious uveitis in the light of the most recent evidence.
Conclusion
Successful management of non-infectious uveitis requires the clinician carefully balance advantages and disadvantages of each new and old therapy while considering individual circumstances. Counselling regarding the benefits and complications of each therapy can help patients make an informed choice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uveitis is a term used to describe a group of heterogeneous diseases characterised by inflammation of the uveal tract and is broadly classified as infectious and non-infectious. Non-infectious uveitis is often related to systemic autoimmune conditions or is idiopathic. Clinical presentation is variable, and symptoms may include blurred vision, ocular pain, photophobia, and significant visual impairment [1]. Uveitis accounts for 15–20% of all cases of legal blindness in the USA [2], with uveitic macular oedema being a common cause of vision loss [2, 3].
The relapsing–remitting disease course makes management of non-infectious uveitis difficult. Traditionally, local or systemic corticosteroids have been the mainstay of treatment, but the associated side effects often limit their use [4].
With this in view, there have been new developments for treatment of non-infectious uveitis. The agents gaining recent recognition are sustained release corticosteroid implants and biologic agents, including agents targeting TNF alpha, a pro-inflammatory cytokine implicated in intraocular inflammation.
Non-infectious uveitis is a heterogeneous disease entity, with each specific aetiology leading to different presentations and complications. One would be remiss to generalise treatments from one disease entity to all non-infectious uveitis.
This article discusses the various medical options available for the treatment of different forms of non-infectious uveitis in the light of the most recent evidence with emphasis on the novel biologic agent adalimumab (ADA).
Corticosteroids
Systemic steroids
Mechanism of action
Corticosteroids suppress inflammation by inhibiting the expression of various pro-inflammatory factors. They also promote the expression of various anti-inflammatory factors.
Summary
Systemic corticosteroid treatment has been one of the reliable treatments for uveitis for many years. Cortisone, hydrocortisone, prednisone, and fludrocortisone are some of the steroids available for oral use. Prednisone is the most common oral steroid used for uveitis. It is initiated at 0.5–1 mg/kg daily, followed by slow tapering once inflammation control is achieved. Ideally, the dose should be less than 0.1 mg/kg daily within 3 months of initiation [5]. Table 1 summarises prednisone’s ideal dosage and tapering schedule, as well some relevant points to consider. Certain baseline investigations must be undertaken before initiating therapy. Blood pressure, weight, fasting glucose (or HbA1C), lipids, and bone mineral density should be recorded prior to therapy. Details of a representative monitoring schedule are listed in Table 1. If chronic suppression requires an excess of 10 mg/day of prednisone/prednisone equivalent, a concurrent immunosuppressive agent should ideally be considered. This would necessitate further baseline testing, listed later in this article in other tables. A physician is obliged to counsel the patient extensively prior to therapy regarding possible side effects and the fact that sudden cessation can precipitate adrenal crisis.
Side effects
Despite their effectiveness, their side effects limit corticosteroid use. Long-term systemic therapy is associated with hypertension, osteoporosis, opportunistic infections, mania, and hyperglycaemia [4].
Local steroids
Periocular and intravitreal injections
Mechanism of action
The mechanism of action of local steroids is identical to that of systemic; the only difference between the two is the mode of delivery to the eye.
Summary
Triamcinolone acetonide injections (TAIs) have been approved by the FDA for intraocular use [6] and have shown to be effective in the treatment of posterior uveitis [7]. Bleriot et al. [8] assessed the safety and efficacy of subconjunctival TAIs retrospectively in 2014. The 12-month follow-up for 31 eyes showed that the mean logMAR visual acuity improved from 0.36 ± 0.27 at baseline to 0.24 ± 0.21 (p = 0.0371) at 12 months and there was no significant increase in IOP or rate of cataract development.
Sen et al. [9] evaluated the effectiveness and complications of periocular depot corticosteroid injections in a retrospective cohort of 1192 eyes of 914 patients with ocular inflammatory disorders and found 72.7% of eyes in remission. Improvement in visual acuity was noted in 50%.
Both intravitreal and orbital floor TAIs have been used in practice [10]. Roesel et al. [11] compared the two in a retrospective study of 97 uveitis patients with macular oedema resistant to other treatments. They reported no significant difference in visual acuity between the two groups at the 3-month follow-up point (p = 0.23), and macular oedema was significantly reduced in the intravitreal group compared to the orbital floor group (p < 0.01). Ocular hypertension (IOP > 21 mmHg) was noted in 21% of the intravitreal group and none of the orbital floor group (p < 0.01).
Side effects
Intraocular hypertension, cataract development, and cataract progression are all possible side effects of these local injections. Clinicians should be wary of the serious adverse effect of endophthalmitis, which is a possibility with multiple intravitreal injections to the same eye. Retinal tears and detachments are also a possibility and should be considered as well.
Sustained release steroid implants
Mechanism of action
The implants are inserted directly into the posterior segment, and release corticosteroids over an extended period (months).
Summary
Ozurdex (IDX), an intravitreal sustained release 0.7 mg dexamethasone implant, was FDA approved in 2010 for non-infectious uveitis involving the posterior segment. Zarranz-Ventura et al. published an 82-eye (63 patient) multicentre retrospective cohort study in 2014. The 63 patients received 142 IDX injections over 35 months. The mean logMAR visual acuity improved from 0.68 ± 0.4 (mean ± standard deviation) at baseline to 0.52 ± 0.5 at 12 months (p < 0.01). At the 12-month follow-up, 40.7% of 54 remaining eyes had undergone 2 injections and 11.2% had required ≥ 3 injections [12].
Retisert (AFI), a non-biodegradable 0.59 mg fluocinolone acetonide pellet, was FDA approved for use in non-infectious posterior uveitis in 2005 [13]. Sangwan et al. published the results of a 3-year, 239-patient, RCT evaluating AFI with non-infectious uveitis in 2015. They compared two implants: the standard 0.59 mg AFI and a newer 2.1 mg AFI. Overall, recurrence rates for eyes treated with any AFI decreased significantly from 42.3% prior to implantation, to 25.9% during the 3-year post-implantation period (p = 0.0003). Significantly more eyes receiving AFI gained ≥ 3 lines of BCVA compared to fellow eyes that did not receive AFI (p ≤ 0.005). However, nearly all eyes receiving AFI (94.9%) required cataract surgery subsequent to treatment, and IOP elevations were noted in noted in 68.6% of study eyes. Comparing the two implants, there was no significant difference in IOP elevations [14].
A similar study comparing AFI and IDX in non-infectious uveitis was published by Arcinue et al. in 2013. It was a 27-eye comparative case series evaluating recurrence rates of uveitis after implantation. They found no statistically significant difference between the two implants’ recurrence rates (p = 0.41). However, eyes receiving IDX were 5 times more likely to require a second implant (p = 0.02). Eyes receiving AFI were likelier to require more glaucoma medications, surgery, or laser (p = 0.02), and were 4.7 times more at risk of cataract progression than IDX eyes (p = 0.04) [15].
Iluvien (IFI) is an injectable intravitreal fluocinolone acetonide. It releases lower amounts of the drug than AFI (0.2 ug or 0.5 ug/day vs. 0.59 ug/day) [16]. It was approved for use in diabetic macular oedema by the FDA in 2014. There are currently no published studies exploring IFI’s utility in non-infectious uveitis. However, there is a pilot phase-I study underway to investigate this [17].
Side effects
Notable side effects have been mentioned previously for local steroid injections. However, AFI appears to have the worst side-effect profile, with glaucoma and cataract progression being more prevalent in AFI compared to the other implants. A summary of steroidal treatment options is presented in Table 2.
Antimetabolites
Methotrexate (MTX)
Mechanism of action
Methotrexate inhibits dihydrofolate reductase and causes a defect in purine and pyrimidine synthesis, thereby inhibiting DNA production. However, a second mechanism is the suppression of amino-imidazole-carboxamide-ribonucleotide transformylase, which causes a build-up on adenosine. Adenosine suppresses lymphocytes, macrophages, dendritic cells, and neutrophils; therefore, this suppresses inflammation.
Summary
In 2001, Samson et al. published one of the largest case series studying MTX use in controlling ocular inflammation in 160 patients with non-infectious uveitis. Inflammation was successfully controlled in 76% of patients. Gangaputra et al. identified 384 patients from the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) cohort retrospectively and evaluated MTX treatment outcomes in non-infectious ocular inflammatory diseases. They reported that ocular inflammatory disease was suppressed in 66% of patients at 1 year. Treatment success was highest for anterior uveitis and scleritis [18].
Side effects
Adverse effects of MTX therapy include fatigue, stomatitis, debilitating nausea and are usually transient. Hepatotoxicity, cytopenia, and interstitial pneumonitis are amongst the more serious effects [18,19,20,21].
Mycophenolate Mofetil (MMF)
Mechanism of action
Mycophenolate Mofetil is an inhibitor of inosine monophosphate dehydrogenase, the rate-limiting enzyme responsible for the de-novo synthesis of guanosine. Lymphocytes are somewhat more dependent on this pathway than other cell types, and the drug has a higher affinity for the isoform present in activated lymphocytes. This results in a cytostatic effect on lymphocytes and a decrease in inflammation.
Summary
The multicentre SITE retrospective cohort conducted in 2010 reported control of ocular inflammation with MMF in 55, 65, and 51% of the 236 patients with anterior, intermediate, and posterior/panuveitis, respectively, within 6 months that improved to a mean of 73% at 1 year [22].
Side effects
GI-related symptoms are most commonly seen. Other less frequently reported included leucopenia and opportunistic infections.
Azathioprine (AZT)
Mechanism of action
Azathioprine is a purine analog, a pro-drug of 6-mercaptopurine. It incorporates into replicating DNA and blocks the replication process. It also hinders the de-novo synthesis of purines, making it more specific to lymphocytes, as they lack the salvage pathway.
Summary
Pasadhika et al.’s [24] retrospective review of the SITE cohort demonstrated that AZT controlled inflammation in 62% of the studied cohort of 145 patients. A RCT of 73 patients by Yazici et al. in 1990 showed inflammation free rates in more than 50% patients [25]. Incidence of side effects was reported to be higher in the AZT group (0.29/PY) by Galor et al. [21] compared to the other antimetabolites (0.14/patient years [PY] for MTX and 0.18/PY for MMP). Rate of discontinuation was similarly higher with AZT in a study conducted by Pasadhika et al. [24]; AZT was discontinued due to side effects at a rate of 0.16/PY, versus 0.13/PY for MTX.
Side effects
Common reported side effects causing discontinuation in Pasadhika et al.’s study included gastrointestinal reactions, bone marrow suppression, elevated liver enzymes, infection, and allergic reactions.
Cyclophosphamide (CYCP)
Mechanism of action
Cyclophosphamide alkylates the guanine base in DNA, leading to cell death. It has a cytotoxic effect on both resting and dividing lymphocytes.
Summary
The SITE retrospective cohort of 215 patients evaluating cyclophosphamide found ocular inflammation in uveitis to be controlled in 50% patients within 6 months that subsequently improved to 81% by 12 months. Steroid sparing effect similarly showed a time dependent improvement from 30.1% at 6 months to 61.2% at 12 months. Ocular remission rates with subsequent discontinuation of therapy were highest with CYCP at 63% compared to SITE cohorts [26].
Side effects
Most common adverse effects include leucopenia and cystitis/haematuria. Opportunistic infections especially Pneumocystis carinii pneumonia have also been noted [26, 27]. Other serious effects include secondary malignancy, teratogenicity, and gonadal dysfunction. Treatment with CYCP is hence recommended in severe, vision-threatening causes of ocular disease [26].
Cyclosporine A (CsA)
Mechanism of action
Cyclosporine blocks the calcineurin pathway in T cells, blocking transcription of DNA. It also blocks the JNK and p38 signalling pathways in T cells, which results in it being a very potent T cell inhibitor.
Summary
There are multiple studies establishing cyclosporine’s efficacy in rapidly controlling ocular inflammation. The SITE CsA report published in 2010 by Kacmaz et al. reported that 33.2% of the 373 patients had complete control of disease by 6 months that went up to improve by 12 months at 51.9% [28]. Cyclosporine is contraindicated in neuro-Behcet’s disease, as CsA has serious neurological side effects [29].
Side effects
Side effects include metabolic abnormalities, nephrotoxicity, gingivitis, and hirsutism. Out of 373 patients in Kacmaz et al’s study, 11% had to cease the drug within a year due to side effects [28]. The most common adverse effect appears to be neurotoxicity, which occurs in up to 40% of patients. The most serious complication is a reversible posterior leukoencephalopathy syndrome [30].
Tacrolimus
Mechanism of action
Like CsA, tacrolimus too is a calcineurin inhibitor. After it binds to FK506 binding protein, the complex formed inhibits calcineurin in a manner similar to CsA.
Summary
Multiple, non-randomised clinical studies have shown tacrolimus’ effectiveness in uveitis cases refractory to CsA, including Behcet’s disease-associated uveitis [31, 32]. The favourable outcomes were maintained in a long-term follow-up study. A recent RCT by Murphy et al. comparing the two calcineurin inhibitors in 37 patients found them to be comparable with response rates of 67% for CsA versus 68% for tacrolimus [33].
Side effects
Tacrolimus, however, has a significantly more favourable side-effect profile than CsA, particularly its effect on systemic blood pressure, serum cholesterol level, hyperglycaemia, and overall cardiovascular morbidity [33, 34].
Biologics
Anti-TNF agents
Tumour necrosis factor plays a key role in the inflammatory process, and molecules targeting it are particularly useful in halting the process and therefore in various forms of non-infectious uveitis. To this effect, various monoclonal antibodies are employed in this class. A side effect of using them is the sensitisation of the body to their use and development of antibodies against these molecules. However, adalimumab, being a fully human antibody, has a lower risk of this happening and is therefore less immunogenic.
Adalimumab (ADA)
Mechanism of action
Adalimumab is a fully human monoclonal antibody targeting TNF alpha.
Summary
Adalimumab’s role in the treatment of non-infectious uveitis was first recognised in 2008 by Diaz-Llopis et al. [35]. Several studies have subsequently established the effectiveness of ADA in non-infectious uveitis associated with autoimmune systemic diseases. In 2009, Rudwaleit et al. published a 1250-patient phase-III prospective study on the use of ADA for anterior uveitis in ankylosing spondylitis and reported 51% of patients as having experienced a significant decrease in anterior uveitis flares (p < 0.001) [36]. In 2012, Diaz-Llopis et al. published a 131-patient prospective multicentre case series evaluating ADA therapy in refractory non-infectious uveitis of multiple aetiologies. They found a statistically significant improvement in the mean intraocular inflammation between baseline and the 6-month visit (p < 0.001). A statistically significant improvement in mean baseline logMAR Best Corrected Visual Acuity (BCVA) and the mean logMAR BCVA at the 6-month visit (p < 0.001) was also reported. In a study by Bawazeer et al., complete resolution of ocular inflammation was reported in 10 out 11 patients with Behcet’s disease-associated uveitis within 4 weeks of initiation of ADA therapy [37]. Suher et al. assessed the use of ADA in a prospective, multicentre trial in 31 subjects with refractory non-infectious uveitis including those with Vogt–Koyanagi–Harada (VKH) disease, and birdshot retinochoroidopathy (BSCR). 68% of the participants achieved clinical response at 10 weeks of treatment initiation, and 39% had sustained effect after 50 weeks [38].
In 2014, Simonini et al. published a meta-analysis comparing ADA, infliximab (IFN), and etanercept (ETA), in paediatric autoimmune chronic uveitis. Data analysed from a total of 23 studies (229 patients) showed ADA to have superior efficacy resulting in improvement of inflammation in 87% of patients, compared to IFN (72%) and ETA (33%) [39].
The major trials evaluating ADA’s efficacy and safety were the VISUAL I and VISUAL II trials. VISUAL I was a multicentre randomised controlled trial (RCT) assessing ADA’s efficacy and safety in 217 patients with active, non-infectious uveitis while receiving systemic corticosteroid treatment. Results showed that patients receiving ADA were less likely to experience treatment failure (p < 0.001) and had a statistically significant improvement in logMAR BCVA compared to the placebo group (26.2, 95% CI 7.0–45.3; p = 0.008). The median time to treatment failure was 13 weeks for the placebo group and 24 weeks for the ADA group. Macular oedema risk was reduced by 67% in the ADA group versus placebo (p = 0.023) [40, 41].
VISUAL II was a 229-patient multicentre RCT which assessed ADA in patients with inactive, non-infectious uveitis while on systemic corticosteroid therapy. The study showed that patients who received ADA were less likely to have treatment failure when compared to the placebo group (p = 0.004). Median time to treatment failure was 8.3 months for the placebo group and could not be estimated for the ADA group, as over 50% of the ADA-treated patients did not experience treatment failure [42].
Based on the findings of the VISUAL I and VISUAL II trials, ADA was approved by the FDA for use in non-infectious uveitis in 2016 and is currently the only non-steroid treatment approved for this purpose [43].
Side effects
In 2012, Burmester et al., in an analysis of 71 clinical trials, noted serious opportunistic infection as the most commonly encountered side effect of ADA. Malignancy risk, which was an initial concern, was not higher than the general population [44]. Notably, there were no serious opportunistic infections reported for psoriasis patients. While this study was published prior to ADA’s approval for uveitis, the dosage and treatment regimen for psoriasis and uveitis is similar (80 mg loading dose, then 40 mg every other week starting one week after loading) [45]. It is therefore reasonable to assume that adverse effects reported for psoriasis would be comparable to those in non-infectious uveitis.
Infliximab (INF)
Mechanism of action
Infliximab is a chimeric (human/mouse) monoclonal antibody targeting TNF alpha.
Summary
Infliximab’s use in uveitis was first reported in 2001 [46]. In 2012, Markomichelakis et al. undertook a prospective, pilot study of 15 patients that assessed the safety and efficacy of intravitreal (IV) INF for sight-threatening uveitis due to Behcet disease. They found that patients suffered no ocular or extra-ocular side effects in the first month and there was a significant increase in logMAR BCVA (mean = 0.30; p < 0.0001). Anterior chamber cells and vitreous haze also decreased significantly (both p < 0.0001). Central macular thickness decreased from a baseline mean of 434–309 µm at the end of follow-up (p < 0.0001) [47]. A second study undertaken by the same author comparing a single infliximab infusion (5 mg/kg, 19 eyes), methylprednisolone (1 g/day for 3 days, 8 eyes), and intravitreal triamcinolone acetonide injection (4 mg, 8 eyes), demonstrated that those receiving infliximab achieved significantly faster resolution of panuveitis attacks in Behcet’s disease than those given corticosteroid therapy. They concluded that single infliximab infusions should thus always be considered, even if only as adjunct [48]. Similar results on its efficacy were demonstrated by Suhler et al. in 4 patients with Behcet’s-related panuveitis, and it has also been shown to be more effective than conventional therapy [49, 50].
In 2016, Vallet et al. published a 160-patient multicentre retrospective chart review comparing the ADA and INF [51]. They found the two agents did not differ significantly in achieving complete response (p = 0.39), and side effects (p = 0.089). Overall response rate was 97% amongst the INF group and 95% in the ADA group.
Infliximab may also be effective for management of uveitis associated with Vogt–Koyanagi–Harada disease, sarcoidosis, HLA-B27 birdshot retinochoroidopathy, par planitis, multifocal choroiditis, and idiopathic uveitis [52].
Side effects
As with ADA, the most serious side effect appears to be opportunistic infection. Infusion reactions are one of the more common side effects [53].
Etanercept (ETA)
Mechanism of action
Etanercept is a dimeric fusion protein consisting of the extracellular ligand-binding portion of the p75 TNF receptor that is covalently bonded to the Fc portion of human IgG1. It functions as a decoy receptor for TNF alpha.
Summary
Etanercept was investigated as a possible agent for non-infectious uveitis in 2001 by Reiff et al. in a prospective study that included 16 paediatric eyes [54]. A 20 patient RCT assessing the efficacy of ETA was published in 2003 by Foster et al. They found no significant difference in the efficacy of ETA as compared to placebo in preventing relapses in patients being tapered from methotrexate (p = 0.66; Fisher exact test) [55]. Etanercept appeared to perform poorly against ADA and INF in the meta-analysis published by Simonini et al., with both ADA (p < 0.001) and INF (p < 0.001) exhibiting superior efficacy compared to ETA [39]. Although etanercept has been successfully shown to control Behcet’s uveitis-related inflammation in different case reports, the resolution was not sustained once the drug was stopped. Based on existing data, etanercept is currently recommended as a second line agent for management of ocular inflammation after infliximab and adalimumab, which are believed to be more effective [21, 56, 57].
Side effects
Injection site reactions are the most common side effect. There are concerns about demyelinating diseases caused by etanercept, but the most serious side effect remains opportunistic infection [53].
Golimumab (GOL)
Mechanism of action
Golimumab is a fully human monoclonal antibody targeting TNF alpha.
Summary
Currently, there are only a small number of studies published using GOL in non-infectious uveitis, with relatively small sample sizes [58,59,60,61]. A retrospective case series of 34 eyes by Miserocchi et al. found that there was a decrease in flare, before and after treatment and that stabilisation of visual acuity in most eyes (n = 26) and improvement in 7. The conclusion was that GOL was a viable candidate for the treatment of non-infectious uveitis in cases recalcitrant to treatment with other biologic agents [62].
Santos-Gomez et al. assessed the use of alternative biologics in 7 patients (golimumab = 4, tocilizumab = 2 and rituximab = 1) with refractory Behcet’s unresponsive to ADA and infliximab. In all 7 patients, complete resolution of ocular inflammation was achieved and maintained up to 1 year of follow-up [63].
Side effects
Injection site reactions are the most common adverse effect, with mild infections being the second most common [64]. Clinicians should always be on the lookout for the potential of serious opportunistic infections.
Immunomodulatory agents
Abatacept (ABA)
Mechanism of action
Abatacept is a humanised CTLA-4-immunoglobulin-Fc fusion protein that binds the CD80 and CD86 ligands, preventing CD28 stimulation on T lymphocytes.
Summary
Tappeiner et al. published a multicentre retrospective analysis on the efficacy of ABA in 21 patients with severe JIA-associated uveitis. They observed that although 11 (52.4%) patients had uveitic inactivity during at least one follow-up visit, 8 had recurrences in subsequent visits. Ten patients did not respond to ABA during the entire course of the follow-up. There was no significant difference in uveitis activity before and after initiating ABA treatment and no significant change in visual acuity [65].
Side effects
Opportunistic infections appear to be a significant concern, but the side effect to keep in mind for ABA is a notable blunting of the response to vaccination; patients failed to achieve seroprotective levels after influenza vaccination while on ABA [66].
Rituximab (RIT)
Mechanism of action
Rituximab is a chimeric (mouse/human) monoclonal antibody that targets CD20.
Summary
Rituximab has shown promising results in uveitis of varying aetiologies [67, 68]. In 2015, Miserocchi et al. published a 15-eye retrospective study which assessed the response of treatment-refractory JIA-related uveitis to RIT. They found mean number of uveitis recurrences decreased from 0.7 to 0.2 episodes per year after treatment with RIT. At the end of follow-up, only 3 patients needed daily low dose prednisolone (2.5–7.5 mg) [69].
A pilot study involving 20 patients with severe manifestations of Behcet’s uveitis reported significant improvement in the total adjusted disease activity index (TADAI) in the rituximab group compared to the cytotoxic group receiving cyclophosphamide and methotrexate [68].
Side effects
Infusion reactions are the most commonly reported adverse effect. However, minor bacterial infections, serious viral infections, arrhythmias, and malignancies have all been reported in past [70].
Interferon alpha-2a
Mechanism of action
Interferon alpha-2a is a cytokine, and its mechanism of action is largely unclear. It has been suggested it may reduce HLA-1 on monocytes. However, the exact mechanism is not yet discovered.
Summary
In 2016, Hasanreisoglu et al. published a 39-patient retrospective study, comparing 23 patients receiving azathioprine-cyclosporine and 16 patients receiving Interferon alpha-2a. They found a significant decrease in uveitis attacks/year after initiation of Interferon treatment (2.4 ± 1.8 vs. 1.3 ± 2.0; p = 0.041), but this was not statistically significant (p > 0.05). They concluded that interferon alpha-2a is a viable alternative to conventional combination therapy for Behcet’s uveitis [71]. A systematic review evaluating efficacy of IFN-alpha in Behcet’s uveitis (n = 182 patients) observed 94% of the cohort achieved partial or complete resolution of inflammation. They suggested IFN-alpha as a reliable and effective modality for management of Behcet’s uveitis with low relapse rates on treatment cessation [72]. The results of a RCT using Interferon alpha-2a in Birdshot Chorioretinopathy-related uveitis are yet awaited [73].
Side effects
The main side effect appears to be a flu-like syndrome that responds well to oral acetaminophen. Hair loss, rash, and possible marrow suppression are also to be considered.
Anti-interleukin agents
Secukinumab
Mechanism of action
Secukinumab is a human monoclonal anti-IL17A antibody.
Summary
Secukinumab (SEC): Secukinumab is a human monoclonal anti-IL17A antibody. Subcutaneous Secukinumab has not shown promise in non-infectious uveitis, as published by Dick et al. in 2013. They published the results of three RCTs; the SHIELD, INSURE and ENDURE trial. The SHIELD trial results showed no statistically significant difference between treatment and placebo groups for Behcet’s uveitis recurrence rates (p = 0.445), and the INSURE trial was terminated early due the lack of efficacy of SEC in the SHIELD trial. ENDURE too showed no significant difference between treatment and placebo groups [78]. Secukinumab is currently not employed for Behcet’s management.
An RCT by Letko et al. published in 2015 hypothesised that a different mode of administration would yield better results. They compared different intravenous (IV) and SC doses of SEC in 37 patients with non-infectious uveitis requiring corticosteroid-sparing immunosuppressive therapy. The IV route in comparison, produced higher response rates (72.7 and 61.5% vs. 33.3%, respectively) and remission rates (27.3 and 38.5% vs. 16.7%, respectively). Their conclusion was that the SC route used by the previous studies was insufficient to deliver substantial therapeutic benefit and that high-dose IV SEC should be used in the future [79].
Side effects
Paradoxically, Dick et al. report that their patients encountered exacerbation of systemic Behcet’s, uveitis, and folliculitis. Nasopharyngitis, headache, and arthralgias seemed to be most common side effects [78].
Gevokizumab (GEV)
Mechanism of action
Gevokizumab is a monoclonal anti-IL1-beta antibody and currently does not have FDA approval for any indication.
Summary
Gevokizumab is a monoclonal anti-IL1-beta antibody and currently does not have FDA approval for any indication. Evidence related to its safety and efficacy is scarce. A phase-III study conducted with gevokizumab for Behcet’s disease uveitis concluded that compared to placebo, gevokizumab did not significantly reduce risk of uveitis exacerbations, the primary outcome being evaluated. However, it was suggested that gevokizumab could positively affect visual outcome and reduce disease severity (EYEGUARD) [80]. Tugal-Tutkun et al. published a 21-patient prospective RCT in 2016, assessing the efficacy of GEV in Behcet’s disease-related uveitis. Response to GEV was defined as improved vitreous haze score by ≥ 2 units, ≥ 15-letter improvement in BCVA, and resolution of retinal inflammation. They reported that most patients responded within 1 week of initiation of treatment and that there was a mean improvement of BCVA from baseline of 22.40 ± 15.37 letters [81].
Side effects
Evidence related to its safety and efficacy is scarce.
Anti-interleukin receptor agents
Anakinra
Mechanism of action
It is a recombinant version of the human IL-1 receptor antagonist protein.
Summary
Although recognised to be effective in animal models of uveitis [74], to date there has been no RCT testing the use of this drug in patients with non-infectious uveitis and a clinical trial of Anakinra for the treatment of Behcet’s disease is now recruiting participants. The evidence related to its use is mostly derived from isolated case reports or series reporting good results [75, 76]. The most recent case series was published by Cantarini et al. in 2015, in which Anakinra was used in 9 patients with drug-resistant Behcet’s disease. Three patients had complete resolution of intraocular inflammation initially, but suffered a relapse after an average period of 24 weeks [77].
Side effects
Anakinra’s most common side effect is injection site reaction. If used in concert with anti-TNF agents, neutropenia and opportunistic infections are side effects that may occur. There have been reports of elevated liver enzymes and lipids.
Daclizumab (DAC)
Mechanism of action
Daclizumab is a humanised monoclonal antibody targeting IL-2 receptors.
Summary
Daclizumab has been studied extensively in the last decade with respect to non-infectious uveitis [82,83,84]. Most of these studies did not report any substantial increase in visual acuity of patients. Yeh et al. conducted a prospective pilot study of high-dose intravenous daclizumab therapy in active uveitis of various etiologies including BSRC, VKH, and idiopathic uveitis. All 5 patients demonstrated a decrease in vitreous haze and intraocular inflammation at the end of final follow-up [85].
Buggage et al. conducted a 17-patient RCT in 2007 to investigate the safety and efficacy of DAC in controlling the ocular manifestations of Behcet’s disease. Primary efficacy outcomes were the number of ocular attacks, and no significant differences were reported between placebo and treatment groups [84].
Despite promising results in managing uveitis, daclizumab was discontinued by the manufacturer in 2009 due to diminishing market demand with available alternative treatments [52].
Side effects
Safety data outlining DAC use in uveitis are relatively limited in the literature. In 2011, Wroblewski et al. published a 39-patient retrospective chart review assessing the efficacy and safety of DAC over a mean of 40.3 months. They reported stabilisation of visual acuity and prevention of uveitic flares in most cases. Cutaneous reactions were a common adverse effect; 4 patients developed solid tumours during treatment [86].
Tocilizumab
Mechanism of action
Tocilizumab is an anti-IL-6 receptor monoclonal antibody.
Summary
Tocilizumab is an IL-6 receptor monoclonal antibody, currently approved for the treatment of RA, as well as polyarticular and systemic JIA. It has also demonstrated efficacy in successfully managing JIA-associated uveitis refractory to methotrexate, adalimumab and etanercept [87]. Resolution has also been reported for additional ocular inflammatory conditions as reported in literature [88, 89].
Side effects
Side effects include injection site reactions, nasopharyngitis, increased liver transaminases, dose-dependent neutropenia, lipid elevations and mouth ulceration. Pancreatitis and Steven–Johnson Syndrome have also been reported as serious adverse effects. There is a rare side effect of intestinal perforation.
A summary of non-steroidal treatment options is presented in Table 3.
Conclusion
At present the treatment of non-infectious uveitis poses a significant challenge to ophthalmologists due to its relapsing nature and varied disease aetiology. Local and systemic steroids have conventionally been used for disease control. However, corticosteroid-sparing agents are now becoming an integral component in the long-term management of non-infectious uveitis. Biologic agents such as adalimumab and infliximab have proven to be highly effective, and there is growing evidence to suggest that other anti-TNF alpha biologics, anti-interleukin, and anti-interleukin receptor agents may also be beneficial. Immunomodulatory agents as well as antimetabolites have a long history of being useful in uveitis related to systemic inflammatory disorders. Successful management of non-infectious uveitis often requires the clinician to carefully balance the advantages and disadvantages of each therapy while considering individual patient circumstances. A summary of the recommended screening tests that should be undertaken for each class of drug is presented in Table 4. Adequate counselling regarding the potential benefits and reported complications of each therapy can help the patient make an informed choice, which is imperative before initiating therapy (Table 3).
References
Keino H, Watanabe T, Taki W, Nakayama M, Nakamura T, Yan K et al (2017) Clinical features of uveitis in children and adolescents at a tertiary referral centre in Tokyo. Br J Ophthalmol 101(4):406–410
Nussenblatt RB (1990) The natural history of uveitis. Int Ophthalmol 14(5–6):303–308
Kollias G, Kontoyiannis D (2002) Role of TNF/TNFR in autoimmunity: specific TNF receptor blockade may be advantageous to anti-TNF treatments. Cytokine Growth Factor Rev 13(4–5):315–321
Poetker DM, Reh DD (2010) A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin North Am 43(4):753–768
McGhee CN (1992) Pharmacokinetics of ophthalmic corticosteroids. Br J Ophthalmol 76(11):681–684
Chambers WA. FDA Approval Letter for Triesence 2007. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2007/022048s000,%20022223s000ltr.pdf
Liu X, Wang M, Zhao C, Gao F, Zhang M (2015) The efficacy and safety of subconjunctival triamcinolone acetonide injections in treatment of uveitic macular edema. Zhonghua Yan Ke Za Zhi 51(10):734–738
Bleriot A, Couret C, Le Meur G, Lebranchu P, Weber M (2014) Safety and efficacy of subconjunctival triamcinolone injections in the management of uveitic macular edema: retrospective study of thirty-one cases. J Fr Ophtalmol 37(8):599–604
Sen HN, Vitale S, Gangaputra SS, Nussenblatt RB, Liesegang TL, Levy-Clarke GA et al (2014) Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology 121(11):2275–2286
Mir TA, Reddy A (2016) Inside the Uveitis Toolbox. Retina Today 2016:33–35
Roesel M, Gutfleisch M, Heinz C, Heimes B, Zurek-Imhoff B, Heiligenhaus A (2009) Intravitreal and orbital floor triamcinolone acetonide injections in noninfectious uveitis: a comparative study. Ophthalmic Res 42(2):81–86
Zarranz-Ventura J, Carreno E, Johnston RL, Mohammed Q, Ross AH, Barker C et al (2014) Multicenter study of intravitreal dexamethasone implant in noninfectious uveitis: indications, outcomes, and reinjection frequency. Am J Ophthalmol 158(6):1136–1145
FDA. Approval Letter for Retisert 2005. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021737s000_APPROV.pdf
Sangwan VS, Pearson PA, Paul H, Comstock TL (2015) Use of the fluocinolone acetonide intravitreal implant for the treatment of noninfectious posterior uveitis: 3-year results of a randomized clinical trial in a predominantly Asian population. Ophthalmol Ther 4(1):1–19
Arcinue CA, Ceron OM, Foster CS (2013) A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J Ocul Pharmacol Ther 29(5):501–507
Kane FE, Burdan J, Cutino A, Green KE (2008) Iluvien: a new sustained delivery technology for posterior eye disease. Expert Opin Drug Deliv 5(9):1039–1046
ClinicalTrials.gov. Pilot Study of a Fluocinolone Acetonide Intravitreal Insert (FA-i) to Treat Intermediate-, Posterior-, or Panuveitis (Iluvien) 2012. https://clinicaltrials.gov/ct2/show/NCT01781936
Gangaputra S, Newcomb CW, Liesegang TL, Kacmaz RO, Jabs DA, Levy-Clarke GA et al (2009) Methotrexate for ocular inflammatory diseases. Ophthalmology 116(11):2188–2198
Samson CM, Waheed N, Baltatzis S, Foster CS (2001) Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology 108(6):1134–1139
Kremer JM, Alarcón GS, Lightfoot RW, Willkens RF, Furst DE, Williams HJ et al (1994) Methotrexate for rheumatoid arthritis. Arthritis Rheum 37(3):316–328
Galor A, Jabs DA, Leder HA, Kedhar SR, Dunn JP, Peters GB 3rd et al (2008) Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology 115(10):1826–1832
Daniel E, Thorne JE, Newcomb CW, Pujari SS, Kacmaz RO, Levy-Clarke GA et al (2010) Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol 149(3):423–432
Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP (2005) Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology 112(8):1472–1477
Pasadhika S, Kempen JH, Newcomb CW, Liesegang TL, Pujari SS, Rosenbaum JT et al (2009) Azathioprine for ocular inflammatory diseases. Am J Ophthalmol 148(4):500–509
Yazici H, Pazarli H, Barnes CG, Tüzün Y, Özyazgan Y, Silman A et al (1990) A controlled trial of azathioprine in Behçet’s syndrome. N Engl J Med 322(5):281–285
Pujari SS, Kempen JH, Newcomb CW, Gangaputra S, Daniel E, Suhler EB et al (2010) Cyclophosphamide for ocular inflammatory diseases. Ophthalmology 117(2):356–365
Chung JB, Armstrong K, Schwartz JS, Albert D (2000) Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegner’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 43(8):1841–1848
Kacmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB et al (2010) Cyclosporine for ocular inflammatory diseases. Ophthalmology 117(3):576–584
Akman-Demir G, Ayranci O, Kurtuncu M, Vanli EN, Mutlu M, Tugal-Tutkun I (2008) Cyclosporine for Behcet’s uveitis: is it associated with an increased risk of neurological involvement? Clin Exp Rheumatol 26(4 Suppl 50):S84–S90
Gijtenbeek JM, van den Bent MJ, Vecht CJ (1999) Cyclosporine neurotoxicity: a review. J Neurol 246(5):339–346
Kilmartin DJ, Forrester JV, Dick AD (1998) Tacrolimus (FK506) in failed cyclosporin A therapy in endogenous posterior uveitis. Ocul Immunol Inflamm 6(2):101–109
Mochizuki M, Masuda K, Sakane T, Ito K, Kogure M, Sugino N et al (1993) A clinical trial of FK506 in refractory uveitis. Am J Ophthalmol 115(6):763–769
Murphy CC, Greiner K, Plskova J, Duncan L, Frost NA, Forrester JV et al (2005) Cyclosporine vs tacrolimus therapy for posterior and intermediate uveitis. Arch Ophthalmol 123(5):634–641
Taylor DO, Barr ML, Radovancevic B, Renlund DG, Mentzer RM Jr, Smart FW et al (1999) A randomized, multicenter comparison of tacrolimus and cyclosporine immunosuppressive regimens in cardiac transplantation: decreased hyperlipidemia and hypertension with tacrolimus. J Heart Lung Transp 18(4):336–345
Diaz-Llopis M, Garcia-Delpech S, Salom D, Udaondo P, Hernandez-Garfella M, Bosch-Morell F et al (2008) Adalimumab therapy for refractory uveitis: a pilot study. J Ocul Pharmacol Ther 24(3):351–361
Rudwaleit M, Rodevand E, Holck P, Vanhoof J, Kron M, Kary S et al (2009) Adalimumab effectively reduces the rate of anterior uveitis flares in patients with active ankylosing spondylitis: results of a prospective open-label study. Ann Rheum Dis 68(5):696–701
Bawazeer A, Raffa LH, Nizamuddin (2010) Clinical experience with adalimumab in the treatment of ocular Behçet disease. Ocul Immunol Inflamm 18(3):226–232
Suhler EB, Lowder CY, Goldstein DA, Giles T, Lauer AK, Kurz PA et al (2013) Adalimumab therapy for refractory uveitis: results of a multicentre, open-label, prospective trial. Br J Ophthalmol 97(4):481–486
Simonini G, Druce K, Cimaz R, Macfarlane GJ, Jones GT (2014) Current evidence of anti-tumor necrosis factor alpha treatment efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach of individual drugs. Arthritis Care Res 66(7):1073–1084
Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P et al (2016) Adalimumab in patients with active noninfectious uveitis. N Engl J Med 375(10):932–943
Brezin AP, Kestelyn P, Van Calster J, Jaffe GJ, Thorne JE, Scales D, Franco P, Dick AD, Nguyen QD, Suhler EB, Camez A, Song AP, Kron M, Tari S, Rosenbaum JT, Heiligenhaus A (2015 ) Adalimumab in patients with active, noninfectious uveitis using high-dose corticosteroids [abstract] 67(suppl 10). http://acrabstracts.org/abstract/adalimumab-in-patients-with-active-noninfectious-uveitis-using-high-dose-corticosteroids/
Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J et al (2016) Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 388(10050):1183–1192
AbbVie’s HUMIRA® (adalimumab) Receives U.S. Food and Drug Administration Approval to Treat Adults with Non-Infectious Intermediate, Posterior and Panuveitis 2016. https://news.abbvie.com/news/abbvies-humira-adalimumab-receives-us-food-and-drug-administration-approval-to-treat-adults-with-non-infectious-intermediate-posterior-and-panuveitis.htm
Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda APM (2013) Adalimumab: long-term safety in 23,458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis 72(4):517–524
Abbvie. Humira Prescribing Information 2016. http://www.rxabbvie.com/pdf/humira.pdf
Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN (2001) Effect of infliximab on sight-threatening panuveitis in Behcet’s disease. Lancet 358(9278):295–296
Markomichelakis N, Delicha E, Masselos S, Sfikakis PP (2012) Intravitreal infliximab for sight-threatening relapsing uveitis in Behcet disease: a pilot study in 15 patients. Am J Ophthalmol 154(3):534–541
Markomichelakis N, Delicha E, Masselos S, Fragiadaki K, Kaklamanis P, Sfikakis PP (2011) A single infliximab infusion vs corticosteroids for acute panuveitis attacks in Behcet’s disease: a comparative 4-week study. Rheumatology 50(3):593–597
Suhler EB, Smith JR, Wertheim MS et al (2005) A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol 123(7):903–912
Tabbara KF, Al-Hemidan AI (2008) Infliximab effects compared to conventional therapy in the management of retinal vasculitis in Behcet disease. Am J Ophthalmol 146(6):845–850
Vallet H, Seve P, Biard L, Baptiste Fraison J, Bielefeld P, Perard L et al (2016) Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: a multicenter study from the French uveitis network. Arthritis Rheumatol 68(6):1522–1530
Pasadhika S, Rosenbaum JT (2014) Update on the use of systemic biologic agents in the treatment of noninfectious uveitis. Biol Targets Ther 8:67–81
Dogra S, Khullar G (2013) Tumor necrosis factor-alpha antagonists: side effects and their management. Indian J Dermatol Venereol Leprol 79(Suppl 7):S35–S46
Reiff A, Takei S, Sadeghi S, Stout A, Shaham B, Bernstein B et al (2001) Etanercept therapy in children with treatment-resistant uveitis. Arthritis Rheum 44(6):1411–1415
Foster CS, Tufail F, Waheed NK, Chu D, Miserocchi E, Baltatzis S et al (2003) Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol 121(4):437–440
Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN (2014) Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology 121(3):785–796
Galor A, Perez VL, Hammel JP, Lowder CY (2006) Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology 113(12):2317–2323
Calvo-Rio V, de la Hera D, Blanco R, Beltran-Catalan E, Loricera J, Canal J et al (2014) Golimumab in uveitis previously treated with other anti-TNF-alpha drugs: a retrospective study of three cases from a single centre and literature review. Clin Exp Rheumatol 32(6):864–868
Faez S, Lobo AM, Sobrin L, Papaliodis GN (2014) Treatment of seronegative spondyloarthropathy-associated uveitis with golimumab: retrospective case series. Clin Exp Ophthalmol 42(4):392–395
Cordero-Coma M, Salom D, Díaz-Llopis M, López-Prats MJ, Calleja S (2011) Golimumab for uveitis. Ophthalmology 118(9):1892
William M, Faez S, Papaliodis GN, Lobo A-M (2012) Golimumab for the treatment of refractory juvenile idiopathic arthritis-associated uveitis. J Ophthal Inflamm Infect 2(4):231–233
Miserocchi E, Modorati G, Pontikaki I, Meroni PL, Gerloni V (2014) Long-term treatment with golimumab for severe uveitis. Ocul Immunol Inflamm 22(2):90–95
Santos-Gomez M, Calvo-Rio V, Blanco R, Beltran E, Mesquida M, Adan A et al (2016) The effect of biologic therapy different from infliximab or adalimumab in patients with refractory uveitis due to Behcet’s disease: results of a multicentre open-label study. Clin Exp Rheumatol 34(6 Suppl 102):S34–S40
Michelon MA, Gottlieb AB (2010) Role of golimumab, a TNF-alpha inhibitor, in the treatment of the psoriatic arthritis. Clin Cosmet Investig Dermatol CCID 3:79–84
Tappeiner C, Miserocchi E, Bodaghi B, Kotaniemi K, Mackensen F, Gerloni V et al (2015) Abatacept in the treatment of severe, longstanding, and refractory uveitis associated with juvenile idiopathic arthritis. J Rheumatol 42(4):706–711
Ribeiro AC, Laurindo IM, Guedes LK, Saad CG, Moraes JC, Silva CA et al (2013) Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res 65(3):476–480
Tomkins-Netzer O, Taylor SR, Lightman S (2013) Can rituximab induce long-term disease remission in patients with intra-ocular non-infectious inflammation? Ophthalmologica 230(3):109–115
Davatchi F, Shams H, Rezaipoor M, Sadeghi-Abdollahi B, Shahram F, Nadji A et al (2010) Rituximab in intractable ocular lesions of Behcet’s disease; randomized single-blind control study (pilot study). Int J Rheum Dis 13(3):246–252
Miserocchi E, Modorati G, Berchicci L, Pontikaki I, Meroni P, Gerloni V (2016) Long-term treatment with rituximab in severe juvenile idiopathic arthritis-associated uveitis. Br J Ophthalmol 100(6):782–786
Kasi PM, Tawbi HA, Oddis CV, Kulkarni HS (2012) Clinical review: Serious adverse events associated with the use of rituximab—a critical care perspective. Crit Care 16(4):231
Hasanreisoglu M, Cubuk MO, Ozdek S, Gurelik G, Aktas Z, Hasanreisoglu B (2017) Interferon Alpha-2a therapy in patients with refractory Behcet uveitis. Ocul Immunol Inflamm 25(1):71–75
Kotter I, Gunaydin I, Zierhut M, Stubiger N (2004) The use of interferon alpha in Behcet disease: review of the literature. Semin Arthritis Rheum 33(5):320–335
ClinicalTrials.gov. Evaluation of Birdshot Retine Choroidopathy Treatment by Either Steroid or Interferon alpha2a (BIRDFERON). https://clinicaltrials.gov/ct2/show/NCT00508040
Rosenbaum JT, Boney RS (1992) ACtivity of an interleukin 1 receptor antagonist in rabbit models of uveitis. Arch Ophthalmol 110(4):547–549
Arida A, Sfikakis PP (2014) Anti-cytokine biologic treatment beyond anti-TNF in Behcet’s disease. Clin Exp Rheumatol 32(4 Suppl 84):149–155
Teoh SCB, Sharma S, Hogan A, Lee R, Ramanan AV, Dick AD (2007) Tailoring biological treatment: anakinra treatment of posterior uveitis associated with the CINCA syndrome. Br J Ophthalmol 91(2):263–264
Cantarini L, Vitale A, Scalini P, Dinarello CA, Rigante D, Franceschini R et al (2015) Anakinra treatment in drug-resistant Behcet’s disease: a case series. Clin Rheumatol 34(7):1293–1301
Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V et al (2013) Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology 120(4):777–787
Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL (2015) Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology 122(5):939–948
News GEaB. Xoma’s Gevokizumab Fails Phase III Trial 2015. http://www.genengnews.com/gen-news-highlights/xoma-s-gevokizumab-fails-phase-iii-trial/81251535/
Tugal-Tutkun IM, Kadayifcilar SM, Khairallah MM, Lee SCMP, Ozdal P, Ozyazgan Y et al (2017) Safety and efficacy of gevokizumab in patients with Behcet’s disease uveitis: results of an exploratory phase 2 study. Ocul Immunol Inflamm 25(1):62–70
Bhat P, Castaneda-Cervantes RA, Doctor PP, Foster CS (2009) Intravenous daclizumab for recalcitrant ocular inflammatory disease. Graefes Arch Clin Exp Ophthalmol 247(5):687–692
Nussenblatt RB, Peterson JS, Foster CS, Rao NA, See RF, Letko E et al (2005) Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: a multicenter noncomparative interventional case series. Ophthalmology 112(5):764–770
Buggage RR, Levy-Clarke G, Sen HN, Ursea R, Srivastava SK, Suhler EB et al (2007) A double-masked, randomized study to investigate the safety and efficacy of daclizumab to treat the ocular complications related to Behcet’s disease. Ocul Immunol Inflamm 15(2):63–70
Yeh S, Wroblewski K, Buggage R, Li Z, Kurup SK, Sen HN et al (2008) High-dose humanized anti-IL-2 receptor alpha antibody (daclizumab) for the treatment of active, noninfectious uveitis. J Autoimmun 31(2):91–97
Wroblewski K, Sen HN, Yeh S, Faia L, Li Z, Sran P et al (2011) Long-term daclizumab therapy for the treatment of noninfectious ocular inflammatory disease. Can J Ophthalmol J 46(4):322–328
Tappeiner C, Heinz C, Ganser G, Heiligenhaus A (2012) Is tocilizumab an effective option for treatment of refractory uveitis associated with juvenile idiopathic arthritis? J Rheumatol 39(6):1294–1295
Papo M, Bielefeld P, Vallet H, Seve P, Wechsler B, Cacoub P et al (2014) Tocilizumab in severe and refractory non-infectious uveitis. Clin Exp Rheumatol 32(4 Suppl 84):S75–S79
Calvo-Rio V, de la Hera D, Beltran-Catalan E, Blanco R, Hernandez M, Martinez-Costa L et al (2014) Tocilizumab in uveitis refractory to other biologic drugs: a study of 3 cases and a literature review. Clin Exp Rheumatol 32(4 Suppl 84):S54–S57
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Therefore, no informed consent was necessary.
Rights and permissions
About this article
Cite this article
Shahab, M.A., Mir, T.A. & Zafar, S. Optimising drug therapy for non-infectious uveitis. Int Ophthalmol 39, 1633–1650 (2019). https://doi.org/10.1007/s10792-018-0984-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-018-0984-1