Abstract
The norepinephrine prodrug droxidopa (NORTHERA™) is approved in the US for the treatment of orthostatic dizziness, lightheadedness, or the ‘feeling that you are about to black out’ in adults with symptomatic neurogenic orthostatic hypotension associated with primary autonomic failure (e.g. Parkinson’s disease, multiple system atrophy or pure autonomic failure), dopamine β-hydroxylase deficiency or nondiabetic autonomic neuropathy. This article reviews the clinical efficacy and tolerability of droxidopa in symptomatic neurogenic orthostatic hypotension, as well as summarizing its pharmacological properties. Oral droxidopa was effective in the shorter-term treatment of patients with symptomatic neurogenic orthostatic hypotension, with improvements seen in symptoms, the impact of symptoms on daily activities and standing systolic blood pressure. More data are needed to confirm the longer-term efficacy of droxidopa. Droxidopa was generally well tolerated, although patients should be monitored for supine hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Synthetic amino acid converted by L-aromatic-amino-acid decarboxylase into norepinephrine |
Effective in the shorter term, improving symptoms, the impact of symptoms on daily activities and standing systolic blood pressure |
More data needed regarding its longer-term efficacy |
Generally well tolerated |
Monitor patients for supine hypertension |
1 Introduction
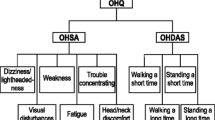
Neurogenic orthostatic hypotension is a symptom of the autonomic nervous system dysfunction seen in conditions such as Parkinson’s disease, pure autonomic failure, multiple system atrophy and autonomic neuropathy [1–3]. Patients with neurogenic orthostatic hypotension experience a sustained reduction in blood pressure (BP) on standing because of impaired compensatory autonomic reflexes [1–3]. Patients affected by neurogenic orthostatic hypotension are often profoundly symptomatic, with symptoms including lightheadedness, dizziness or syncope, visual disturbances, weakness, fatigue, an inability to perform daily activities and fall-related injuries [2, 3].
The deficient activation of vascular adrenoceptors that gives rise to neurogenic orthostatic hypotension may reflect degenerative loss of postganglionic sympathetic neurons (such as in pure autonomic failure), which is usually associated with low resting norepinephrine (noradrenaline) levels, or loss of central pathways that regulate sympathetic drive (such as in multiple system atrophy), which may be associated with normal supine norepinephrine levels [1]. No increase in plasma norepinephrine levels is seen on standing in either patient group [1].
The norepinephrine prodrug droxidopa (L-threo-3,4-dihydroxyphenylserine; L-DOPS) [NORTHERA™] is approved in the US for the treatment of orthostatic dizziness, lightheadedness, or the ‘feeling that you are about to black out’ in adults with symptomatic neurogenic orthostatic hypotension associated with primary autonomic failure (e.g. Parkinson’s disease, multiple system atrophy or pure autonomic failure), dopamine β-hydroxylase deficiency or nondiabetic autonomic neuropathy [4].
This article reviews the clinical efficacy and tolerability of droxidopa in symptomatic neurogenic orthostatic hypotension, as well as summarizing its pharmacological properties.
2 Pharmacodynamic Properties
Droxidopa is a synthetic amino acid that is converted by L-aromatic-amino-acid decarboxylase (DOPA decarboxylase) into norepinephrine (see also Sect. 3.2) [1, 5, 6]. DOPA decarboxylase is widely expressed (e.g. expressed in the parenchymal cells of the liver, kidneys and gastrointestinal tract), meaning that even if postganglionic sympathetic neurons are not intact, administration of droxidopa may increase norepinephrine levels by the conversion of droxidopa to norepinephrine outside sympathetic neurons [6–8].
Although the precise mechanism of action of droxidopa in symptomatic neurogenic orthostatic hypotension is unknown, it is thought that the pharmacological effects of droxidopa are mediated through norepinephrine, which increases BP by inducing peripheral arterial and venous vasoconstriction, rather than through the parent drug or other metabolites [4]. Norepinephrine levels increased in patients with symptomatic neurogenic orthostatic hypotension who received droxidopa (see also Sect. 3.1) [9–11].
In 19 patients with severe symptomatic neurogenic orthostatic hypotension (associated with pure autonomic failure or multiple system atrophy) who received a single, individually optimized dose of droxidopa 200–2,000 mg (mean dose 1,137 mg), mean BP was significantly (p < 0.001) increased when supine and after standing for 1 or 3 min [9]. Both supine and standing BP increased by ≈40 mmHg. The pressor effect started 1 h after administration of droxidopa and lasted for 6 h when standing and for 8 h when supine. Peak standing BP occurred 3.5 h after administration of droxidopa (p < 0.00001 vs. placebo). Heart rate did not significantly differ between droxidopa and placebo when patients were supine, standing for 1 min or standing for 3 min [9].
It appears that a peripheral mechanism of action is responsible for the pressor activity of droxidopa, as the pressor response to droxidopa was abolished by coadministration of relatively high doses (200 mg) of the DOPA decarboxylase inhibitor carbidopa [6] (see also Sect. 4), which does not cross the blood–brain barrier [6, 9]. Peripherally, droxidopa-induced norepinephrine is thought to act as both a neurotransmitter at postganglionic sympathetic neurons and as a circulating hormone [6, 8].
3 Pharmacokinetic Properties
3.1 Absorption and Distribution
In healthy volunteers, peak plasma concentrations (Cmax) of droxidopa were reached 1–4 h postdose, with a mean time to Cmax (tmax) of ≈2 h [4]. When droxidopa was administered with a high-fat meal, tmax was delayed by ≈2 h, Cmax was reduced by 35 % and the area under the plasma concentration–time curve was reduced by 20 % [4]. The droxidopa Cmax was associated with increased systolic and diastolic BP [4].
Following administration of a single oral dose of droxidopa 400 mg to patients with severe symptomatic neurogenic orthostatic hypotension, a droxidopa Cmax of 1,942 ng/mL was reached in 3 h, with droxidopa concentrations remaining detectable for ≥22 h following administration of droxidopa [9]. A significant (p < 0.05) increase from baseline in plasma norepinephrine levels was seen 2 h after administration of droxidopa 400 mg (from 294 to 806 pg/mL), with norepinephrine levels peaking at 1,250 pg/mL, 6 h after administration of droxidopa (p < 0.005 vs. placebo) [9].
Droxidopa crossed the blood–brain barrier [12, 13]. Plasma protein binding of droxidopa was 75 and 26 % at concentrations of 100 and 10,000 ng/mL [4]. Droxidopa had an estimated apparent volume distribution of about 200 L [4].
3.2 Metabolism and Elimination
Droxidopa is metabolized via the catecholamine system [4]. Droxidopa is metabolized by DOPA decarboxylase to yield the main active metabolite norepinephrine, by catechol-O-methyltransferase (COMT) to yield methoxylated dihydroxyphenylserine (3-OM-DOPS) and by L-threo-3,4-dihydroxyphenylserine aldolase to yield protocatechualdehyde [4, 14].
The major route of elimination for droxidopa and its metabolites was renal, with a mean elimination half-life of ≈2.5 h [4]. In animal studies, ≈75 % of a radiolabelled dose of droxidopa was excreted in the urine within 24 h of oral administration [4].
3.3 Special Patient Populations
The pharmacokinetics of droxidopa were not affected to a clinically significant extent by age, body mass index or sex [4]. Hepatic function (assessed using liver function tests) did not appear to affect droxidopa exposure, according to the results of a population pharmacokinetic analysis [4]. The droxidopa dosage does not need to be adjusted in patients with mild to moderate renal impairment; experience with droxidopa in patients with severe renal impairment is limited [4].
4 Potential Drug Interactions
This section briefly summarizes drug interactions that may potentially be associated with droxidopa. It should be noted that no dedicated drug–drug interaction studies have been conducted for droxidopa [4], and most studies were not powered to test for efficacy in the presence versus absence of concomitant agents.
It is expected that the risk of supine hypertension would be increased if droxidopa was administered in combination with other agents that increase BP, such as norepinephrine, ephedrine, midodrine and triptans [4].
Importantly, in clinical trials in which droxidopa was coadministered with levodopa/DOPA decarboxylase inhibitor combination drugs (at usual dosages), there was no significant need for altered droxidopa doses and no increase in associated adverse events, despite the clearance of droxidopa being decreased and both droxidopa and 3-OM-DOPS exposure being increased [4]. However, the US prescribing information states that dosage adjustment may be needed in patients receiving both droxidopa and a DOPA decarboxylase inhibitor [4]. In patients with autonomic failure [7] or pure autonomic failure or multiple system atrophy [9] who received relatively high doses of carbidopa (200 mg), the increase in norepinephrine levels seen with droxidopa was abolished [7] or markedly attenuated [9], and BP was not increased [7].
Coadministration of the peripheral COMT inhibitor entacapone with droxidopa did not affect plasma droxidopa concentrations, the droxidopa-induced increase in plasma norepinephrine levels or systolic BP [7]. The US prescribing information states that dopamine agonists, amantadine derivatives and monoamine oxidase (MAO)-B inhibitors do not affect the clearance of droxidopa and dosage adjustments are not required [4].
In clinical trials, significant (p < 0.05) improvements in the Orthostatic Hypotension Questionnaire (OHQ) composite score (see Sect. 5) occurred with droxidopa, regardless of concomitant administration of fludrocortisone or dopaminergics [15]. A significant (p < 0.001) improvement in the OHQ composite score was seen in patients who did not receive concomitant droxidopa enzymatic degradation agents (e.g. COMT inhibitors or MAO inhibitors), but not in patients who received concomitant droxidopa enzymatic degradation agents [15].
5 Therapeutic Efficacy
Early trials demonstrated the potential of droxidopa in patients with neurogenic orthostatic hypotension [9, 16–19]. However, these trials will not be discussed further, given the availability of phase III trials.
Four short-term, randomized, double-blind, multicentre, phase III trials (studies 301 [20], 302 [21], 306a [22] and 306b [23]) examined the efficacy of droxidopa in patients with neurogenic orthostatic hypotension. Patients in studies 301 and 302 had neurogenic orthostatic hypotension associated with Parkinson’s disease, pure autonomic failure, multiple system atrophy, nondiabetic autonomic neuropathy or dopamine β-hydroxylase deficiency [20, 21]. Of the randomized patients, the primary clinical diagnosis included Parkinson’s disease (40.7 % [20] and 43.6 % [21] of patients), pure autonomic failure (33.3 % [20] and 17.8 % [21]), multiple system atrophy (16.0 % [20] and 29.7 % [21]), nondiabetic autonomic neuropathy (4.9 % [20] and 5.0 % [21]), dopamine β-hydroxylase deficiency (0 % [20] and 1.0 % [21]) or ‘other’ (4.9 % [20] and 3.0 % [21]). Patients in studies 306a and 306b had neurogenic orthostatic hypotension associated with Parkinson’s disease; dosages of drugs for Parkinson’s disease remained stable [22, 23]. Key inclusion and exclusion criteria for these trials are shown in Table 1.
Following open-label dose optimization and washout phases, responders in study 301 received double-blind droxidopa or placebo for 1 week [20]. Study 302 had a randomized, withdrawal design; after a dose-optimization phase and a 7-day open-label phase, responders were randomized to receive double-blind droxidopa or placebo for 14 days [21]. Following a randomized, double-blind, dose-optimization phase in study 306, patients continued to receive droxidopa or placebo in an 8-week maintenance phase. A preplanned futility analysis was conducted in the 51 patients initially enrolled in study 306 [22]. This portion of the study (306a) was then halted because prespecified stopping criteria for futility were met; interim results should be considered exploratory [22]. Blinding was maintained until the end of the study for the final 171 patients, and data were analysed as a separate study (study 306b) [23]. Additional information regarding the design of these trials is presented in Table 1.
Primary endpoints were the change in the OHQ composite score from randomization to week 1 (study 301 [20]) or to maintenance week 8 (study 306a [22]) or the change in the score for item 1 of the Orthostatic Hypotension Symptom Assessment (OHSA) [i.e. dizziness, lightheadedness, feeling faint or feeling like you might black out] from randomization to maintenance week 1 (study 306b [23]) or after a 14-day withdrawal phase (study 302 [21]).
Data from a longer-term extension study (study 303) is available as an abstract [24], supplemented by information from US FDA medical review documents [14]. Study 303 was an extension of studies 301 and 302 (mostly enrolling patients from study 302), in which, after 3 months’ open-label treatment with droxidopa (n = 103), there was a 14-day withdrawal phase in which patients were randomized in a double-blind manner to continue droxidopa (n = 38) or receive placebo (n = 37) [14]. This was followed by a 9-month open-label treatment phase [14]. The primary endpoint in study 303 was the mean change in the OHQ composite score from randomization to the end of the 14-day withdrawal phase [14].
5.1 Effects on Symptoms and Symptom Impact on Daily Activities
Droxidopa improved symptoms and the impact of symptoms on daily activities in patients with neurogenic orthostatic hypotension associated with various underlying conditions, according to the results of study 301 [20]. One week after randomization, the composite OHQ score and the OHSA item 1 score decreased (improved) to a significantly greater extent with droxidopa than with placebo (Table 2) [20]. Significantly more droxidopa than placebo recipients had an improvement in the composite OHQ score of ≥3 units (27.2 vs. 11.4 %; p = 0.016) or ≥4 units (17.3 vs. 2.5 %; p = 0.003) [20].
Patients receiving droxidopa versus placebo had a significantly greater improvement in the mean OHSA composite score (−1.68 vs. −0.95; p = 0.01); in addition to dizziness/lightheadedness, significant (p < 0.05) improvements were seen in vision disturbance, weakness and fatigue [20]. The mean Orthostatic Hypotension Daily Activity Scale (OHDAS) composite score also improved to a significantly greater extent with droxidopa than with placebo (−1.98 vs. −0.92; p = 0.003); significant (p < 0.01) improvements were seen in all four symptom-impact items [20].
In study 302, the mean score for item 1 of the OHSA was >6 at baseline and 2.1 at the time of randomization (Table 2) [21]. However, the change in the OHSA item 1 score from the time of randomization to the end of the 14-day withdrawal phase did not significantly differ between droxidopa and placebo recipients; scores worsened in both treatment groups (Table 2). During the withdrawal phase, the OHQ composite score worsened to a significantly smaller extent with droxidopa than with placebo, according to results of a post hoc analysis (Table 2) [21].
In an integrated analysis of studies 301 and 302 (available as an abstract), the OHQ composite score, the OHSA composite score and the OHDAS composite score improved to a significantly (p < 0.01) greater extent with droxidopa than with placebo [25]. Significant (p < 0.05) improvement was seen for three of six symptoms (dizziness/lightheadedness, weakness, fatigue) and for all four symptom-impact items [25].
Exploratory analysis of study 306a revealed no significant difference between droxidopa and placebo recipients in the change from randomization to maintenance week 8 in the OHQ composite score in patients with neurogenic orthostatic hypotension associated with Parkinson’s disease (Table 2) [22]. Although there was also no significant between-group difference in terms of the change from randomization to maintenance week 1 in the OHSA item 1 score (p = 0.24) (Table 2), it should be noted that study 306a was not powered for this endpoint, and study results did signal a possible benefit [22], meaning that the change in the OHSA item 1 score was selected as the primary endpoint in study 306b.
In study 306b, the improvement from randomization to maintenance week 1 in the OHSA item 1 score was significantly greater with droxidopa than with placebo (Table 2) [23]. Significantly (p < 0.05) more patients receiving droxidopa than placebo had improvements in this endpoint of ≥2 units, ≥3 units, ≥4 units, ≥25 % or ≥50 %. There were no significant differences between droxidopa and placebo recipients in the mean change in the OHSA item 1 score from randomization to maintenance weeks 2 (−1.9 vs. −1.6), 4 (−2.0 vs. −1.5) or 8 (−2.1 vs. −1.5). The changes from randomization to maintenance week 8 in the OHQ composite score are shown in Table 2 [23].
In a meta-analysis of studies 306a and 306b (available as an abstract) [197 patients in the modified intent-to-treat (mITT) population], a significant improvement in the mean OHSA item 1 score was seen with droxidopa versus placebo at week 1 (between-group difference of −1.2; p = 0.008), but not at week 8 (between-group difference of −0.8; p = 0.077) [26].
5.1.1 Extension Study
In study 303, the mean OHQ composite scores in patients randomized to droxidopa and placebo were 6.38 vs. 6.27, respectively, at baseline, 3.26 vs. 2.92, respectively, at month 3 (i.e. after 3 months’ open-label therapy with droxidopa) and 3.82 vs. 3.83, respectively, at the end of the randomized withdrawal phase [14]. The worsening in the OHQ composite score from randomization until the end of the withdrawal period did not significantly differ between droxidopa and placebo recipients (+0.57 vs. +0.90) [14]. Mean OHQ composite scores were 2.79, 3.24 and 3.02 at months 6, 9 and 12, respectively (i.e. during the subsequent open-label treatment phase) [24].
5.2 Effect on Blood Pressure
The mean increase from randomization to study end in standing systolic BP was significantly greater with droxidopa than with placebo in study 301 (+11.2 vs. +3.9 mmHg; p < 0.001) [20]. Supine systolic BP increased to a significantly greater extent with droxidopa than with placebo (+7.6 vs. +0.8 mmHg; p < 0.001) (see also Sect. 6) [20].
Among patients subsequently randomized to droxidopa and placebo in study 302, mean standing systolic BP increased by 22.6 and 25.5 mmHg, respectively, and mean standing diastolic BP increased by 11.4 and 11.7 mmHg, respectively, during the dose-optimization phase [21]. From randomization to study end, mean changes in standing systolic BP (−7.6 vs. −5.2 mmHg) and standing diastolic BP (−2.6 vs. −2.5 mmHg) did not significantly differ between droxidopa and placebo recipients. Changes in standing systolic BP, in particular, were highly variable in both droxidopa and placebo recipients [21].
In study 306a, the mean change from randomization to maintenance week 1 in standing systolic BP was significantly greater with droxidopa than with placebo (+8.4 vs. −4.1 mmHg; p = 0.04), although there was no significant between-group difference in the mean change from randomization to the end of the study (+7.0 vs. +7.7 mmHg) [22].
In study 306b, the mean change from randomization to maintenance week 1 in lowest standing systolic BP significantly favoured droxidopa versus placebo recipients (+6.4 vs. +0.7 mmHg; p = 0.032); the mean change from randomization to maintenance week 8 in the corresponding treatment groups was +5.0 and +0.9 mmHg (p = 0.160) [23].
In a meta-analysis of studies 306a and 306b, a significant improvement in mean standing systolic BP was seen with droxidopa versus placebo at week 1 (between-group difference of +6.8 mmHg; p = 0.014), but not at week 8 (between-group difference of +2.2 mmHg) [26].
5.3 Effect on Falls
In study 306a, the proportion of patients reporting falls was 54 % with droxidopa and 59 % with placebo [22]. The total number of falls was 79 in droxidopa and 192 in placebo recipients, with an average number of falls per patient per week of 0.4 and 0.8 in the corresponding treatment groups (relative risk 0.5; p = 0.16) [22]. Among patients who experienced repeat falls (i.e. at least two falls during study drug treatment), the mean number of falls per patient per week was 1.0 with droxidopa versus 1.9 with placebo [22].
In study 306b, the mean number of falls per week was 0.39 in droxidopa recipients and 1.09 in placebo recipients (p = 0.853) [23]. Droxidopa and placebo recipients had a total of 229 and 716 reported falls, respectively, with fall-related injury occurring in 16.9 and 25.6 % of patients in the corresponding treatment groups [23].
In a meta-analysis of studies 306a and 306b (available as an abstract) [197 mITT patients], the average number of falls per patient per week (0.38 vs. 1.73) and the total number of falls (325 vs. 908) did not significantly differ between droxidopa and placebo recipients [27].
6 Tolerability
Data concerning the tolerability of oral droxidopa in patients with neurogenic orthostatic hypotension were obtained from the clinical trials discussed in Sect. 5 and analyses reported in the US prescribing information [4]. Data concerning the longer-term tolerability of droxidopa were obtained from study 303 [24] and study 304 [28]. Study 304 was an extension of studies 301, 302 and 306 and is available as an abstract [28]. In this interim analysis (n = 213), the mean duration of droxidopa exposure was 189 days and the mean droxidopa dose was 416 mg three times daily [28]. An analysis of studies 303 and 304 reported in the US prescribing information included 422 patients with a mean total droxidopa exposure of ≈1 year [4].
Oral droxidopa was generally well tolerated in patients with symptomatic neurogenic orthostatic hypotension. The most commonly reported adverse reactions in droxidopa recipients in studies, 301, 302 and 306 were headache, dizziness, nausea and hypertension (Fig. 1) [4]. The adverse reactions that most commonly led to treatment discontinuation in droxidopa recipients included hypertension, increased BP and nausea [4].
Tolerability of oral droxidopa in patients with neurogenic orthostatic hypotension. Results of (a) a pooled analysis of studies 301 and 302 and (b) an analysis of study 306, as reported in the US prescribing information [4]. Shown are adverse reactions reported in >5 % of droxidopa recipients and with a ≥3 % higher incidence with droxidopa than with placebo. The duration of randomized treatment was 1–2 weeks in studies 301 and 302 and 8–10 weeks in study 306. δ = Incidence of 0 %
Serious adverse events were reported in two droxidopa recipients during the dose-escalation phase of study 301 (nausea and vomiting in one patient and urinary tract infection and urinary obstruction in a second patient) [20]. However, no serious adverse events were reported during the double-blind phase of this trial [20]. Serious adverse events were reported in 2.2 % of droxidopa recipients during the open-label droxidopa treatment phase of study 302, and in 0 % of droxidopa recipients and 2.0 % of placebo recipients during the double-blind treatment phase [21]. No serious adverse events were reported in study 306a [22], and serious treatment-emergent adverse events were reported in 5.6 % of droxidopa recipients and 4.9 % of placebo recipients in study 306b [23]. Cardiac events (most commonly palpitations) were reported in 3.0 % of droxidopa recipients during the dose-escalation phase of study 301, with no cardiac events reported during the double-blind phase [20].
Oral droxidopa was generally well tolerated in the longer term [28]. In the extension studies (studies 303 and 304), the most commonly reported adverse events in droxidopa recipients were falls (24 % of patients), urinary tract infections (15 %), headache (13 %), syncope (13 %) and dizziness (10 %) [4].
The US prescribing information carries a boxed warning regarding the risk of supine hypertension with droxidopa (see also Sect. 8) [4]. In study 301, supine hypertension (systolic BP >180 mmHg) was present in 0 % of droxidopa recipients and in 2.5 % of placebo recipients at randomization and in 4.9 and 2.5 % of patients in the corresponding treatment groups at study end [20]. No droxidopa recipients had supine systolic BP of >200 mmHg, compared with two placebo recipients [20]. In study 302, supine hypertension was reported in 10.9 % of patients after 7 days’ open-label treatment with droxidopa, and in 14.0 % of droxidopa recipients and 5.9 % of placebo recipients at study end [21]. In study 306a, supine hypertension was reported in one droxidopa recipient and one placebo recipient during the dose-optimization phase [22]. In study 306b, supine hypertension was reported in 7.9 % of droxidopa recipients and 4.9 % of placebo recipients during the dose-optimization phase and weeks 1 and 2 of the maintenance phase, with no episodes reported after week 2 [23]. In study 304, increased BP, hypertension and hypertensive crisis was reported in 1.4, 2.8 and 1.4 % of patients, respectively [28].
No clinically significant changes in laboratory parameters or ECG recordings were reported in studies 301 [20], 302 [21], 306a [22] or 306b [23].
7 Dosage and Administration
Droxidopa is approved in the US for the treatment of orthostatic dizziness, lightheadedness, or the ‘feeling that you are about to black out’ in adults with symptomatic neurogenic orthostatic hypotension associated with primary autonomic failure (e.g. Parkinson’s disease, multiple system atrophy or pure autonomic failure), dopamine β-hydroxylase deficiency or nondiabetic autonomic neuropathy [4].
It is recommended that oral droxidopa be initiated at a dosage of 100 mg three times daily (when the patient arises in the morning, at midday and in the late afternoon ≥3 h prior to bedtime) [4]. Droxidopa should be administered consistently, either with or without food. The droxidopa dosage should be titrated to the symptomatic response in increments of 100 mg three times daily every 24–48 h, up to a maximum dosage of 600 mg three times daily [4].
The efficacy of droxidopa beyond 2 weeks is uncertain, and the continued effectiveness of droxidopa should be assessed periodically [4].
The US prescribing information carries a boxed warning regarding the risk of supine hypertension [4]. Local prescribing information should be consulted for further information concerning warnings and precautions relating to droxidopa.
8 Current Status of Droxidopa in Symptomatic Neurogenic Orthostatic Hypotension
The goal of treatment in symptomatic neurogenic orthostatic hypotension is to improve symptoms, rather than to restore normal BP [3, 29]. The goal is to maintain cerebral perfusion pressure within the range of cerebral vascular autoregulation so that consciousness is maintained, not to boost systemic BP. Nonpharmacological approaches to the treatment of symptomatic neurogenic orthostatic hypotension include getting up slowly, elevating the head of the bed, increasing fluid and salt intake, and wearing compression garments/stockings [2, 3].
Pharmacological options for the treatment of symptomatic neurogenic orthostatic hypotension are limited. Midodrine, which is converted to the α1-receptor agonist desglymidodrine, is approved in the US for the treatment of symptomatic orthostatic hypotension [3, 29, 30]. Although midodrine improves standing systolic BP in patients with orthostatic hypotension, its effects on symptoms are inconsistent and it is associated with a risk of supine hypertension [2, 3]. Off-label drugs used in the treatment of symptomatic neurogenic orthostatic hypotension include fludrocortisone, pyridostigmine, desmopressin, dihydroergotamine, indomethacin and erythropoietin [2, 3, 29].
Droxidopa has been available in Japan for use in neurogenic orthostatic hypotension since 1989 [5], and was recently approved in the US for the treatment of orthostatic dizziness, lightheadedness, or the ‘feeling that you are about to black out’ in adults with symptomatic neurogenic orthostatic hypotension [4].
After 1 week’s therapy, droxidopa improved symptoms and the impact of symptoms on daily activities in patients with symptomatic neurogenic orthostatic hypotension associated with various underlying conditions, according to the results of study 301 (Sect. 5.1). Standing systolic BP was also improved with droxidopa (Sect. 5.2). In study 302, the OHSA item 1 score worsened in both droxidopa and placebo recipients during the randomized withdrawal phase, suggesting that the efficacy of droxidopa may diminish over time [14]. It should be noted that the OHSA item 1 score did not return to baseline in placebo recipients during the withdrawal phase of this study, raising the possibility that droxidopa has a carryover effect [14]. Both studies 301 and 302 used an enrichment design, whereby only patients classified as responders were entered into the randomized phase of these trials.
In patients with symptomatic neurogenic orthostatic hypotension associated with Parkinson’s disease, the OHSA item 1 score was significantly improved with droxidopa versus placebo from randomization to maintenance week 1 in study 306b, with no significant between-group difference in terms of the change from randomization to maintenance week 8 in the OHQ composite score in either study 306a or 306b (Sect. 5.1). Droxidopa might be useful to reduce falls among patients with Parkinson’s disease who are repeat fallers (Sect. 5.3). A clinical trial designed to examine the efficacy of droxidopa in repeat fallers would be of interest [22].
The efficacy of droxidopa in the longer term is unclear. In the extension study (study 303), although a numerical reduction in the OHQ composite score was seen with droxidopa from baseline to the end of the 3-month open-label phase, the worsening in the OHQ composite score seen in the randomized withdrawal phase did not significantly differ between droxidopa and placebo recipients (Sect 5.1.1); it has been suggested that study 303 was not sufficiently powered to show a significant difference between droxidopa and placebo [14]. The US prescribing information states that the effectiveness of droxidopa beyond 2 weeks is uncertain, and patients should be evaluated periodically to determine if droxidopa is still providing benefit [4].
Oral droxidopa was generally well tolerated in patients with symptomatic neurogenic orthostatic hypotension (Sect. 6). Regarding the risk of supine hypertension, the US prescribing information states that droxidopa recipients should be advised to raise the head of their bed when sleeping or resting; BP should be monitored in both the supine and the head-elevated sleeping position [4]. If supine hypertension persists, the dosage of droxidopa should be reduced or it should be discontinued [4].
In conclusion, oral droxidopa is effective in the shorter-term treatment of patients with symptomatic neurogenic orthostatic hypotension, with improvements seen in symptoms, the impact of symptoms on daily activities and standing systolic BP. More data are needed to confirm the longer-term efficacy of droxidopa. Droxidopa was generally well tolerated, although patients should be monitored for supine hypertension.
Data selection sources:
Relevant medical literature (including published and unpublished data) on droxidopa was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) [searches last updated 15 December 2014], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Droxidopa, NORTHERA, L-threo-3,4-dihydroxyphenylserine, L-DOPS, Threo-dopas*, L*dihydroxyphenylserine, orthostatic hypotension, postural hypotension.
Study selection: Studies in patients with symptomatic neurogenic orthostatic hypotension who received droxidopa. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Freeman R, Landsberg L. The treatment of orthostatic hypotension with dihydroxyphenylserine. Clin Neuropharmacol. 1991;14(4):296–304.
Berger MJ, Kimpinski K. A practical guide to the treatment of neurogenic orthostatic hypotension. Can J Neurol Sci. 2014;41(2):156–63.
Schroeder C, Jordan J, Kaufmann H. Management of neurogenic orthostatic hypotension in patients with autonomic failure. Drugs. 2013;73(12):1267–79.
Lundbeck NA Ltd. NORTHERA™ (droxidopa) capsules, for oral use: US prescribing information. 2014. http://www.northera.com. Accessed 10 Dec 2014.
Goldstein DS. L-Dihydroxyphenylserine (L-DOPS): a norepinephrine prodrug. Cardiovasc Drug Rev. 2006;24(3–4):189–203.
Kaufmann H. The discovery of the pressor effect of DOPS and its blunting by decarboxylase inhibitors. J Neural Transm. 2006;70(Suppl):477–84.
Goldstein DS, Holmes C, Sewell LT, et al. Effects of carbidopa and entacapone on the metabolic fate of the norepinephrine prodrug L-DOPS. J Clin Pharmacol. 2011;51(1):66–74.
Kaufmann H. L-dihydroxyphenylserine (droxidopa): a new therapy for neurogenic orthostatic hypotension. The US experience. Clin Auton Res. 2008;18(Suppl 1):19–24.
Kaufmann H, Saadia D, Voustianiouk A, et al. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108(6):724–8.
Goldstein DS, Holmes C, Kaufmann H, et al. Clinical pharmacokinetics of the norepinephrine precursor L-threo-DOPS in primary chronic autonomic failure. Clin Auton Res. 2004;14(6):363–8.
Suzuki T, Higa S, Sakoda S, et al. Pharmacokinetic studies of oral L-threo-3,4-dihydroxyphenylserine in normal subjects and patients with familial amyloid polyneuropathy. Eur J Clin Pharmacol. 1982;23(5):463–8.
Suzuki T, Sakoda S, Ueji M, et al. Treatment of parkinsonism with L-threo-3,4-dihydroxyphenylserine: a pharmacokinetic study. Neurology. 1984;34(11):1446–50.
Kato T, Karai N, Katsuyama M, et al. Studies on the activity of L-threo-3,4-dihydroxyphenylserine (L-DOPS) as a catecholamine precursor in the brain: comparison with that of L-DOPA. Biochem Pharmacol. 1987;36(18):3051–7.
US Food and Drug Administration. Droxidopa: medical review. Application number: 203202Orig1s000. 2014. http://www.fda.gov. Accessed 10 Dec 2014.
LeWitt P, Gorny S. Analysis of efficacy and safety outcomes in patients treated with droxidopa in combination with other drug classes [abstract no. 1294]. Mov Disord. 2012;27(Suppl 1):S425–6.
Kaufmann H, Oribe E, Yahr MD. Differential effect of L-threo-3,4-dihydroxyphenylserine in pure autonomic failure and multiple system atrophy with autonomic failure. J Neural Transm. 1991;3(2):143–8.
Mathias CJ, Senard J-M, Braune S, et al. L-threo-dihydroxyphenylserine (L-threo-DOPS; droxidopa) in the management of neurogenic orthostatic hypotension: a multi-national, multi-center, dose-ranging study in multiple system atrophy and pure autonomic failure. Clin Auton Res. 2001;11:235–42.
Mathias CJ, Senard JM, Cortelli P. A double-blind, randomized, placebo-controlled study to determine the efficacy and safety of droxidopa in the treatment of orthostatic hypotension associated with multiple system atrophy or Parkinson’s disease [abstract]. Clin Auton Res. 2007;17:272.
Wikstrom L, Bjerle P, Boman K. L-threo-DOPS treatment of orthostatic hypotension in Swedish patients with familial amyloidotic polyneuropathy (TTR-met30). Amyloid J Protein Fold Disord. 1996;3:162–6.
Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83(4):328–35.
Biaggioni I, Freeman R, Mathias CJ, et al. Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa. Hypertension. 2014. doi:10.1161/HYPERTENSIONAHA.114.04035.
Hauser RA, Hewitt LA, Isaacson S. Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson’s disease (NOH306A). J Parkinsons Dis. 2014;4(1):57–65.
Hauser RA, Isaacson S, Lisk JP, et al. Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson’s disease (nOH306B). Mov Disord. 2014. doi:10.1002/mds.26086.
Isaacson S, Shill H, Vernino S, et al. Durability of effect with long-term, open-label droxidopa treatment in patients with symptomatic neurogenic orthostatic hypotension (NOH 303) [abstract no. 1291]. Mov Disord. 2012;27(Suppl 1):S424–5.
Mathias C, Low P, Freeman R, et al. Integrated efficacy analysis of droxidopa in 2 double-blind, placebo-controlled phase 3 studies in patients with neurogenic orthostatic hypotension [abstract no. 1296]. Mov Disord. 2012;27(Suppl 1):S426.
Isaacson S, Hauser R, Szakacs C, et al. Droxidopa treatment impact on orthostatic symptoms and standing systolic blood pressure in patients with Parkinson’s disease (PD) and symptomatic neurogenic orthostatic hypotension (NOH) [abstract]. Neurology. 2013;80(19):e203.
Hauser RA, Jerome LP, Schwieterman WD, et al. Impact of droxidopa treatment on falls and fall related injuries in patients with Parkinson’s disease and symptomatic neurogenic orthostatic hypotension (study 306) [abstract no. 481]. Mov Disord. 2013;28(Suppl 1):S171.
Shill H, Vernino S, Hutchman R, et al. A multicenter, open-label study to assess the long-term safety of droxidopa in patients with symptomatic neurogenic orthostatic hypotension (NOH 304) [abstract no. 1302]. Mov Disord. 2012;27(Suppl 1):S428.
Lamarre-Cliche M. Drug treatment of orthostatic hypotension because of autonomic failure or neurocardiogenic syncope. Am J Cardiovasc Drugs. 2002;2(1):23–35.
Upsher-Smith Laboratories Inc. Midodrine HCl tablets: US prescribing information. 2012. http://www.upsher-smith.com/. Accessed 10 Dec 2014.
Kaufmann H, Malamut R, Norcliffe-Kaufmann L, et al. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22(2):79–90.
Disclosure
The preparation of this review was not supported by any external funding. Gillian Keating is a salaried employee of Adis/Springer. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: S.H. Fox, Movement Disorder Clinic, University of Toronto, Toronto Western Hospital, Toronto, ON, Canada; S. Grill, Parkinson’s and Movement Disorders Center of Maryland, Elkridge, MD, USA; P.A. Low, Mayo Clinic, Rochester, MN, USA.
Rights and permissions
About this article
Cite this article
Keating, G.M. Droxidopa: A Review of Its Use in Symptomatic Neurogenic Orthostatic Hypotension. Drugs 75, 197–206 (2015). https://doi.org/10.1007/s40265-014-0342-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0342-1