Abstract
Sucroferric oxyhydroxide (Velphoro®), an iron-based oral phosphate binder, is available for the control of serum phosphorus levels in patients with chronic kidney disease (CKD) on dialysis. In a pivotal phase III trial, sucroferric oxyhydroxide 1000–3000 mg/day for 24 weeks was noninferior to sevelamer carbonate 4800–14,400 mg/day with regard to lowering serum phosphorus levels. Additionally, sucroferric oxyhydroxide at maintenance dosages was significantly more effective than low dosage sucroferric oxyhydroxide (250 mg/day) with regard to maintaining controlled serum phosphorus levels during weeks 24–27 of treatment. Sucroferric oxyhydroxide had a numerically lower mean daily pill burden and better treatment adherence than sevelamer carbonate. Treatment with sucroferric oxyhydroxide was generally well tolerated over 24 weeks’ treatment, with the most frequently reported treatment-emergent adverse events being mild, transient diarrhoea and discoloured faeces. In a 28-week extension study, the efficacy and tolerability profile of sucroferric oxyhydroxide remained similar to sevelamer carbonate for up to 52 weeks. In conclusion, sucroferric oxyhydroxide is a valuable treatment option for hyperphosphataemia in CKD patients on dialysis, providing an effective and generally well tolerated noncalcium-based phosphate binder therapy with a lower pill burden than sevelamer carbonate and the potential for improved treatment adherence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Oral, iron-based phosphate binder with a practically insoluble active moiety (polynuclear iron[III]-oxyhydroxide) |
Noninferior efficacy to sevelamer carbonate in lowering serum phosphorus levels |
Significantly more effective in maintaining controlled serum phosphorus levels with maintenance dosage than with low dosage |
Numerically lower pill burden and better treatment adherence than sevelamer carbonate |
Generally well tolerated, with a similar long-term tolerability profile to sevelamer carbonate |
Minimal iron absorption, with no evidence of iron accumulation over 52 weeks |
1 Introduction
Chronic kidney disease (CKD) patients experience a progressive deterioration of mineral homeostasis due to declining kidney function that leads to abnormal serum and tissue levels of phosphorus [1]. International clinical practice guidelines suggest lowering serum phosphorus levels towards the normal range in CKD patients on dialysis [1]. To achieve this, restriction of dietary phosphate is recommended and increased dialytic removal of phosphate in patients with persistent hyperphosphataemia is suggested [1]. However, as dietary phosphate restriction is complicated and often inadequate for maintaining controlled serum phosphorus levels, oral phosphate binder therapy is a necessary component of hyperphosphataemia management for most CKD patients on dialysis [2–4]. Calcium-based binders (e.g. calcium carbonate and calcium acetate) have been the mainstay of phosphate binder therapy for many years, and are recommended as first-line therapy in the UK treatment guidelines [5]; however, at high dosages they are associated with an increased risk of hypercalcaemia and vascular calcification [6]. This has led to the development of noncalcium-based phosphate binders in recent years [3].
Sucroferric oxyhydroxide (Velphoro®) is an oral, iron-based phosphate binder that is indicated for the treatment of hyperphosphataemia in CKD patients undergoing dialysis in the USA [7] and EU [8]. A new drug application for sucroferric oxyhydroxide in this indication has been submitted in Japan [9]. This article provides an overview of the pharmacological properties of sucroferric oxyhydroxide and reviews the clinical data underpinning its use in hyperphosphataemia in CKD patients undergoing dialysis. In this review, the dose of sucroferric oxyhydroxide is expressed in terms of iron content (i.e. 250 mg or 500 mg per tablet). Although the dose of sucroferric oxyhydroxide has been described in terms of tablet weight in earlier studies, the dose description in this article is exclusively based on iron content to maintain consistency and avoid misinterpretation.
2 Pharmacodynamic Properties
Sucroferric oxyhydroxide binds dietary phosphate in the gastrointestinal (GI) tract by ligand exchange involving its hydroxyl groups and/or associated water molecules [7, 8]. Serum phosphorus and calcium-phosphate product levels are consequently decreased due to reduced dietary phosphate absorption, with bound phosphate being eliminated in the faeces [7, 8].
In vitro, sucroferric oxyhydroxide had high phosphate binding capacity over the physiologically relevant pH range found throughout the GI tract, indicating that phosphate binding could start in the stomach [10]. Phosphate adsorption peaked at a final pH value of 2.6, with a stoichiometric phosphate to iron ratio of 0.47 mmol phosphorus/mmol iron (0.26 mg phosphorus/mg iron). Based on this finding, three sucroferric oxyhydroxide 500 mg tablets had an estimated in vitro binding capacity of 126 mmol (390 mg) of phosphorus, which would meet the average daily phosphorus binding requirements for CKD dialysis patients of ≈120 mmol (371 mg) [10].
In patients with stable preterminal renal failure and hyperphosphataemia, oral administration of powdered sucroferric oxyhydroxide 500 mg three times daily resulted in a median decrease in urinary phosphate of 37 % and a calculated binding capacity of ≈1.33 mmol phosphorus for sucroferric oxyhydroxide 250 mg [11].
3 Pharmacokinetic Properties
Sucroferric oxyhydroxide consists of a mixture of polynuclear iron(III)-oxyhydroxide (≈33 %), sucrose (≈30 %), starches (≈28 %) and water (≤10 %), and has an iron content of ≈21 % [10]. The active moiety, polynuclear iron(III)-oxyhydroxide, is practically insoluble and not intended to be absorbed or metabolized, while the sucrose and starches are digested to absorbable carbohydrates (i.e. glucose, fructose and maltose) [7, 8]. Sucroferric oxyhydroxide is formulated as a chewable tablet to allow for optimal phosphate adsorption without the need for additional fluid intake [12]. In vitro, sucroferric oxyhydroxide tablets had adequate chewability that was comparable to other commercially available chewable phosphate binder tablets [12].
No classical pharmacokinetic studies have been performed on sucroferric oxyhydroxide due to its insolubility and degradation characteristics [7, 8]. Mononuclear iron can potentially be released from the surface of polynuclear iron(III)-oxyhydroxide as a degradation product and absorbed [7, 8]. In vitro, there was minimal iron release from sucroferric oxyhydroxide (0.26–0.35 %) under conditions simulating administration on a full stomach during passage through the GI tract (final pH 2.6–8.0) [10]. Iron release of ≈0.3 % corresponded to 4.5 mg of iron released for three sucroferric oxyhydroxide 500 mg tablets. Under conditions simulating administration on an empty stomach (i.e. initial pH 1.2), iron release from sucroferric oxyhydroxide was 67 and 6 % in the absence and presence of phosphate. X-ray photoelectron spectroscopy showed that iron phosphate was predominantly formed when sucroferric oxyhydroxide was exposed to a phosphate solution under acidic conditions, whereas iron(III)-oxyhydroxide was present under alkaline conditions [10].
In healthy volunteers and CKD patients, there was very low iron uptake at 21 days after oral administration of radiolabelled sucroferric oxyhydroxide, with healthy volunteers having ≈10-fold higher iron uptake than CKD patients [13]. The median expected iron absorption from sucroferric oxyhydroxide 2000 mg/day was ≈1.2 and 0.4 mg/day for nondialysis and haemodialysis (HD) CKD patients, respectively. Therefore, patients receiving sucroferric oxyhydroxide in a clinical setting are thought to have a very low risk of iron overload [13].
In a pivotal phase III trial in CKD patients with hyperphosphataemia on dialysis (Sect. 5), there were significantly (p < 0.0001) greater increases from baseline in median transferrin saturation levels with sucroferric oxyhydroxide than sevelamer carbonate at week 24, while increases from baseline in serum ferritin did not significantly differ between treatment groups and there were no significant changes from baseline in iron or haemoglobin levels [14]. Increases in iron parameters occurred early during treatment and stabilized with ongoing therapy, suggesting there was no iron accumulation with sucroferric oxyhydroxide [14]. In a 28-week extension of this trial (Sect. 5.4), there was no evidence of iron accumulation with sucroferric oxyhydroxide over 52 weeks of treatment, with transferrin saturation and serum ferritin levels remaining stable in these patients [15]. In subgroup analysis of iron parameters by region, baseline median serum ferritin levels were at least 75 % higher in patients from the USA than patients from Europe and other countries, with numerically larger increases from baseline at week 24 observed in patients from the USA than in patients from other regions [14]. During the phase III trial, concomitant intravenous iron products were received by 71 % of sucroferric oxyhydroxide and 74 % of sevelamer carbonate recipients, with a generally higher proportion of patients receiving intravenous iron in the USA (73 %) compared with Europe (53 %) and other countries (33 %) [14]. In post hoc analysis at week 52, changes in iron parameters were mainly attributed to concomitant intravenous iron treatment [16].
4 Drug Interactions
Potential drug interactions have been identified in vitro between sucroferric oxyhydroxide and alendronic acid, doxycycline, levothyroxine sodium, cephalexin, doxercalciferol and paricalcitol [7, 8]. In vitro studies have also demonstrated that there are no relevant interactions between sucroferric oxyhydroxide and any of the following medications: ciprofloxacin, enalapril, hydrochlorothiazide, metformin, metoprolol, nifedipine, quinidine, cinacalcet, clopidogrel or pioglitazone [7, 8]. The US prescribing information recommends administration of doxycycline ≥1 h before sucroferric oxyhydroxide, and states that levothyroxine sodium should not be prescribed with sucroferric oxyhydroxide [7]. The EU label suggests that any known or potentially interacting medication should be administered ≥1 h before or 2 h after sucroferric oxyhydroxide [8].
In healthy volunteers, there were no clinically relevant effects on the systemic exposure of digoxin, furosemide, losartan, omeprazole or warfarin when these agents were administered simultaneously or 2 h after a single dose of sucroferric oxyhydroxide 1000 mg [17]. In this study, peak serum concentrations (Cmax) of digoxin, furosemide, losartan and omeprazole were decreased when administered with sucroferric oxyhydroxide and food compared with administration 2 h after food and sucroferric oxyhydroxide. While it was considered possible that the differences in Cmax were due to the effect of food, a direct effect of sucroferric oxyhydroxide on the absorption rate of these medications could not be excluded [17].
In post hoc analysis from a phase III trial in CKD patients on dialysis (Sect. 5), sucroferric oxyhydroxide had no effect on the lipid-lowering effects of simvastatin, atorvastatin or other statins, despite earlier in vitro data indicating that an interaction occurs between sucroferric oxyhydroxide and atorvastatin [18]. Another post hoc analysis from this trial revealed that there was no interaction between sucroferric oxyhydroxide and stable oral vitamin D agonist therapy [19]. In these analyses, the active control drug (sevelamer carbonate) impacted the serum lipid parameters during treatment with statins [18] and interacted with oral vitamin D agonists [19].
5 Therapeutic Efficacy
Sucroferric oxyhydroxide, when administered at 1000, 1500, 2000 or 2500 mg/day for 6 weeks, significantly (p ≤ 0.05) decreased mean serum phosphorus levels from baseline (primary endpoint) in a phase II, dose-finding trial in CKD patients on HD (n = 150), with efficacy that was comparable to sevelamer hydrochloride 4800 mg/day and significantly (p ≤ 0.01) better than sucroferric oxyhydroxide 250 mg/day [20]. However, given the availability of a pivotal phase III trial [14] and its 28-week extension [15], this phase II trial is not discussed further.
The efficacy of sucroferric oxyhydroxide in the treatment of hyperphosphataemia in adult (≥18 years) CKD patients undergoing dialysis was compared with sevelamer carbonate in a randomized, open-label, multinational, active-controlled, parallel-group, prospective two-stage phase III trial [14] plus a 28-week extension [15]. Patients had a history of hyperphosphataemia treated with stable phosphate binder therapy for ≥1 month, and had been receiving maintenance HD three times per week or peritoneal dialysis (PD) for ≥3 months. Key exclusion criteria included hypercalcaemia (total serum calcium >2.75 mmol/L) while receiving calcium-free phosphate binders, hypocalcaemia, intact parathyroid hormone (iPTH) levels >88 pmol/L and serum ferritin levels >4494 pmol/L. After a washout period of 2–4 weeks during which previous phosphate binders were stopped, eligible patients had serum phosphorus levels ≥1.94 mmol/L [14].
In stage 1 (baseline to week 24), patients received sucroferric oxyhydroxide 1000–3000 mg/day (2–6 chewable tablets daily) or sevelamer carbonate 4800–14,400 mg/day (6–18 tablets daily) [14]. Sucroferric oxyhydroxide was started at 1000 mg/day (one chewable 500 mg tablet taken twice daily with the largest meals of the day) and sevelamer carbonate was started at 4800 mg/day (two 800 mg tablets taken three times daily). Stage 1 consisted of a dose-titration phase (weeks 0–8; the dosage of either drug could be titrated for efficacy and tolerability), a maintenance phase (weeks 8–12; dosage adjustments were allowed only for tolerability) and a second maintenance phase (weeks 12–24; dosage titration for efficacy and tolerability was permitted). Sucroferric oxyhydroxide could be titrated by 500 mg/day every 2 weeks (within a range of 1000–3000 mg/day), while sevelamer carbonate could be titrated by 2400 mg/day (within a range of 2400–14,400 mg/day). In stage 2 (weeks 24–27), HD patients from the sucroferric oxyhydroxide treatment group were re-randomized to continue sucroferric oxyhydroxide at the week 24 maintenance dosage (MD) or low dosage 250 mg/day (LD) (minimal or no effects were observed at this dosage in the phase II trial [20]); dosage adjustments were not permitted [14].
In stage 1, the main objective was to demonstrate noninferiority of sucroferric oxyhydroxide compared with sevelamer carbonate by assessing the changes from baseline in mean serum phosphorus levels at week 12 (secondary endpoint) [14]. In stage 2, the efficacy objective was to establish superiority of sucroferric oxyhydroxide MD compared with sucroferric oxyhydroxide LD by assessing serum phosphorus from weeks 24–27 (primary endpoint) [14]. Treatment adherence was assessed by determining the actual tablet intake during the maintenance treatment period relative to the expected tablet intake, with the number of tablets returned by patients used for calculation purposes. Patients receiving 70–120 % of the expected tablet intake were recorded as being adherent [14].
In the full analysis set (FAS) in stage 1 (n = 1041), patients had a mean age of 56 years and 92 and 8 % of patients were undergoing HD and PD [14].
5.1 Noninferiority of Sucroferric Oxyhydroxide Versus Sevelamer Carbonate (Stage 1)
Sucroferric oxyhydroxide was noninferior to sevelamer carbonate in lowering serum phosphorus levels in CKD dialysis patients with hyperphosphataemia [14]. At week 12, the least-squares mean difference in change from baseline serum phosphorus levels between sucroferric oxyhydroxide and sevelamer carbonate treatment groups met predefined noninferiority criteria (Table 1). The serum phosphorus-lowering effect was maintained at week 24 for both treatment groups. For the entire study period, there were no significant interactions with treatment effect for baseline demographic and disease covariates, including sex, age, ethnicity, geographical region, baseline serum phosphorus levels, dialysis modality, time from first dialysis, aetiology of end-stage renal disease, number of previous phosphate binders received and previous use of sevelamer carbonate [14].
5.2 Other Outcomes (Stage 1)
Patients receiving sucroferric oxyhydroxide had a numerically lower mean pill burden than those receiving sevelamer carbonate during stage 1 of this trial [14]. From baseline to week 24, FAS patients in the sucroferric oxyhydroxide and sevelamer carbonate groups had mean pill burdens of 3.1 and 8.1 tablets/day. Over the same study period, treatment adherence was 83 and 77 % in patients receiving sucroferric oxyhydroxide and sevelamer carbonate [14].
In CKD, hyperphosphataemia is associated with elevated PTH levels and decreased 1,25-dihydroxy-vitamin D [1,25(OH)2D] levels as a result of impaired conversion from 25-hydroxy-vitamin D [25(OH)D] [1]. At week 24 of the study, patients in both treatment groups had significant (p < 0.05) decreases from baseline in median serum iPTH levels, with a significantly (p = 0.04) greater reduction in iPTH levels with sucroferric oxyhydroxide than sevelamer carbonate [14]. At week 24, median serum 25(OH)D levels had also significantly (p < 0.0001) decreased from baseline in the sucroferric oxyhydroxide and sevelamer carbonate groups, with sevelamer carbonate recipients having a significantly (p = 0.019) greater reduction in serum 25(OH)D levels than sucroferric oxyhydroxide recipients. Patients in the sevelamer carbonate group had significant (p = 0.0065) decreases from baseline in median serum 1,25(OH)2D levels at week 24, but median serum 1,25(OH)2D levels did not significantly decrease from baseline in patients receiving sucroferric oxyhydroxide [14].
5.3 Superiority of Sucroferric Oxyhydroxide Maintenance Dosage Versus Low Dosage (Stage 2)
Treatment with sucroferric oxyhydroxide MD for 3 weeks was significantly more effective than sucroferric oxyhydroxide LD with regard to maintaining serum phosphorus levels following 24 weeks of treatment (Table 1) [14]. There was no significant change in mean serum phosphorus levels from weeks 24–27 for patients in the sucroferric oxyhydroxide MD treatment group, while patients in the sucroferric oxyhydroxide LD group had a significantly greater increase in serum phosphorus levels and the between-group difference significantly favoured sucroferric oxyhydroxide MD treatment (Table 1). Covariance analyses demonstrated no significant interaction with treatment effects when week 24 serum phosphorus levels or other baseline patient characteristics (Sect. 5.1) were used as covariates [14].
5.4 Extension Study
The efficacy of sucroferric oxyhydroxide was maintained throughout a 28-week extension and the total treatment period of 52 weeks [15]. Patients who completed treatment to weeks 24 or 27 (excluding those receiving sucroferric oxyhydroxide LD) were enrolled in a 28-week extension in which they continued to receive maintenance dosages of either sucroferric oxyhydroxide (n = 384) or sevelamer carbonate (n = 260). At week 52, there was no significant difference between treatment groups in the change from extension study baseline in mean serum phosphorus levels (0.02 vs. 0.09 mmol/L with sucroferric oxyhydroxide vs. sevelamer carbonate). In a pooled analysis of data from the initial 24-week trial and its 28-week extension, changes from baseline in mean serum phosphorus levels also did not significantly differ over the total 52-week treatment period between the sucroferric oxyhydroxide (–0.70 mmol/L) and sevelamer carbonate (–0.66 mmol/L) groups. Mean serum phosphorus levels for both treatment groups remained within the Kidney Disease Outcomes Quality Initiative target range of 1.13–1.78 mmol/L at each timepoint throughout the 28-week extension. Among patients completing 52 weeks’ treatment, 52 and 55 % of sucroferric oxyhydroxide and sevelamer carbonate recipients were within this target range at week 52 [15]. In subgroup analysis, the efficacy of sucroferric oxyhydroxide and sevelamer carbonate were similar in HD versus PD patients (abstract [22] and abstract plus poster [23] presentations).
During the 28-week extension, sucroferric oxyhydroxide recipients required a numerically lower mean daily pill number compared with sevelamer carbonate recipients to maintain control of serum phosphorus levels (4.0 vs. 10.1 tablets) [15]. Over this period, treatment adherence was 86 and 77 % in the sucroferric oxyhydroxide and sevelamer carbonate groups. In the pooled analysis, the mean pill burden with sucroferric oxyhydroxide was 62 % lower than that of sevelamer carbonate over 52 weeks’ treatment (3.3 vs 8.7 tablets/day). During this period, treatment adherence was 83 % with sucroferric oxyhydroxide and 80 % with sevelamer carbonate [15].
At week 52, there were significant (p < 0.001) increases in mean iPTH levels from extension study baseline in both treatment groups [15]. Bone-specific alkaline phosphatase levels significantly (p < 0.001) decreased from extension study baseline to week 52 with sucroferric oxyhydroxide, but did not significantly change with sevelamer carbonate. There was no significant difference in mean total serum calcium levels from extension study baseline to week 52 in either treatment group. The between-group differences were not significant for any of these parameters [15].
6 Tolerability
Sucroferric oxyhydroxide was generally well tolerated in the phase III trial and its 28-week extension discussed in Sect. 5. The incidence of treatment-emergent adverse events (TEAEs) was 83 and 76 % in patients receiving sucroferric oxyhydroxide and sevelamer carbonate over 24 weeks (stage 1) [14]. The incidence of serious TEAEs was 18 and 20 % in the sucroferric oxyhydroxide and sevelamer carbonate groups, although sucroferric oxyhydroxide recipients had a numerically higher incidence of GI TEAEs (45 vs. 34 %) and withdrawal due to TEAEs (16 vs. 7 %) than sevelamer carbonate recipients, the latter being primarily related to GI TEAEs (e.g. diarrhoea). However, nonadherence to study treatment was reported in 15 and 21 % of sucroferric oxyhydroxide and sevelamer carbonate recipients. Withdrawal due to hyperphosphataemia occurred in 1 % of patients receiving sucroferric oxyhydroxide and none of the patients receiving sevelamer carbonate; this was thought to be due to the low starting dosage of sucroferric oxyhydroxide. The mean daily number of tablets in the sucroferric oxyhydroxide group was 3.6 by weeks 12–24, indicating that most of these patients required three tablets daily for effective control of serum phosphorus levels [14].
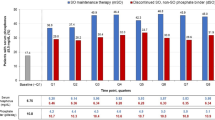
Over 24 weeks’ treatment, the most common adverse events (AEs) with sucroferric oxyhydroxide were diarrhoea, discoloured faeces and hyperphosphataemia, whereas nausea and constipation were the most common AEs with sevelamer carbonate (Fig. 1) [14]. Treatment-related AEs were reported in 40 % of patients receiving sucroferric oxyhydroxide and 20 % of those receiving sevelamer carbonate. The difference between the treatment groups was mainly due to the incidence of discoloured faeces and diarrhoea during sucroferric oxyhydroxide treatment. All reports of sucroferric oxyhydroxide-related discoloured faeces occurred during the titration phase (weeks 0–8), and the event rarely resulted in treatment withdrawal (<1 %). Given that sucroferric oxyhydroxide is an oral iron preparation, the occurrence of discoloured faeces was to be expected [14]. While this event may potentially mask GI bleeding, sucroferric oxyhydroxide does not affect faecal occult blood tests [8]. Of the patients who reported diarrhoea during treatment, most cases were mild and transient in both the sucroferric oxyhydroxide (69 %) and sevelamer carbonate (58 %) groups [14]. Additionally, most cases of diarrhoea occurred early for both treatment groups and resolved without study drug discontinuation. It should also be noted that 38 % of patients in this trial had received sevelamer carbonate during the 12 months before study entry. These patients may have already been acclimated to sevelamer carbonate-related AEs, which may have affected the reporting of AEs in this treatment group. Serious treatment-related GI AEs occurred in two sucroferric oxyhydroxide recipients and none of the sevelamer carbonate recipients. One sucroferric oxyhydroxide patient was hospitalized for assessment of faecal discolouration and recovered without study drug discontinuation, while the other patient experienced a duodenal ulcer with GI bleeding that later resolved [14].
Treatment-emergent adverse events with a ≥5 % incidence over 24 weeks in a phase III trial in chronic kidney disease patients on dialysis [14]. PA21 sucroferric oxyhydroxide, SEV-carb sevelamer carbonate
During the 28-week extension, the incidence of TEAEs with sucroferric oxyhydroxide decreased to 74 % [15] from 83 % during weeks 0–24 [14], and was consistent with the incidence of TEAEs with sevelamer carbonate (77 %) [15]. Serious TEAEs were reported in 20 % of patients in both treatment groups, and <1 % of these events were considered related to treatment; all serious treatment-related AEs were GI disorders. Treatment-related AEs were reported in 15 and 9 % of sucroferric oxyhydroxide and sevelamer carbonate recipients [15].
The most common TEAE in both treatment groups during the extension study was hyperphosphataemia, occurring in 12 and 11 % of sucroferric oxyhydroxide and sevelamer carbonate recipients [15]. Aside from hyperphosophataemia, the most common TEAEs with sucroferric oxyhydroxide were hypertension (10 vs. 8 % with sevelamer carbonate) and diarrhoea (8 vs. 6 %), while the most common events with sevelamer carbonate were secondary hyperparathyroidism (9 vs. 4 % with sucroferric oxyhydroxide) and hypotension (8 vs. 5 %) [15]. The incidence of diarrhoea and discoloured faeces, which were the most frequent treatment-related AEs with sucroferric oxyhydroxide during the first 24 weeks’ treatment [14], decreased over time in the extension study [15].
In the extension study, the incidence of TEAEs resulting in withdrawal was 8 % (decreasing from 16 % over the first 24 weeks), while 5 % of sevelamer carbonate recipients experienced TEAEs that resulted in withdrawal [15]. In both treatment groups, hyperphosphataemia was the most common TEAE causing withdrawal (occurring in 3 % of patients from either group), while GI TEAEs led to withdrawal in 2 and <1 % of sucroferric oxyhydroxide and sevelamer carbonate recipients. Other than a GI haemorrhage that occurred in one sevelamer carbonate recipient, all GI TEAEs resulting in withdrawal were of mild to moderate intensity. In a pooled analysis of data from the initial 24-week trial and its 28-week extension, the incidence of TEAEs resulting in withdrawal over 52 weeks was 21 and 10 % with sucroferric oxyhydroxide and sevelamer carbonate [15].
In post hoc analysis at week 52, sucroferric oxyhydroxide recipients had a significantly (p < 0.05) higher relative risk (RR) of mild diarrhoea (RR 2.3) and moderate diarrhoea (RR 1.9) than sevelamer carbonate recipients, with no significant difference in RR reported for severe diarrhoea, although there was a significantly (p < 0.05) lower risk of hyperparathyroidism (RR 0.6), nausea (RR 0.7), decreased appetite (RR 0.5) and constipation (RR 0.5) with sucroferric oxyhydroxide than sevelamer carbonate (abstract presentation) [24].
7 Dosage and Administration
In the USA [7] and EU [8], oral sucroferric oxyhydroxide is indicated for the control of serum phosphorus levels in adult patients with CKD on dialysis. In the EU, the use of sucroferric oxyhydroxide as part of a multiple therapeutic approach is suggested; this may include calcium supplementation, 1,25-dihydroxy-vitamin D3 or one of its analogues, or calcimimetics to control renal bone disease development [8].
Each woodberry flavoured sucroferric oxyhydroxide chewable tablet contains 500 mg iron [7, 8]. The recommended starting dosage is three sucroferric oxyhydroxide tablets (1500 mg) per day, administered as one tablet three times daily. Sucroferric oxyhydroxide tablets must be chewed and not swallowed whole; however, the tablets may be crushed to aid with chewing and swallowing. Additional fluid intake above the usual amount is not required. Sucroferric oxyhydroxide must be taken with meals, with the total daily dose divided across the meals of the day to maximize dietary phosphate binding [7, 8]. The EU label also recommends that patients remain adherent to their prescribed diets [8]. If one or more doses of sucroferric oxyhydroxide are missed, the medication should be resumed with the patient’s next meal [7, 8].
The dosage of sucroferric oxyhydroxide should be titrated in increments or decrements of one tablet per day until acceptable serum phosphorus levels are reached (≤1.78 mmol/L), with subsequent regular monitoring [7, 8]. In the EU, dosage titration every 2–4 weeks is recommended [8], while the US label states that titration can start from 1 week after treatment initiation with adjustments made at weekly intervals as needed [7]. In clinical studies, an average dosage of 3–4 tablets/day (1500–2000 mg/day) was required to control serum phosphorus levels [7, 8]. The maximum recommended dosage in the EU is 6 tablets/day (3000 mg/day) [8].
Sucroferric oxyhydroxide is contraindicated in patients with haemochromatosis or any other iron accumulation disorder in the EU [8], and the US label recommends monitoring the effects on iron homeostasis in these patients [7].
Local prescribing information should be consulted for detailed information, including warnings and precautions, contraindications, potential drug interactions and administration in special patient populations.
8 Place of Sucroferric Oxyhydroxide in the Management of Hyperphosphataemia in Chronic Kidney Disease Patients Undergoing Dialysis
Management of hyperphosphataemia in CKD patients on dialysis is essential due to the associated increased risk of cardiovascular and all-cause mortality, with most patients requiring oral phosphate binder therapy for adequate control of serum phosphorus levels [2–4, 25, 26]. Phosphate binders account for approximately one-half of a typically high daily pill burden in CKD dialysis patients [27]. Higher pill burdens have been associated with lower health-related quality of life (HR-QOL) scores and significantly reduced treatment adherence [27, 28], which is common in CKD dialysis patients [2, 3, 25]. In a study of HD patients receiving phosphate binder therapy, increased adherence was associated with lower mean phosphorus levels and a higher proportion of patients with serum phosphorus ≤1.78 mmol/L [28]. This study concluded that phosphate binder formulations with lower pill burdens and equivalent phosphate binding capacity are likely to result in increased patient adherence and potentially improved control of serum phosphorus levels [28].
An ideal phosphate binder should effectively bind dietary phosphate across a physiological pH range, have minimal systemic absorption, an acceptable tolerability profile, a low pill burden and be cost effective [2, 3, 25]. Of the currently available phosphate binders, calcium-based binders are effective, well tolerated and inexpensive [4, 25]. However, they can be associated with a positive calcium balance that can lead to oversuppression of PTH, adynamic bone disease and vascular calcification due to calcium deposition in tissues and vessels [4, 25], and clinical guidelines recommend avoiding high dosages in certain patients populations [1]. Sevelamer is an anion-exchange resin that effectively binds phosphate while avoiding the risk of hypercalcaemia due to its nonabsorbable properties [4, 25]. However, sevelamer-based binders (i.e. sevelamer hydrochloride and sevelamer carbonate) are expensive and have an increased risk of GI AEs compared with calcium-based binders; sevelamer hydrochloride has also been attributed to an increased incidence of metabolic acidosis [3, 25]. Moreover, sevelamer-based binders require high pill burdens (6–12 tablets/day) for effective management of hyperphosphataemia [3].
Sucroferric oxyhydroxide, an iron-based phosphate binder, has been approved for the treatment of hyperphosphataemia in CKD patients undergoing dialysis [7, 8]. In a pivotal phase III trial, sucroferric oxyhydroxide 1000–3000 mg/day had noninferior efficacy to sevelamer carbonate 4800–14,400 mg/day in lowering serum phosphorus levels in CKD patients on dialysis over 24 weeks (Sect. 5.1). Furthermore, sucroferric oxyhydroxide MD (1000–3000 mg/day) was significantly more effective than sucroferric oxyhydroxide LD (250 mg/day) with regard to maintaining serum phosphorus levels for a further 3 weeks (Sect. 5.3). Sucroferric oxyhydroxide had a numerically lower mean daily pill burden than sevelamer carbonate, and treatment adherence was observed in a numerically higher proportion of sucroferric oxyhydroxide versus sevelamer carbonate recipients (Sect. 5.2). In a 28-week extension, the efficacy of sucroferric oxyhydroxide was maintained over 52 weeks of treatment with a numerically lower pill burden than sevelamer carbonate (Sect. 5.4).
The reduced pill burden of sucroferric oxyhydroxide compared with sevelamer carbonate has the potential for improved treatment adherence in dialysis patients [14], and may also result in improved patient HR-QOL [27]. While sevelamer carbonate is also available as a powder, which may help to reduce its pill burden [2, 4], sucroferric oxyhydroxide is formulated as a chewable tablet that does not require any additional fluids (Sect. 7), which could potentially provide a further clinical benefit for CKD patients by avoiding excess fluid intake [12].
Sucroferric oxyhydroxide was generally well tolerated in clinical trials (Sect. 6). Although TEAEs were reported with numerically higher frequency in patients receiving sucroferric oxyhydroxide than sevelamer carbonate during the phase III trial, this difference was principally due to a higher incidence of mild, transient diarrhoea and discoloured faeces with sucroferric oxyhydroxide during early treatment. Other reported GI TEAEs, including constipation and nausea, were reported with numerically higher frequency with sevelamer carbonate than sucroferric oxyhydroxide. Over 24 weeks’ treatment, hyperphosphataemia also occurred with numerically higher frequency with sucroferric oxyhydroxide than sevelamer carbonate; however, this was thought to be due to a study design limitation, where the dose frequency at the initiation of the dosage titration phase for sucroferric oxyhydroxide (twice daily) was less than equivalent to that used for sevelamer carbonate (three times daily). Other limitations of the phase III clinical trial included its open-label design and that a large proportion (more than one-third) of patients had previously received sevelamer carbonate in the 12 months before study enrolment [14]. These patients were potentially already accustomed to the adverse events associated with sevelamer carbonate, which may have affected the reported incidence of TEAEs in this treatment group [14]. In the 28-week extension study, the incidence of TEAEs were generally similar across treatment groups over 52 weeks of treatment, with hyperphosphataemia being the most commonly reported event in both treatment groups (Sect. 6).
Sucroferric oxyhydroxide administration is associated with minimal iron absorption in CKD patients on dialysis, and in the phase III trial, there were larger increases from baseline in median transferrin saturation levels in patients receiving sucroferric oxyhydroxide, especially those receiving concomitant intravenous iron (Sect. 3). However, there was no evidence of iron accumulation over 52 weeks [15], and given that iron deficiency is prevalent in CKD patients, mild iron absorption from sucroferric oxyhydroxide may be beneficial in these patients [26]. Longer-term studies are needed to determine whether iron uptake is associated with any potential drug toxicity [26].
There is limited pharmacoeconomic data available regarding the use of sucroferric oxyhydroxide in CKD dialysis patients with hyperphosphataemia. However, a recent modelled analysis assessing cost implications over a time horizon of 10 years has indicated that treatment with sucroferric oxyhydroxide was cost effective compared with sevelamer carbonate from the perspective of the National Health Service in Scotland (abstract presentation) [29]. Total costs per patient were slightly lower with sucroferric oxyhydroxide than sevelamer carbonate, while the estimated quality-adjusted life years remained the same for both treatment groups [29]. Additional studies are required to confirm the cost effectiveness of sucroferric oxyhydroxide in CKD dialysis patients with hyperphosphataemia.
In conclusion, sucroferric oxyhydroxide was effective in lowering serum phosphorus levels in CKD patients undergoing dialysis and was generally well tolerated over 52 weeks, with noninferior efficacy, generally lower pill burden and increased adherence compared to sevelamer carbonate. Thus, sucroferric oxyhydroxide is a valuable option for the treatment of hyperphosphataemia in CKD patients undergoing dialysis.
Data selection sources:
Relevant medical literature (including published and unpublished data) on sucroferric oxyhydroxide was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) [searches last updated 23 February 2015], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Sucroferric oxyhydroxide, Velphoro, PA21, ferric oxyhydroxide, iron oxyhydroxide, phosphate binder, Vifor.
Study selection: Studies in patients with hyperphosphataemia and chronic kidney disease undergoing dialysis who received sucroferric oxyhydroxide. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–130.
Covic A, Rastogi A. Hyperphosphatemia in patients with ESRD: assessing the current evidence linking outcomes with treatment adherence. BMC Nephrol. 2013;14:153.
Ketteler M, Wuthrich RP, Floege J. Management of hyperphosphataemia in chronic kidney disease-challenges and solutions. Clin Kidney J. 2013;6(2):128–36.
Cupisti A, Gallieni M, Rizzo MA, et al. Phosphate control in dialysis. Int J Nephrol Renovasc Dis. 2013;6:193–205.
National Institute for Health and Care Excellence. Hyperphosphataemia in chronic kidney disease: management of hyperphosphataemia in patients with stage 4 or 5 chronic kidney disease (NICE clinical guideline 157). 2013. http://www.nice.org.uk. Accessed 23 Feb 2015.
Moe SM, Chertow GM. The case against calcium-based phosphate binders. Clin J Am Soc Nephrol. 2006;1(4):697–703.
Fresenius Medical Care North America. Velphoro (sucroferric oxyhydroxide) chewable tablet for oral use: US prescribing information. 2014. http://www.velphoro.us. Accessed 23 Feb 2015.
Vifor Fresenius Medical Care Renal Pharma France. Velphoro 500 mg chewable tablets: EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 23 Feb 2015.
Kissei Pharmaceutical Co. Ltd. New drug application submitted for “PA21 (development code)” for treatment of hyperphosphatemia [media release]. 19 Nov 2014. http://kissei.co.jp/e_contents.
Wilhelm M, Gaillard S, Rakov V, et al. The iron-based phosphate binder PA21 has potent phosphate binding capacity and minimal iron release across a physiological pH range in vitro. Clin Nephrol. 2014;81(04):251–8.
Hergesell O, Ritz E. Stabilized polynuclear iron hydroxide is an efficient oral phosphate binder in uraemic patients. Nephrol Dial Transplant. 1999;14(4):863–7.
Lanz M, Baldischweiler J, Kriwet B, et al. Chewability testing in the development of a chewable tablet for hyperphosphatemia. Drug Dev Ind Pharm. 2014;40(12):1623–31.
Geisser P, Philipp E. PA21: a novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney disease. Clin Nephrol. 2010;74(1):4–11.
Floege J, Covic AC, Ketteler M, et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int. 2014;86:638–47.
Floege J, Covic AC, Ketteler M, et al. Long-term effects of iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015. doi:10.1093/ndt/gfv006.
Sprague SM, Covic A, Floege J, et al. Concomitant intravenous iron use drives changes in iron indices in a phase 3 study of PA21 [abstract plus poster]. In: National Kidney Foundation 2014 Spring Clinical Meeting. 2014.
Chong E, Kalia V, Willsie S, et al. Drug-drug interactions between sucroferric oxyhydroxide and losartan, furosemide, omeprazole, digoxin and warfarin in healthy subjects. J Nephrol. 2014;27(6):659–66.
Levesque V, Chong EMF, Moneuse P. Post-hoc analysis of pharmacodynamic interaction of PA21 with statins in a phase 3 study of PA21 in dialysis patients with hyperphosphatemia [abstract no. SA-PO568]. In: American Society of Nephrology Kidney Week 2013. 2013.
Floege J, Botha J, Chong E, et al. PA21 does not interact with oral vitamin D receptor agonists: a post hoc analysis of a phase 3 study [abstract no. SP257]. Nephrol Dial Transplant. 2014;29(Suppl 3):iii157.
Wuthrich RP, Chonchol M, Covic A, et al. Randomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(2):280–9.
Vifor Inc. A phase 3 study to investigate the safety and efficacy of PA21, a phosphate binder, in dialysis patients [ClinicalTrials.gov identifier NCT01324128]. US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/. Accessed 23 Feb 2015.
Covic AC, Floege J, Ketteler M, et al. Efficacy and safety of PA21, a novel iron-based phosphate binder in CKD patients on peritoneal- and hemodialysis [abstract]. In: 15th Congress of the International Society of Peritoneal Dialysis. 2014.
Floege J. Efficacy and safety of the novel iron-based phosphate binder PA21 in peritoneal- and hemodialysis-dependent CKD patients [abstract plus poster]. In: 11th European Peritoneal Dialysis Meeting. 2013.
Covic A, Ketteler M, Rastogi A, et al. Comparison of safety profiles of PA21 and sevelamer carbonate in a post hoc analysis of a phase 3 study [abstract no. SP245]. Nephrol Dial Transplant. 2014;29(Suppl 3):iii153.
Malberti F. Hyperphosphataemia: treatment options. Drugs. 2013;73(7):673–88.
Nastou D, Fernandez-Fernandez B, Elewa U, et al. Next-generation phosphate binders: focus on iron-based binders. Drugs. 2014;74(8):863–77.
Chiu YW, Teitelbaum I, Misra M, et al. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–96.
Wang S, Alfieri T, Ramakrishnan K, et al. Serum phosphorus levels and pill burden are inversely associated with adherence in patients on hemodialysis. Nephrol Dial Transplant. 2014;29(11):2092–9.
Gutzwiller FS, Braunhofer PG, Szucs TD, et al. Health economic evaluation of non-calcium-based phosphate binders in Scotland [abstract no. SP596]. Nephrol Dial Transplant. 2014;29(Suppl 3):iii271.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit. Sarah Greig and Greg Plosker are salaried employees of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: B. S. Spinowitz, Nephrology, New York Hospital, Queens, New York, NY, USA; R. P. Wüthrich, Division of Nephrology, University Hospital, Zurich, Switzerland.
Rights and permissions
About this article
Cite this article
Greig, S.L., Plosker, G.L. Sucroferric Oxyhydroxide: A Review in Hyperphosphataemia in Chronic Kidney Disease Patients Undergoing Dialysis. Drugs 75, 533–542 (2015). https://doi.org/10.1007/s40265-015-0366-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0366-1