Abstract
The 13-valent pneumococcal conjugate vaccine (Prevenar 13®, Prevnar 13®) [PCV13] consists of 13 serotype-specific polysaccharides of Streptococcus pneumoniae (pneumococcus), each covalently conjugated to a non-toxic immunogenic carrier protein. PCV13 has a well established immunogenicity and tolerability profile in adults, particularly those ≥50 years of age. Results of CAPiTA, a randomized, double-blind, placebo-controlled trial in >84,000 older adults aged ≥65 years, showed that PCV13 was effective in preventing vaccine-type pneumococcal community-acquired pneumonia (CAP), vaccine-type pneumococcal nonbacteraemic (noninvasive) CAP and vaccine-type invasive pneumococcal disease (IPD). These findings, along with changes in pneumococcal serotype distribution and epidemiology of pneumococcal disease, prompted the US Advisory Committee on Immunization Practices (ACIP) to recommend PCV13 in series with 23-valent pneumococcal polysaccharide vaccine (PPVS23) for all adults aged ≥65 years. PCV13 also has a role in preventing pneumococcal disease (pneumonia and IPD) in younger adults with immunocompromising conditions and potentially in those with other underlying medical conditions that increase the risk of pneumococcal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Immunogenicity well established in adults, particularly those ≥50 years of age |
Approved for prevention of vaccine-type pneumococcal disease (IPD and pneumonia) in adults ≥50 years in the USA or ≥18 years in the EU |
Demonstrated efficacy in preventing vaccine-type pneumococcal CAP, vaccine-type pneumococcal nonbacteraemic (noninvasive) CAP and vaccine-type IPD in adults aged ≥65 years in CAPiTA |
Recommended by ACIP (in series with PPSV23) for all adults aged ≥65 years |
Well established tolerability profile in adults |
1 Introduction
Despite targeted recommendations for the use of pneumococcal vaccines for age and risk groups, Streptococcus pneumoniae (pneumococcus) continues to be a leading cause of serious illness, including bacteraemia, meningitis and pneumonia, among adults [1, 2]. Invasive pneumococcal disease (IPD) is defined as an infection confirmed by the isolation of S. pneumoniae from a normally sterile body site, and its clinical presentations include bacteraemia, meningitis and bacteraemic pneumonia [3, 4]. S. pneumoniae is the most common cause of community-acquired pneumonia (CAP) in the USA and Europe [3, 4]. Pneumococcal CAP typically presents as noninvasive disease; nonbacteraemic pneumonia occurs in ≈75 % of cases [5]. The predominant manifestation of IPD is bacteraemic pneumonia, representing ≈70 % of all cases of IPD in the USA [5]. There are an estimated 40,000 cases of IPD annually in the USA, of which 13,500 occur in adults ≥65 years of age [1]. The annual number of IPD-related deaths is ≈4500 among adults aged ≥50 years in the USA [6]. Globally, pneumococcal infection is estimated to be responsible for ≈1.6 million deaths annually [3, 4]. Most of these deaths occur in poor countries and include a disproportionate number of children <2 years of age [3]. Other high-risk groups for IPD and pneumococcal pneumonia include individuals who are elderly (≥65 years of age), immunocompromised, or have certain chronic medical conditions, such as diabetes [3, 4, 7, 8]. In addition to the chronic conditions listed as indications for pneumococcal vaccination in guidelines from the US Advisory Committee on Immunization Practices (ACIP) for prevention of pneumococcal disease in adults (Sect. 7), recent data also revealed increased rates of infections for other chronic conditions not included in ACIP guidelines, as well as in individuals with multiple at-risk conditions [9]. When compared with healthy age-matched adults in a retrospective cohort study, the accumulation of concurrent at-risk conditions (risk stacking) increased the risk ratio for all-cause pneumonia, pneumococcal pneumonia and IPD to the level of an immunocompromised individual [9].
Two vaccines are widely available and approved for the prevention of pneumococcal disease in adult populations [1, 2]. The 23-valent pneumococcal polysaccharide vaccine (PPSV23), which has been available in many countries for about three decades, consists of 23 serotype-specific pneumococcal capsular polysaccharide antigens [1, 2]. Although some studies with PPSV23 have demonstrated protection against IPD in adults, its efficacy against noninvasive pneumonia is unclear or lacking [10–17]. There are also concerns about the extent to which the protective effectiveness of PPSV23 varies by age of recipient, time since vaccination and the presence of underlying disease [18]. In addition, vaccines composed of purified capsular polysaccharides are not immunogenic in young children <2 years of age [7, 19], nor do they provide booster responses on revaccination [20]. The 13-valent pneumococcal conjugate vaccine (Prevenar 13®, Prevnar 13®) [PCV13] consists of 13 serotype-specific polysaccharides of S. pneumoniae, each covalently conjugated to a non-toxic immunogenic carrier protein (diphtheria CRM197 protein). Seven serotypes included in PCV13 are common to its 7-valent predecessor (PCV7) [serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F] and six are additional serotypes (1, 3, 5, 6A, 7F and 19A) [21, 22]. Twelve of the serotypes included in PCV13 (all but 6A) are also contained in PPSV23 [2, 18]. The current article focuses on PCV13 for the prevention of vaccine-type pneumococcal disease, including pneumonia and IPD, in adults.
2 Immunogenicity
The immunogenicity of PCV13 in adults, particularly in those ≥50 years of age, is well established [21–23]. Clinical trials with PCV13 in adults used a serotype-specific opsonophagocytosis assay (OPA) as a surrogate to assess protective efficacy against pneumococcal disease, as there is no established antibody threshold of serotype-specific pneumococcal polysaccharide immunoglobulin G (IgG) concentration associated with protection in adults [22]. OPA geometric mean titres (GMTs) were determined 1 month after vaccination and reflect functional immune responses (OPA titres are expressed as the reciprocal of the highest serum dilution that reduces survival of the pneumococci by ≥50 %) [22].
The immunogenicity of PCV13 has been demonstrated in adults ≥18 years of age in five phase III trials conducted in the USA and Europe, including individuals who were or were not previously vaccinated with PPSV23 [21, 22, 24–28]. Each study included healthy adults and immunocompetent adults with stable underlying conditions or risk factors known to predispose individuals to pneumococcal infection. The studies focused on different age groups, most of which were older adults ≥50 years of age.
Two randomized, head-to-head comparative trials, one in PPSV23-naive adults aged 60–64 years (n = 831) [27] and the other in PPSV23-experienced adults aged ≥70 years (n = 936) [24], demonstrated that serotype-specific functional OPA antibody responses with PCV13 were noninferior to those with PPSV23 for the 12 serotypes common to both vaccines (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23) [24, 27]. Noninferiority of PCV13 to PPSV23 was met if the lower limit of the 95 % CI of the GMT ratio (GMR) was >0.5. In addition, the functional immune response to serotype 6A (unique to PCV13) was demonstrated, as there was a fourfold increase in OPA GMT compared with the pre-vaccination baseline titre [24, 27]. Both trials also showed that OPA GMTs were statistically significantly greater in PCV13 than PPSV23 recipients for the majority of serotypes common to both vaccines and for serotype 6A [24, 27]. The study in PPSV23-naive individuals also included a group of 403 adults aged 50–59 years who received open-label PCV13 [27]. For all 13 vaccine serotypes, functional OPA antibody responses with PCV13 were noninferior when comparing the younger (aged 50–59 years) to the older (aged 60–64 years) group (i.e. the lower limit of the 95 % CI for GMR was >0.5) [27]. In the study of older individuals who had previously received PPSV23 (≥5 years before study entry), participants in both groups also received a follow-on dose of PCV13 after 1 year, and responses to follow-on vaccination were generally lower in those randomized to PPSV23 than in those who received PCV13 at enrollment [24].
Findings of another phase III trial in 720 PPSV23-naive adults aged 60–64 years indicate that the order of administration of PCV13 and PPSV23 may be important for individuals who receive the vaccines in sequence 1 year apart [26]. Participants were randomized to one of three vaccination schedules: (i) PCV13 at year 0 and PCV13 at year 1; (ii) PCV13 at year 0 and PPSV23 at year 1; and (iii) PPSV23 at year 0 and PVC13 at year 1. Results showed that initial PCV13 administration augmented the functional OPA antibody responses to subsequent administration of PPSV23 for many of the serotypes common to both vaccines. In contrast, initial PPSV23 administration diminished functional immune responses to subsequent PCV13 administration for all serotypes. Another finding of potential clinical relevance was that responses after subsequent vaccination with PCV13 or PPSV23 were usually noninferior to responses after the initial PPV13 dose, suggesting that an interval longer than 1 year between vaccine administrations may be appropriate [26]. The findings of this trial are also supported by results of an extension of the head-to-head comparative trial in PPSV23-naive adults, which showed that re-vaccination with either PCV13 or PPSV23 at ≈4 years after initial vaccination with PCV13 resulted in recall anti-pneumococcal responses, whereas this did not occur in individuals who received initial and subsequent vaccination with PPSV23 [29].
Two additional phase III trials assessed functional immune responses when PCV13 was administered concomitantly with trivalent inactivated influenza vaccine [25, 28]. One study, conducted in the USA, included 1116 participants aged 50–59 years who were randomized to receive PCV13 with inactivated influenza vaccine at month 0 and placebo at month 1 or inactivated influenza vaccine with placebo at month 0 and PCV13 at month 1 [28]. The other study, conducted in Europe, included 1160 participants aged ≥65 years and was of similar design [25]. In both studies, PCV13 administered concurrently with inactivated influenza vaccine produced antibody responses that were noninferior to those observed with sequential administration, other than for influenza strain A/H3N2 and pneumococcal serotype 19F in those aged ≥65 years [25, 28]. Although antibody responses were numerically higher with sequential than concomitant administration, the clinical significance of this is unknown.
In addition to the phase III studies, an open-label study in PPSV23-naive adults ≥50 years of age (n = 324) in Mexico showed robust immune responses in terms of OPA GMTs for vaccine serotypes at 1 month following PCV13 administration [30]. For most serotypes, overall immune responses were higher than those previously reported in similar adult populations in the USA or Europe.
Findings of a noncomparative trial in PPSV23-experienced HIV-infected adults ≥18 years of age with CD4 cell counts ≥200 cells/mm3 and HIV viral loads <50,000 copies/mL support the use of PCV13 in this population [31]. A total of 329 HIV-infected patients received ≥1 dose of PCV13 and 279 received all 3 planned doses of PCV13 administered at 6-month intervals. PCV13 induced IgG and functional OPA antibody responses after dose 1 and were similar or modestly higher after doses 2 and 3 [31]. Vaccination with PCV13 also induced IgG and OPA antibody responses in pneumococcal vaccine-naïve HIV-infected patients [32].
3 Protective Efficacy and Effectiveness
3.1 CAPiTA Study
The efficacy of PCV13 in preventing vaccine-type pneumococcal CAP, vaccine-type nonbacteraemic (noninvasive) CAP and vaccine-type IPD in older adults (≥65 years of age) was demonstrated in a large (n = 84,496), randomized, double-blind, placebo-controlled trial known as CAPiTA [33]. The study was conducted at community-based sites throughout the Netherlands and participants were enrolled between September 2008 and January 2010. PCV13 or placebo was administered as a single intramuscular dose. The use of a placebo was considered appropriate because at the time of the trial no pneumococcal vaccine was recommended for routine use in older adults in the Netherlands. Among the exclusion criteria were previous pneumococcal vaccination, the presence of specified immunocompromising conditions, and residence in a nursing home, long-term care facility, or other institution, or requirement of semiskilled nursing care (i.e. the study was conducted in a relatively healthy population ≥65 years of age). A safety subgroup of participants who were followed with home visits to collect additional tolerability data (see Sect. 4) was enrolled separately but was included in the overall study population. Although none of those in the safety subgroup received concurrent administration of trivalent inactivated influenza vaccine, it was administered concurrently with PCV13 or placebo in 30.4 % of the overall study population (all during the first 2.5 months of study enrolment). It was expected that most study participants would receive annual influenza vaccination, as the rate in the Netherlands is high for this age group.
The primary endpoint of CAPiTA was the prevention of a first episode of confirmed vaccine-type CAP in the per-protocol population [33]. Confirmed vaccine-type CAP was defined as the presence of ≥2 prespecified clinical criteria, findings on chest radiography consistent with CAP, and a positive vaccine serotype-specific urinary antigen test or isolation of vaccine-type S. pneumoniae from blood or another sterile site. Two secondary endpoints and a number of exploratory endpoints were also evaluated in the per-protocol population, which was defined as participants who met the criteria for the modified intention-to-treat (mITT) population, were eligible for the study, received a study vaccination and had no other major protocol violations. The mITT population (defined as patients who developed CAP or IPD with onset of symptoms ≥14 days after vaccination) was used for analysis of all episodes of CAP, an exploratory endpoint that included pneumococcal and nonpneumococcal pneumonia. Surveillance for suspected CAP and IPD was conducted at 59 sentinel centres and the mean follow-up period was 4 years. Baseline characteristics were similar between participants who received PCV13 (n = 42,240) and those who received placebo (n = 42,256). During the 4-year follow-up period, 1552 study participants in the PCV13 group and 1680 in the placebo group visited sentinel centres for suspected pneumonia or IPD.
For the primary endpoint of confirmed vaccine-type CAP, vaccine efficacy was 45.6 % (95.2 % CI 21.8–62.5; p < 0.001), as the number of episodes detected at sentinel centres among placebo recipients was almost halved for PCV13 recipients (90 vs. 49) (Table 1) [33]. PCV13 was also statistically superior to placebo in reducing the number of episodes of confirmed vaccine-type nonbacteraemic and noninvasive CAP (vaccine efficacy 45.0 %) and confirmed vaccine-type IPD (vaccine efficacy 75.0 %), both of which were secondary endpoints (Table 1). PCV13 also demonstrated efficacy in preventing some, but not all, exploratory endpoints involving infection with any pneumococcal strain (Table 1). As might be expected, PCV13 did not demonstrate efficacy in preventing CAP from any cause (vaccine efficacy 5.1 %; 95 % CI −5.1 to 14.2), which was also an exploratory endpoint. The number of deaths associated with pneumococcal disease was very small and precluded a meaningful analysis of the effect of PCV13 on mortality. There were two deaths in each group from confirmed vaccine-type pneumococcal CAP or vaccine-type IPD. There were six and seven deaths in the PCV13 and placebo groups, respectively, from confirmed pneumococcal CAP or IPD.
The study authors identified several limitations of their trial [33], including that it was conducted in one country in a homogenous population with a low incidence of pneumococcal disease, and the degree of vaccine efficacy may vary in other populations where the susceptibility to pneumococcal disease and epidemiologic characteristics of the vaccine-associated serotypes may differ. In addition, the higher sensitivity of the serotype-specific urinary antigen detection assay used in the trial may have led to a small overestimation of the proportion of vaccine-type serotypes and, in turn, vaccine efficacy against all pneumococcal CAP. Also, the per-protocol analysis excluded events that occurred in patients who developed an immunosuppressing condition after enrolment in the trial, as the study was not designed to show vaccine efficacy in this population.
3.2 Postmarketing Surveillance Studies
Evidence for protective effectiveness of PCV13 against IPD and noninvasive pneumococcal disease became available from surveillance studies following the introduction of PCV13 in 2010 in various countries, although most of the initial reports focused on children [34]. However, several recent postmarketing studies also provide evidence for protective effectiveness of PCV13 against pneumococcal disease in adults (indirectly via herd protection) [35–40].
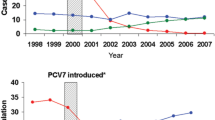
Two years after its introduction into the US paediatric immunization programme, PCV13 was associated with statistically significant reductions in hospital admissions for invasive and noninvasive pneumococcal disease, not only in children but also in some adult age groups, according to findings of a retrospective time series analysis that used data from a private inpatient discharge record database capturing ≈20 % of all admissions to hospitals in the USA [35]. The analysis extracted age-specific data on the incidences of hospital admissions per month for all-cause pneumonia, IPD, noninvasive pneumococcal or lobar pneumonia and empyema for the period 2005–2012 then used a multiple regression model to estimate how much of the change could be attributed to the vaccine. The modelling analysis of changes in outcomes attributable to PCV13 showed statistically significant (p < 0.05) reductions of 37 % for IPD (95 % CI 20–51), 32 % (95 % CI 17–44) for noninvasive pneumococcal or lobar pneumonia and 12 % (95 % CI 6–17) for all-cause pneumonia among adults aged 18–39 years. There were also significant reductions in hospital admissions for noninvasive pneumococcal or lobar pneumonia in adults aged 40–64 years (by 25 %; 95 % CI 16–33) and in older adults aged ≥65 years (by 34 %; 95 % CI 27–41), and for IPD in those aged ≥65 years (by 29 %; 95 % CI 16–40). These findings suggest a strong and rapid development of herd protection.
In another time-series model (2004–2013) comparing IPD rates before and 3 years after the introduction of PCV13 into the US paediatric immunization schedule, the vaccine was associated with reductions in IPD overall as well as IPD caused by the six additional pneumococcal serotypes unique to PCV13 (i.e. those not included in PCV7) in children and adults [36]. Among adults, the incidence of IPD overall decreased by 32 % (95 % CI 22–40) in individuals aged 18–49 years, 18 % (95 % CI 10–26) in those aged 50–64 years and 12 % (95 % CI −1 to 22) in older adults aged ≥65 years. Corresponding reductions in IPD cases caused by serotypes unique to PCV13 for these age groups were 72 % (95 % CI 69–75), 62 % (95 % CI 59–65) and 58 % (95 % CI 52–64). With respect to serotype replacement, there was no significant increase in IPD cases caused by non-PCV13 serotypes in most adult groups, although there was a 26 % (95 % CI 13–44) increase for the 50–64 years old cohort during 2012–2013. The analysis also estimated that during the first 3 years after its introduction, PCV13 probably prevented a total of 30,000 IPD cases (two-thirds in adults) and saved 3000 lives (97 % in adults).
In South Africa, where PCV7 was introduced into the paediatric immunization programme in 2009 and replaced by PCV13 in 2011, national laboratory surveillance data were used to calculate the change in IPD incidence between the prevaccine baseline period (2005–2008) and the postvaccine years 2011 and 2012 [37]. In addition to a marked reduction in the rates of IPD in children, there was also a 34 % reduction (95 % CI 29–39) in the incidence of IPD from baseline to 2012 among adults aged 25–44 years, which was primarily driven by a 57 % reduction (95 % CI 50–63) in PCV7-serotype disease. The reduction in IPD (in terms of cases per 100,000 person-years) among HIV-infected adults exceeded the rate reduction among their HIV-uninfected counterparts by a factor of almost 180.
PCV13 was associated with greater benefits than expected in terms of reducing IPD incidence and pneumococcal-related mortality in a Danish nationwide population-based cohort study that linked national laboratory surveillance and vital statistics data [38]. The analysis estimated changes in IPD incidence and mortality between the prevaccine baseline period (2000–2007), the period when PCV7 was included in routine childhood immunization (2008–2010) and the initial period when PCV13 replaced PCV7 (2011–2013). Overall in the total population, there was a 21 % reduction (95 % CI 17–25) in IPD incidence and a 28 % reduction (95 % CI 18–37) in IPD-related mortality after the introduction of PCV13 compared with baseline. The reduction in mortality occurred primarily in the adult population aged >18 years. Among older adults aged ≥65 years, the proportion of IPD cases caused by non-PCV13 serotypes increased from 19.7 % (95 % CI 18.7–20.8) at baseline to 29.4 % (95 % CI 27.5–31.4) after the introduction of PCV13.
In Israel, where PCV7 was introduced into the national childhood immunization programme in 2009 and was gradually replaced by PCV13 beginning in late 2010, national laboratory surveillance data showed a 21 % reduction in the incidence of IPD from 2009 to 2013 among adults aged ≥18 years [39]. The reduction was more marked in younger than in older adults, and there was no effect on the overall case-fatality rate over the 4-year study period. More than 90 % of individuals who developed IPD had comorbidities. Although IPD incidence was lower in summer than in winter, the impact of vaccination on reducing the incidence of IPD was apparent in both seasons. Serotype replacement was observed during the study period in that the incidence of PCV7 serotype IPD cases decreased by 70 % and there was an initial increase in episodes caused by the additional six serotypes unique to PCV13 (i.e. prior to the introduction of PCV13), but by the fourth year there was a 57 % reduction compared with the first year. However, from the first to fourth years there was also a 52 % increase in IPD episodes caused by non-PCV13 serotypes.
A surveillance study conducted in Navarre, Spain also showed that in the period following the introduction of PCV13 (2010–2013), there was a significant reduction in IPD cases in the overall population, including a 23 % reduction (p = 0.024) in older adults aged ≥65 years, compared with the period 2004–2009 [40]. About half of the older adults who developed IPD had comorbidities. In the adult population, a moderate change in the pattern of serotypes causing IPD was noted following replacement of PCV7 with PCV13, including statistically significant (p < 0.05) increases in cases caused by non-PCV13 serotypes 6C and 15A in older adults.
A nation-wide population-based study in Switzerland, where PCV7 was introduced into the childhood vaccination programme in 2007 and replaced by PCV13 in 2011, showed a marked reduction in adult IPD cases caused by PCV7 serotypes and an increase in those caused by non-PCV7 serotypes when comparing laboratory surveillance data from 2012 with that from 2003 [41]. The analysis also showed that case fatality was significantly (p < 0.05) increased for serotypes 3, 19A and 19F compared with serotypes 1 and 7F.
Nasopharyngeal colonization by S. pneumoniae is a prerequisite for IPD and pneumococcal pneumonia and can lead to transmission of the bacteria from the colonized individual to other contacts [4, 42]. Studies conducted in Alaska [43] and England [44] evaluated the impact of PCV13 on pneumococcal nasopharyngeal (PNP) carriage in adults as well as children. In Alaska, where PCV13 replaced PCV7 as part of routine childhood immunization in 2010, PNP carriage of the six additional serotypes unique to PCV13 decreased significantly over the course of the study from 2008 to 2012 among children as well as unvaccinated adults, suggesting indirect protection from PCV13 [43]. Broadly similar findings were reported in the study conducted in England [44].
4 Tolerability
Pooled tolerability data for PCV13 in adults are available from seven clinical studies that included a total of 91,593 participants, of whom 48,806 received PCV13 [22]. The large majority of PCV13 recipients (92.8 %) were older adults aged ≥65 years; 5.4 % were aged 50–64 years and 1.8 % were 18–49 years of age. Overall, <4 % of PCV13 recipients had previously received PPSV23 (in all cases ≥3 years prior to study vaccination). Local reactions and systemic adverse events were solicited daily after study vaccination for 2 weeks in most studies (but for only 1 week in one of the trials).
In general, older adults aged ≥65 years reported fewer adverse events than younger adults, particularly those in the youngest age range of 18–29 years [22]. Across all age groups, adverse events that were considered very common (reported by ≥1 of 10 participants) included decreased appetite, headaches, diarrhoea, arthralgia, myalgia, rash, general disorders, including chills and fatigue, and administration site conditions, such as vaccination-site erythema, vaccination-site induration/swelling, vaccination-site pain/tenderness and limitation of arm movement. Vomiting was also very common in adults aged 18–49 years and pyrexia was very common in younger adults 18–29 years of age, but both of these adverse events were found to be common (reported by ≥1/100 to <1/10) in all other age groups. In general, local reactions tended to be more severe in younger age groups.
Pooled tolerability data showed that prior exposure to PPSV23 did not affect the frequency of reported adverse events following study vaccination [22]. However, there was an increased frequency for some adverse events (e.g. headache, decreased appetite, arthralgia) when PCV13 was administered concurrently with trivalent inactivated influenza vaccine than with either vaccine alone [22]. An open-label safety study in 1049 older adults (≥68 years) who had been previously immunized with PPSV23 (≥3 years earlier) also showed that PCV13 can be safely administered in this patient population, as the tolerability profile following PCV13 administration was generally similar to that of other elderly PPSV23-naive and—experienced study populations [45].
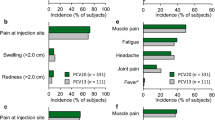
Tolerability data solicited from the safety subgroup in CAPiTA (Sect. 3.1) indicate a higher frequency of prespecified local reactions and systemic events in the PCV13 group than in the placebo group, although most were mild or moderate in severity [33]. The study reports that in the safety subgroup, 188 of 1006 PCV13 recipients and 144 of 1005 placebo recipients (18.7 vs. 14.3 %; p = 0.01) experienced at least one adverse event within 1 month after vaccination, which reflected between-group differences in the frequency of injection-site reactions and muscular pain. Supplementary data from the trial indicate that 38.4 % of PCV13 recipients and 8.4 % of placebo recipients in the safety population reported any local reaction within 7 days after vaccination (p < 0.001). There were no statistically significant differences in the frequency of serious adverse events within 6 months after vaccination with PCV13 or placebo in the safety population (7.0 vs. 6.0 %) or within 1 month after vaccination in the overall study population (0.8 vs. 0.7 %). There were no serious adverse events considered to be related to study vaccine.
5 Pharmacoeconomic Considerations
Several fully published, modelled pharmacoeconomic analyses of PCV13 in adults have been conducted in various European countries [46–51] and in the USA [52–57] (Table 2). All of the analyses incorporated data from a number of different sources, such as published clinical trials and expert opinion, although none were recent enough to include data from CAPiTA. Most were cost-effectiveness analyses (CEAs) comparing various pneumococcal vaccination strategies in specific adult populations, with results expressed in terms of an incremental cost-effectiveness ratio (ICER) [47, 50–56]. ICERs were reported in terms of the incremental cost per quality-adjusted life-year (QALY) gained, and results were considered cost effective if ICER values were below commonly used cost-effectiveness thresholds (e.g. $US50,000, €50,000 or £30,000 per QALY gained) or if the vaccination strategy of interest dominated (i.e. was less costly and less expensive than) the comparator strategy. In addition to the CEAs, a smaller number of budget impact analyses (BIAs) have been conducted with PCV13 in adults, which also compared various vaccination strategies and estimated effects of PCV13 vaccination on net costs and clinical outcomes, but did not consider effects on quality-adjusted life-expectancy or provide ICERs [46, 48, 49, 57].
Overall, base-case results of the CEAs were generally favourable for PCV13 in most studies, but they varied widely across analyses, ranging from PCV13 being dominant to PCV13 not being cost effective (Table 2). This variability in results generally reflected differences in data sources or model input parameters, patient populations, comparator vaccination strategies, study perspective, time horizon and geographic location. Most CEAs included herd effects from childhood immunity in the base case [47, 50, 51, 53–56], and analyses with a lifetime horizon incorporated vaccine waning, whereby efficacy was assumed to be nil after a specific time period of 5–15 years [47, 50–53, 55, 56], but costs and outcomes were extrapolated over a lifetime horizon.
European BIAs provided somewhat mixed results. A German study showed that replacing PPSV23 with PCV13 would increase the net budget by ≈€60–152 million over 5 years with equivocal effects on IPD [46], whereas BIAs with 5-year time horizons conducted in Italy [48] and Spain [49] showed that PCV13 reduced costs and pneumococcal disease compared with no vaccination (Table 2). In addition, a BIA with a lifetime horizon conducted in the USA showed that replacing PPSV23 with a single dose of PCV13 in adults aged ≥50 years would save billions of dollars and reduce pneumococcal disease [57] (Table 2).
In an effort to address the inherent uncertainties of results from modelled analyses, the studies included sensitivity and/or scenario analyses to assess the impact on base-case results when making plausible modifications to key input parameters. Results of sensitivity analyses of the CEAs generally showed that base-case results were robust to reasonable changes in most parameter values, although in some analyses results were highly sensitive to changes in PCV13 cost [47, 50, 51, 54], predicted herd effects of childhood vaccination [50, 52, 55], vaccine effectiveness [50–56], parameters related to inpatient CAP [47, 51] and life expectancy [56].
6 Dosage and Administration
PCV13 is administered by intramuscular injection in the deltoid muscle of the upper arm in adults [21, 22]. It is administered as one single dose of 0.5 mL in adults, as the need for revaccination with a subsequent dose of the vaccine has not been established in this population. In the EU adult population, PCV13 is indicated for active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae serotypes covered by the vaccine in adults ≥18 years of age and the elderly [22]. For the US adult population, PCV13 is also approved for the prevention of vaccine-type pneumococcal disease (IPD and pneumonia), but for a narrower age range of ≥50 years [21]. Local prescribing information should be consulted for additional information on contraindications, warnings and precautions, and use in special patient populations.
7 Place of 13-Valent Pneumococcal Conjugate Vaccine in Preventing Pneumococcal Disease in Adults
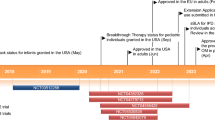
As noted in Sect. 6, PCV13 is approved in a number of countries for the prevention of IPD and pneumonia caused by vaccine pneumococcal serotypes in adults, albeit for different age groups depending on the country. PPSV23 has been available for many years and, until recently, has been the recommended pneumococcal vaccine for older adults. The 2012 recommendations for the use of pneumococcal vaccines from ACIP in the USA included the following: (i) PPSV23 for healthy adults aged ≥65 years; (ii) PPSV23 for immunocompetent adults aged 50–64 years with certain underlying medical conditions, such as diabetes or asthma (and revaccination at age 65 years); and (iii) PCV13 followed by PPSV23 (≥8 weeks later) for adults aged ≥19 years with immunocompromising conditions, functional and anatomic asplenia, cerebrospinal leaks or cochlear implants [2]. However, on the basis of CAPiTA results showing protective efficacy against vaccine-type pneumococcal CAP, vaccine-type pneumococcal nonbacteraemic CAP and vaccine-type IPD (Sect. 3.1), ACIP updated their adult pneumococcal vaccination guidelines in 2014/15 to recommend routine use of PCV13 (in series with PPSV23) in all adults aged ≥65 years (Table 3; see ACIP immunization recommendations for full details) [1, 58]. The effectiveness of PPSV23 in preventing IPD in older adults has been demonstrated, but its effectiveness in preventing noninvasive pneumococcal pneumonia in adults ≥65 years of age has been inconsistent [58].
The ACIP recommendation to use PCV13 in series with PPSV23 is also driven by changes in pneumococcal serotype distribution and epidemiology of pneumococcal disease among adults aged ≥65 years [1, 58]. PCV13 replaced PCV7 in the US paediatric immunization schedule in 2010, and by 2013 the incidence of IPD caused by pneumococcal serotypes unique to PCV13 among adults aged ≥65 years had decreased by ≈50 % (via indirect effects) [1, 58]. In the same year, 38 % of IPD cases in adults ≥65 years of age were caused by pneumococcal serotypes unique to PPSV23, hence the need for potentially broader protection by using both vaccines in series [58]. Interestingly, data from US adults aged ≥50 years with CAP suggest that pneumococcal serotypes causing noninvasive pneumonia in this population may differ markedly from those causing IPD, with PCV7 serotypes over-represented and serotype 5 contributing substantially to the observed cases of noninvasive pneumococcal pneumonia [59]. Other data from US adults (median age 58 years) hospitalized with CAP after the introduction of PCV13 for infant vaccination showed that PCV13 serotypes were detected in 9 % (178/2044) of patients, including only 2 % (40) with PCV7 serotypes [reported as an abstract] [60]. The most common serotypes were 19A (31 %), 5 (20 %), 3 (17 %) and 7F (15 %) [60]. A prospective observational cohort study conducted over 2 years (2008–2010) in two teaching hospitals in the UK included 920 adults (median age 72 years) hospitalized with CAP and showed that the most common pneumococcal serotypes were 14, 1, 8, 3 and 19A [61]. In Spain, a prospective single-centre study conducted over a 4-year period (2010–2014) showed that after the introduction of PCV13 for infant vaccination, there was a reduction in the proportion of cases of pneumococcal pneumonia and IPD in adults that were caused by PCV13 serotypes; however, vaccine serotypes were still responsible for more than half of the cases of pneumonia and ≈40 % of IPD cases in adults [62].
Findings of a recent retrospective cohort study are also noteworthy, as they suggest that the list of underlying medical conditions for which pneumococcal vaccination is recommended in immunocompetent adults by ACIP could be expanded in future guidelines [9]. The analysis identified several conditions not currently listed in ACIP recommendations, including rheumatoid arthritis, systemic lupus erythematosus, Crohn’s disease, and neuromuscular or seizure disorders, that were associated with increased risk of all-cause pneumonia and pneumococcal disease. There was also evidence of risk stacking, whereby the rate of all-cause pneumonia, pneumococcal pneumonia and IPD substantially increased with the accumulation of two or more concurrent risk conditions [9].
Recommendations for pneumococcal immunization outside routine childhood immunization programmes in Europe are complex and vary widely among countries in terms of age groups, risk groups recommended for vaccination and which vaccine should be administered, as highlighted in a recent comprehensive review of government recommendations for 16 Western European countries [63]. The author of the review suggests that greater clarity, simplification and dissemination of these recommendations could improve pneumococcal vaccine uptake and help reduce the high burden of pneumococcal disease in adults via direct and indirect effects [63]. In general, most of the 16 countries have immunization programmes with age- and risk-based elements. PPV23 is widely recommended, but many countries now include PCV (e.g. PCV13) for certain groups of children and adults, either alone or in addition to PPV23 (Table 3) [63].
In conclusion, PCV13 has a well established immunogenicity and tolerability profile in adults, particularly those ≥50 years of age. Results of CAPiTA, a randomized, double-blind, placebo-controlled trial in >84,000 older adults aged ≥65 years, showed that PCV13 was effective in preventing vaccine-type pneumococcal CAP, vaccine-type pneumococcal nonbacteraemic (noninvasive) CAP and vaccine-type IPD. These findings, along with changes in pneumococcal serotype distribution and epidemiology of pneumococcal disease in the USA, prompted ACIP to recommend PCV13 in series with PPVS23 for all adults aged ≥65 years. PCV13 also has a role in preventing pneumococcal disease (pneumonia and IPD) in younger adults with immunocompromising conditions and potentially in those with other underlying medical conditions that increase the risk of pneumococcal disease.
Data selection sources:
Relevant medical literature (including published and unpublished data) on 13-valent pneumococcal conjugate vaccine in adults was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 27 July 2015], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Prevenar-13, Prevnar-13, pneumococcal polysaccharide conjugate vaccine, 13-valent pneumococcal conjugate vaccine, PCV, PCV-13, 13-valent, adults.
Study selection: Studies in adults who received PCV13. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant immunogenicity data are also included.
References
Kim DK, Bridges CB, Harriman KH. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older: United States, 2015. Ann Intern Med. 2015;162(3):214–23.
Centers for Disease Control Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816–9.
World Health Organization. Pneumococcal disease. http://www.who.int/ith/diseases/pneumococcal/en/. Accessed 7 Apr 2015.
Lynch JP 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med. 2010;16(3):217–25.
Said MA, Johnson HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8(4):e60273.
Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043–51.
van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374(9700):1543–56.
Muhammad RD, Oza-Frank R, Zell E, et al. Epidemiology of invasive pneumococcal disease among high-risk adults since the introduction of pneumococcal conjugate vaccine for children. Clin Infect Dis. 2013;56(5):e59–67.
Shea KM, Edelsberg J, Weycker D, et al. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014;1(1):ofu024.
Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348(18):1747–55.
Honkanen PO, Keistinen T, Miettinen L, et al. Incremental effectiveness of pneumococcal vaccine on simultaneously administered influenza vaccine in preventing pneumonia and pneumococcal pneumonia among persons aged 65 years or older. Vaccine. 1999;17(20–21):2493–500.
Huss A, Scott P, Stuck AE, et al. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48–58.
Mangtani P, Cutts F, Hall AJ. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect Dis. 2003;3(2):71–8.
Conaty S, Watson L, Dinnes J, et al. The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials. Vaccine. 2004;22(23–24):3214–24.
Moberley S, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:Cd000422.
Russell KL, Baker CI, Hansen C, et al. Lack of effectiveness of the 23-valent polysaccharide pneumococcal vaccine in reducing all-cause pneumonias among healthy young military recruits: a randomized, double-blind, placebo-controlled trial. Vaccine. 2015;33(9):1182–7.
Leventer-Roberts M, Feldman BS, Brufman I, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive disease and hospital-treated pneumonia among people aged ≥65 years: a retrospective case-control study. Clin Infect Dis. 2015. doi:10.1093/cid/civ096.
Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis. 2008;47(10):1328–38.
Leinonen M, Sakkinen A, Kalliokoski R, et al. Antibody response to 14-valent pneumococcal capsular polysaccharide vaccine in pre-school age children. Pediatr Infect Dis. 1986;5(1):39–44.
Goldblatt D. Conjugate vaccines. Clin Exp Immunol. 2000;119(1):1–3.
Pfizer Inc. Prevnar 13 (pneumococcal 13-valent conjugate vaccine): US prescribing information. 2015. http://labeling.pfizer.com/showlabeling.aspx?id=501. Accessed 27 July 2015.
Prevenar 13 suspension for injection: summary of product characteristics (updated 10 Mar 2015). https://www.medicines.org.uk/emc/history/22689. Accessed 27 July 2015.
Sanford M. Pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed): in older adults. Drugs. 2012;72(9):1243–55.
Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013;31(35):3585–93.
Schwarz TF, Flamaing J, Rumke HC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥65 years. Vaccine. 2011;29(32):5195–202.
Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60–64 years of age. Vaccine. 2014;32(20):2364–74.
Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. 2013;31(35):3577–84.
Frenck RW Jr, Gurtman A, Rubino J, et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin Vaccine Immunol. 2012;19(8):1296–303.
Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594–602.
Tinoco JC, Juergens C, Ruiz Palacios GM, et al. Open-label trial of immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults ≥50 years of age in Mexico. Clin Vaccine Immunol. 2015;22(2):185–92.
Glesby MJ, Watson W, Brinson C, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in HIV-infected adults previously vaccinated with pneumococcal polysaccharide vaccine. J Infect Dis. 2015;212(1):18–27.
Bhorat AE, Madhi SA, Laudat F, et al. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in HIV-infected individuals naive to pneumococcal vaccination. AIDS. 2015;29:1345–54.
Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults [plus supplementary appendix]. N Engl J Med. 2015;372(12):1114–25.
Plosker GL. 13-valent pneumococcal conjugate vaccine: a review of its use in infants, children, and adolescents. Paediatr Drugs. 2013;15(5):403–23.
Simonsen L, Taylor RJ, Schuck-Paim C, et al. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med. 2014;2(5):387–94.
Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–9.
von Gottberg A, de Gouveia L, Tempia S, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371(20):1889–99.
Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014;59(8):1066–73.
Regev-Yochay G, Paran Y, Bishara J, et al. Early impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneumococcal disease: a nationwide surveillance study. Vaccine. 2015;33(9):1135–42.
Guevara M, Ezpeleta C, Gil-Setas A, et al. Reduced incidence of invasive pneumococcal disease after introduction of the 13-valent conjugate vaccine in Navarre, Spain, 2001–2013. Vaccine. 2014;32(22):2553–62.
Meichtry J, Born R, Küffer M, et al. Serotype epidemiology of invasive pneumococcal disease in Swiss adults: a nationwide population-based study. Vaccine. 2014;32(40):5185–91.
Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–54.
Gounder PP, Bruce MG, Bruden DJT, et al. Effect of the 13-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae—Alaska, 2008–2012. J Infect Dis. 2014;209(8):1251–8.
van Hoek AJ, Sheppard CL, Andrews NJ, et al. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine. 2014;32(34):4349–55.
Schwarz TF, Pauksens K, Juergens C, et al. Safety of a 13-valent pneumococcal conjugate vaccine in elderly adults previously immunized with a 23-valent pneumococcal polysaccharide vaccine: an open-label trial. World J Vaccines. 2013;3(4):123–9.
Jiang Y, Gauthier A, Annemans L, et al. A public health and budget impact analysis of vaccinating at-risk adults and the elderly against pneumococcal diseases in Germany. Expert Rev Pharmacoecon Outcomes Res. 2012;12(5):631–43.
Kuhlmann A, Theidel U, Pletz MW, et al. Potential cost-effectiveness and benefit-cost ratios of adult pneumococcal vaccination in Germany. Health Econ Rev. 2012;2(1):4.
Liguori G, Parlato A, Zamparelli AS, et al. Adult immunization with 13-valent pneumococcal vaccine in Campania region, South Italy: an economic evaluation. Hum Vaccines Immunother. 2014;10(2):492–7.
Pradas R, Gil de Miguel A, Alvaro A, et al. Budget impact analysis of a pneumococcal vaccination programme in the 65-year-old Spanish cohort using a dynamic model. BMC Infect Dis. 2013;13:175.
Rozenbaum MH, van Hoek AJ, Fleming D, et al. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ. 2012;345:e6879.
Rozenbaum MH, Hak E, van der Werf TS, et al. Results of a cohort model analysis of the cost-effectiveness of routine immunization with 13-valent pneumococcal conjugate vaccine of those aged ≥65 years in the Netherlands. Clin Ther. 2010;32(8):1517–32.
Chen J, O’Brien MA, Yang HK, et al. Cost-effectiveness of pneumococcal vaccines for adults in the United States. Adv Ther. 2014;31(4):392–409.
Cho B-H, Stoecker C, Link-Gelles R, et al. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–21.
Smith KJ, Wateska AR, Nowalk M, et al. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804–12.
Smith KJ, Wateska AR, Nowalk MP, et al. Modeling of cost effectiveness of pneumococcal conjugate vaccination strategies in U.S. older adults. Am J Prev Med. 2013;44(4):373–81.
Smith KJ, Nowalk MP, Raymund M, et al. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950–6.
Weycker D, Sato R, Strutton D, et al. Public health and economic impact of 13-valent pneumococcal conjugate vaccine in US adults aged ≥50 years. Vaccine. 2012;30(36):5437–44.
Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822–5.
Sherwin RL, Gray S, Alexander R, et al. Distribution of 13-valent pneumococcal conjugate vaccine Streptococcus pneumoniae serotypes in US adults aged ≥50 years with community-acquired pneumonia. J Infect Dis. 2013;208(11):1813–20.
Grijalva CG, Wunderink RG, Williams D, et al. Distribution of pneumococcal serotypes detected through urine analysis among US adults hospitalized with pneumonia after introduction of PCV13 [abstract no. ISPPD-0225]. Pneumonia. 2014;3:251.
Bewick T, Sheppard C, Greenwood S, et al. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax. 2012;67(6):540–5.
Payeras A, Villoslada A, Garau M, et al. Evolution of pneumococcal infections in adult patients during a four-year period after vaccination of a pediatric population with 13-valent pneumococcal conjugate vaccine. Int J Infect Dis. 2015;33:22–7.
Castiglia P. Recommendations for pneumococcal immunization outside routine childhood immunization programs in Western Europe. Adv Ther. 2014;31(10):1011–44.
Acknowledgments
During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes based on any comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Greg Plosker is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: A. Torres, Servei de Pneumologia, Hospital Clinic IDIBAPS, Barcelona, Spain; G. Zhanel, Department of Medical Microbiology, University of Manitoba, Winnipeg, MB, Canada.
Rights and permissions
About this article
Cite this article
Plosker, G.L. 13-Valent Pneumococcal Conjugate Vaccine: A Review of Its Use in Adults. Drugs 75, 1535–1546 (2015). https://doi.org/10.1007/s40265-015-0449-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0449-z