Abstract
Background
ACT-709478 is a selective, orally available T-type calcium channel blocker being studied as a potential new treatment in epilepsy. ACT-709478 had previously been investigated in a single-ascending dose study up to a dose of 400 mg.
Objectives
The aim of this study was to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple doses of ACT-709478. In addition, the drug-drug interaction potential of multiple doses of ACT-709478 with the cytochrome P450 3A4 substrate midazolam was investigated.
Methods
This double-blind, placebo-controlled, randomized study included 46 healthy male and female subjects. Ascending multiple oral doses of ACT-709478 were administered to 10 (cohorts 1–2) or 12 (cohorts 3–4) subjects (two taking placebo per cohort). In cohorts 1–2, 30 or 10 mg ACT-709478 was administered once daily for 12 days. An up-titration regimen was used in cohorts 3–4 with administration of 10, 30, and 60 mg for 7 days each in both cohorts and an additional dose level of 100 mg ACT-709478 once daily for 8 days in cohort 4. Single doses of midazolam were administered at baseline and concomitantly to 60 mg and 100 mg ACT-709478 in cohort 4. Blood sampling for pharmacokinetic evaluations and safety assessments (clinical laboratory, vital signs, adverse events, and electrocardiogram) were performed regularly. Holter electrocardiograms were recorded at baseline and for 24 h at steady state and central nervous system effects were assessed with pharmacodynamic tests at baseline and steady state.
Results
ACT-709478 was absorbed with a time to reach the maximum plasma concentration of 3.5–4.0 h and eliminated with a half-life of 45–53 h. Steady state was reached after 5–7 days of dosing and exposure increased dose-proportionally. An accumulation index of approximately three fold was observed in cohorts 1 and 2. Exposure to midazolam was lower upon concomitant administration of 60 and 100 mg ACT-709478 compared to midazolam alone while the half-life and time to reach the maximum plasma concentration of midazolam remained unchanged, suggesting a weak induction at the gastrointestinal but not hepatic level. Pharmacokinetic parameters of 1-hydroxymidazolam were not affected by ACT-709478 administration. The most frequent adverse events were dizziness, somnolence, and headache. A tolerability signal was detected in cohort 1 (30 mg once daily); therefore, the dose was decreased to 10 mg once daily in cohort 2. The subsequently established up-titration regimen, starting with 10 mg once daily, considerably improved tolerability. Multiple doses up to 100 mg once daily were well tolerated. No treatment-related effects were detected on vital signs, clinical laboratory tests, Holter electrocardiogram variables, or in the pharmacodynamic tests.
Conclusions
ACT-709478 exhibits good tolerability up to 100 mg once daily using an up-titration regimen and pharmacokinetic properties that support further clinical investigations. A weak induction of gastrointestinal cytochrome P450 3A4 activity was observed, unlikely to be of clinical relevance.
ClinicalTrials.gov Identifier
NCT03165097.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The orally available triple T-type calcium channel blocker ACT-709478 could provide a new treatment modality for idiopathic generalized epilepsies. |
Upon multiple dosing, ACT-709478 showed dose-proportional exposure, a half-life of 45–53 h, and no clinically relevant interaction with cytochrome P450 3A4. |
A weekly up-titration regimen considerably improved tolerability of ACT-709478 up to 100 mg once daily. |
1 Introduction
Epilepsy is a disease affecting approximately 50 million patients worldwide. It is a heterogeneous group of disorders comprising both common and very rare forms [1, 2]. Although several different classes of antiepileptic drugs are available for the treatment of epileptic seizures, up to 30% of patients have an incomplete response and experience long-term consequences including an increased risk of premature death or injuries, psychosocial dysfunctions, and a reduced quality of life [3, 4]. Therefore, new antiepileptic drugs are still needed, particularly for drug-resistant epilepsy or rare syndromes, but also with regard to improved tolerability, or reduced drug interaction potential [5, 6].
T-type calcium (Ca2+) channels are low-voltage-activated channels that belong to the family of voltage-gated Ca2+ channels together with the high-voltage-activated L, N, P/Q, and R types. Three T-type Ca2+ channel subtypes with different electrophysiological properties have been described: Cav3.1, Cav3.2, and Cav3.3 [7, 8]. They are widely expressed in the brain [9] and have an important role in neuronal network activities [10]. Abnormal thalamocortical oscillations mediated by T-type Ca2+ channels have been observed during idiopathic generalized epilepsy seizures, particularly absence seizures, in both humans and animals [11,12,13] and other types of epilepsy with spike-and-wave discharges [10]. Furthermore, numerous pathological T-type channel variants have been described, linking these channels to a number of chronic neuronal disorders including congenital forms of seizure disorders [10]. Therefore, selectively blocking the three T-type Ca2+ channels might be beneficial in the treatment of epilepsy. Currently, no selective T-type Ca2+ channel blockers are available for therapeutic applications.
Chemical optimization of pyrazole carboxamides resulted in the clinical candidate ACT-709478 (N-(1-((5-cyanopyridin-2-yl)methyl)-1H-pyrazol-3-yl)-2-(4-(1-(trifluoromethyl)cyclopropyl)phenyl)acetamide), which is a selective, brain-penetrant, and orally available triple T-type Ca2+ channel blocker, i.e., inhibiting the three T-type Ca2+ channel subtypes with similar potency [14]. Of the compounds investigated, ACT-709478 proved to have the best balanced physicochemical and metabolic characteristics with an acceptable solubility profile (5 mg/mL at pH 7.0), good permeability, and a low intrinsic clearance in humans, rats, and dogs as shown in in vitro tests [14]. Additionally, preliminary data suggested that ACT-709478 is a low-clearance drug partially metabolized by cytochrome P450 (CYP) 3A4 [15]. Moreover, ACT-709478 exhibited excellent efficacy in the Wistar Albino Glaxo from Rijswijk rats (WAG/Rij), a model of nonconvulsive absence-like epilepsy. In the Wistar Albino Glaxo from Rijswijk rat model, an oral dose of 10 mg/kg decreased the cumulative duration of seizures over 12 h post-dose by 93%. In the audiogenic seizure sensitive juvenile DBA/2J mouse model of generalized convulsive seizures, a reduction in seizure severity was observed; however, a higher exposure was needed to reduce seizure activity [14].
Previously, ACT-709478 had been investigated in a first-in-human study with ascending single doses of 1–400 mg in healthy male subjects [15]. ACT-709478 was safe and well tolerated over the tested dose range. The maximum plasma concentration (Cmax) was reached within 3 h at lower (≤ 60 mg) dose levels and at 24 h at higher (> 60 mg) dose levels with a range of 1–72 h and with multiple peaks observed up to 72 h post-dose. The half-life (t½) ranged from 36 to 43 h and was consistent across dose levels, while Cmax and the area under the plasma concentration–time curve (AUC) from 0 to infinity (AUC0-∞) increased in a less than dose-proportional manner. Administration with food increased Cmax by 60% without impacting AUC0-∞. Furthermore, a pharmacodynamic (PD) battery of objective (saccadic peak velocity, unstable tracking, simple reaction time tests) and subjective (visual analog scale [VAS] Bond & Lader, VAS Bowdle, and Karolinska Sleepiness Scale) tests was performed, indicating absence of reduced vigilance or psychedelic effects.
The potential of ACT-709478 to cause drug–drug interactions (DDIs) as a perpetrator was predicted to be low. Based on in vitro tests, the half maximal inhibitory concentration values for inhibiting CYP enzymes are higher than the concentrations needed for the pharmacological effect [14]. However, an induction of CYP3A4 enzymes at the level of the gut could not be excluded. Because CYP3A4 is known to be involved in the metabolism of a wide variety of xenobiotic compounds, including many therapeutic drugs, it is important to study DDI potential at early stages of drug development. Midazolam is a sensitive substrate of both hepatic and intestinal CYP3A4 and is recommended as probe substrate for DDI studies [16]. Midazolam is extensively metabolized by CYP3A isozymes (CYP3A4/CYP3A5) to one major metabolite, 1-hydroxymidazolam. If measured in addition to the parent midazolam, the metabolite is giving further indications on the inhibition or induction potential of the tested drug [17].
Here, we report the results of the multiple-ascending dose study with ACT-709478 in healthy subjects including tolerability, safety, and pharmacokinetics (PK). Additionally, potential central nervous system effects were assessed by a PD test battery and the CYP3A4 DDI potential was explored with co-administration of midazolam.
2 Methods
2.1 Study Population
In this study, healthy male and female subjects aged between 18 and 55 years and with a body mass index from 18.0 to 29.9 kg/m2 were eligible. Subjects were of good health as assessed by medical history, physical examination, electrocardiogram (ECG), vital signs, and clinical laboratory tests. Women were only eligible with negative pre-dose pregnancy tests. Treatment with any medication within 2 weeks prior to study drug administration was not permitted. Written informed consent prior to any study-mandated procedure was given by all subjects. The study was approved by the German Health Authority and the Local Ethics Committee of Berlin and was conducted in accordance with the guidelines of good clinical practice and the principles of the Declaration of Helsinki (ClinicalTrials.gov Identifier: NCT03165097).
2.2 Study Design
This was a single-center, double-blind, placebo-controlled, randomized, multiple-ascending oral dose phase I study including a DDI part. The study drug was administered to four sequential cohorts of 10 (cohorts 1–2) and 12 subjects (cohorts 3–4), with a 1:1 sex ratio and including one male and one female subject taking placebo in each cohort.
The starting dose of 30 mg was selected based on the safety and tolerability data of the single-ascending dose (SAD) study and the PK predictions derived from population-PK modeling of the SAD data. Because of the long t½ of the compound, accumulation was expected and, therefore, lower doses were assumed to be required for an exposure comparable to the SAD study. The decisions on the next dose level were based on an interim analysis of blinded tolerability, safety, PK, and PD data of the previous cohort(s). In cohorts 1 and 2, 30 and 10 mg ACT-709478, respectively, were administered once daily (o.d.) for 12 days. An up-titration regimen was used in cohorts 3 (days 1–7: 10 mg; days 8–14: 30 mg; days 15–21: 60 mg; total treatment duration of 21 days) and cohort 4 (days 2–8: 10 mg; days 9–15: 30 mg; days 16–22: 60 mg; days 23–30: 100 mg; total treatment duration of 29 days). Furthermore, single doses of 4 mg midazolam were administered in cohort 4 at baseline (day 1) and concomitantly to 60 mg (day 22) and 100 mg (day 30) ACT-709478 to explore its CYP3A4 DDI potential. The study drug was administered as capsules of 10 and 100 mg ACT-709478. Midazolam was dispensed as an oral solution (2 mg/mL).
Subjects were confined to the study center on day −1. In the morning, ACT-709478, or matching placebo, was administered after consumption of a light breakfast. The subjects were discharged from the center 72 h after the last study drug administration, and returned for ambulatory visits until the end-of-study visit that took place 11 days after the last dose of study drug (i.e., on day 23 for cohorts 1 and 2, on day 32 for cohort 3, and on day 41 for cohort 4). A staggered dosing scheme was applied within each cohort with two sentinel subjects, one receiving ACT-709478 and one matching placebo.
2.3 Pharmacokinetic Assessments
Blood samples for ACT-709478 measurements in plasma were collected in EDTA tubes pre-dose and at 1, 2, 3, 4, 6, 8, 12, and 16 h post-dose on day 1 (cohorts 1–2) and at 1, 2, 3, 4, 6, 8, 12, 16, 24, 30, 36, 48, 60, 72, 96, 120, 168, 216, and 264 h post-dose in all cohorts after reaching steady state (days 12, 21, and 29 in cohorts 1–2, 3, and 4, respectively). Furthermore, trough samples were collected on all dosing days. In cohort 4, additional blood samples were taken for the midazolam and 1-hydroxymidazolam determination on day 1, 22, and 30 pre-dose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 h post-dose. After centrifugation, the plasma was transferred into polypropylene tubes and stored at −80 °C. In addition, a metabolite screening was performed by liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) with pooled plasma samples of cohort 4.
ACT-709478, midazolam, and 1-hydroxymidazolam plasma concentrations were determined using previously described validated LC–MS/MS assays [15, 18]. In brief, for the ACT-709478 plasma samples, protein precipitation was performed and an aliquot was injected onto the LC–MS/MS system (pump: Agilent Technologies Inc., Santa Clara, CA, USA; MS/MS: Thermo Fisher Scientific, San Jose, CA, USA). Chromatographic separation was achieved using a ReproShell C18 column (Dr. Maisch HPLC GmbH, Ammerbuch, Germany) and a gradient elution with water and acetonitrile, both containing 0.1% formic acid. The lower limit of quantification was 0.5 ng/mL and the quality control samples showed an inter-batch precision from 2.5 to 6.5% and an inter-batch accuracy from 95.1 to 102.4% of the nominal concentrations. For midazolam and 1-hydroxymidazolam, a liquid–liquid extraction method was used followed by an LC–MS/MS analysis (pump: Shimadzu, Duisburg, Germany; MS/MS: AB Sciex, Concord, ON, Canada) using a Kinetex C18 column (Phenomenex, Aschaffenburg, Germany) and a gradient elution with methanol containing ammonium formate and methanol containing water. The lower limit of quantification for both analytes was 0.1 ng/mL. The quality control samples showed an inter-batch precision for midazolam and 1-hydroxymidazolam from 2.2 to 5.8% and from 0.7 to 3.8% and an inter-batch accuracy from 93.8 to 111.0% and from 94.4 to 116.5% of the nominal concentrations, respectively.
2.4 Pharmacodynamic Assessments
A PD test battery including the objective assessment of saccadic peak velocity and the subjective assessments VAS Bond & Lader, VAS Bowdle, and Karolinska Sleepiness Scale was used. The tests were performed on day −1 (training), pre-dose (baseline), at 3, 6, and 12 h post-dose on day 1 (cohorts 1–2), and at the same time points at steady state (days 12, 21, and 29 in cohorts 1–2, 3, and 4, respectively).
Saccadic peak velocity was performed to determine the sedative effects using a computer-based method for saccadic eye movement measurement originally described by Baloh et al. [19]. It has been shown to be one of the most sensitive variables for measuring the sedative effects of drugs [20,21,22]. Saccadic eye movements were recorded as previously described [15]. A light stimulus at variable intervals was projected at 15°–20° of the eyeball rotation and the peak velocity (degree/second), the highest velocity reached during the saccade, was measured.
VAS Bond & Lader, VAS Bowdle, and the Karolinska Sleepiness Scale were completed on paper. The VAS Bond & Lader test has previously been used to investigate drug effects on the scores of alertness, mood, and calmness [20, 22, 23]. The scores are calculated by means of 16 VAS, i.e., 100 mm horizontal lines with two words representing opposite feelings placed at both ends, which allow the subjects to indicate their subjective feelings. The VAS Bowdle test has previously been applied to evaluate psychedelic drug effects [24,25,26]. It consists of 13 VAS, i.e., 100 mm horizontal lines, which allow the subjects to rate their subjective feelings regarding a described statement with 0 mm reflecting “not at all” and 100 mm reflecting “extremely”. The scores were combined to assess “internal perception”, “external perception”, “feeling high”, or “drowsy”. The Karolinska Sleepiness Scale is a validated test to assess sleepiness [27]. The subjects are asked to rate their sleepiness on a 9-point scale ranging from 1 “very alert” to 9 “very sleepy, great effort to keep awake, fighting sleep”.
2.5 Tolerability and Safety Assessments
Tolerability and safety were continuously assessed by monitoring the nature, frequency, and intensity of adverse events (AEs). Laboratory variables, vital signs, and 12-lead ECGs were evaluated throughout the study and changes from baseline were calculated. Heart rate telemetry was performed for 6 h post-dose.
2.6 Holter Electrocardiogram Assessment
Continuous Holter ECGs were recorded at baseline (pre-dose on day 1 for 1.5 h), for 24 h following dosing on day 1 (cohorts 1–2) and at steady state (days 12, 21, and 29 for cohorts 1–2, 3, and 4, respectively) using GI M12R Recorders (Global Instrumentation LLC., Manlius, NY, USA). Triplicate ECGs were extracted by a central ECG laboratory (eResearch Technology, Philadelphia, PA, USA) from the continuous recordings at the following time points when subjects were resting in a supine position: pre-dose at 75, 60, and 45 min, at 1, 2, 3, 4, 6, 8, 12, and 24 h post-dose on day 1 (cohorts 1–2), and at the same time points at steady state (days 12, 21, and 29 in cohorts 1–2, 3, and 4, respectively).
2.7 Statistical Analysis
Pharmacokinetic parameters of ACT-709478, midazolam, and 1-hydroxymidazolam were derived by a non-compartmental analysis of the concentration–time profiles using WinNonlin version 8.0 (Pharsight Inc., Mountain View, CA, USA). The measured individual plasma concentrations were used to directly obtain Cmax and time to reach Cmax (tmax). AUC values were calculated according to the linear trapezoidal rule, using the measured concentration values above the lower limit of quantification, without any weighting. The t½ of all analytes was calculated as follows: t½ = ln [2] /λz, where λz represents the terminal elimination rate constant. Assessment of steady-state concentrations of ACT-709478 was based on a visual inspection of the trough concentrations. The accumulation index was calculated as follows: AUC during a dose interval (AUCτ) day 12/AUCτ day 1 (cohorts 1–2 only).
Dose proportionality of ACT-709478 was explored by comparing Cmax and AUCτ values across cohorts, using a power model described by Gough et al. [28]. Moreover, sex differences in PK parameters were investigated.
The effect of 60 and 100 mg ACT-709478 on AUC from 0 to 24 h (AUC0–24), Cmax, and t½ of midazolam and 1-hydroxymidazolam was explored using the geometric mean ratios and 90% confidence intervals (CIs) of the combined administration and midazolam alone as the reference. The effect on tmax was explored using the median difference and its 90% CI.
The PD endpoints were analyzed by mixed-effects model analyses of variance with sex, treatment and dose, time, and treatment and dose by time as fixed effects, subject as random effect, and baseline value as covariate. The PD endpoints from cohorts 1–2, 3, and 4 were analyzed separately because of the different study designs.
Tolerability and safety were analyzed descriptively by treatment group. Subjects treated with placebo were pooled for the analysis.
A concentration-QT corrected interval (QTc) analysis was performed with the Holter data using a linear mixed-effects modeling approach with ΔQTcF (change from baseline in QT interval corrected with Fridericia’s formula) as the dependent variable, time-matched ACT-709478 plasma concentration, centered baseline QTcF, study drug, and the time point as covariates, and a random intercept and slope per subject. Additionally, a central tendency analysis for heart rate and the ECG intervals QTcF, PR, and QRS was performed based on a linear mixed-effects model with the ECG variables as the dependent variable, time point, study drug, and time by treatment interaction as fixed effects, and baseline ECG variables as a covariate. Categorical outliers of ECG variables were analyzed descriptively.
3 Results
3.1 Subjects
A total of 46 subjects in four cohorts were enrolled in this study. Six subjects discontinued the study drug (two, one, and two taking active treatment in cohorts 1, 3, and 4; one taking placebo in cohort 1), two of whom were replaced (one subject in cohort 3 and the placebo subject). All subjects were included in the tolerability and safety analysis whereas only the discontinued subjects from cohort 1 were included in the PK, PD, and cardiodynamic analysis (i.e., corresponding assessments on day 1 performed). All 46 subjects (24 female and 22 male) were white with a mean (range) age and body mass index of 43 (23–55) years and 24.6 (18.5–29.8) kg/m2, respectively.
3.2 Pharmacokinetics
3.2.1 ACT-709478 Pharmacokinetics
The PK parameters following multiple-dose administration of 10, 30, 60, and 100 mg ACT-709478 are summarized in Table 1. ACT-709478 was absorbed with a median tmax of 3.5–4.0 h across cohorts. Similarly, t½ was comparable among all dose levels, ranging from 45 to 53 h. The mean Cmax and AUCτ values at steady state increased in a dose-proportional manner with the 90% CI for the slope determined in the power model of 0.75–0.92 and 0.81–0.99, respectively, both within the critical interval of 0.70–1.3. Furthermore, no statistically significant differences in any of the PK parameters between male and female subjects were observed.
The trough concentration–time profiles are shown in Fig. 1. Steady state was reached in all cohorts and dose levels after 5–7 days of dosing, except for the 100 mg dose level in cohort 4, in which the mean trough plasma concentration fluctuated slightly up to the last day of dosing. An accumulation index of 2.4 and 3.2 was determined for cohorts 1 and 2, respectively. Moreover, no relevant active metabolites were detected in the pooled plasma samples of cohort 4.
3.2.2 Midazolam Pharmacokinetics
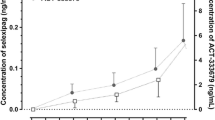
The mean plasma concentration–time profiles of midazolam and 1-hydroxymidazolam in the absence of ACT-709478 and in combination with 60 mg and 100 mg ACT-709478 at steady state are shown in Fig. 2 and the PK parameters and geometric mean ratios are displayed in Table 2. The midazolam and 1-hydroxymidazolam PK profiles were characterized by a fast absorption with a median tmax of 1 h for both compounds and a rapid clearance from plasma with a mean t½ of 5.9 and 4.6 h, respectively. The plasma concentrations of the metabolite were lower compared with the parent compound (Cmax 3.7 ng/mL vs 12.0 ng/mL). After multiple-dose administration of 60 mg and 100 mg ACT-709478, mean Cmax and AUC0–24 of midazolam were decreased by 7 and 21% and by 9 and 30%, respectively, compared with midazolam alone. The lower bound of the 90% CI of the geometric mean ratio was below the 80–125% bioequivalence interval for both PK parameters (60 mg: 0.93 [0.81–1.08] for Cmax, 0.79 [0.70–0.91] for AUC0–24; 100 mg: 0.91 [0.75–1.10] for Cmax, 0.70 [0.59–0.84] for AUC0–24). In contrast, the mean t½ and median tmax of midazolam remained unchanged. Furthermore, similar exposures to 1-hydroxymidazolam were observed following administration of midazolam alone and in combination with ACT-709478.
3.3 Pharmacodynamics
Statistically significant differences compared with placebo were observed for isolated subjective PD test parameters in cohort 1 only. At higher doses, no relevant findings were detected. As a consequence, no clear dose dependency could be established for any treatment-emergent sign of reduced vigilance or psychedelic effects. The results of the saccadic peak velocity assessments are shown in Fig. 3.
Mean change (± standard deviation) from baseline to steady state (days 12, 21, and 29 for cohorts 1–2, cohort 3, and cohort 4, respectively) in saccadic peak velocity after once-daily multiple‐dose administration of ACT‐709478 (n = 6, 7, 10, and 7 for cohorts 1, 2, 3, and 4, respectively) or placebo (n = 9)
3.4 Tolerability and Safety
The total number of AEs in the study (whether related to the study drug) was 475 reported by 40 subjects (87%) of which 39 AEs were reported by six subjects (67%) taking placebo while seven AEs were reported by four subjects (33%) after administration of midazolam alone or midazolam plus placebo. The reported AEs were of mild (323) or moderate (149) intensity with the exception of three severe AEs (taking active treatment: drug-related headache, not drug-related deep vein thrombosis; taking placebo: vasovagal syncope) in cohort 1. The number of AEs under active treatment per cohort and intensity are shown in Fig. 4. In cohort 1 (30 mg o.d.), a tolerability signal was observed. All eight subjects taking ACT-709478 (100%) reported an overall of 115 AEs. The tolerability findings were mostly related to AEs classified as nervous system disorders. There was an increase in the onset of AEs from day 3 to 6, but not thereafter, when steady state was reached, suggesting improved tolerability after the first days of exposure and thus supporting weekly dose increases in an up-titration regimen. A lower dose was studied in cohort 2 (10 mg o.d.) that was well tolerated with six subjects taking active treatment (75%) reporting eight AEs. Therefore, the 10 mg dose was chosen as the starting dose for the up-titration regimen in further cohorts. With the up-titration regimen in cohort 3 (10, 30, and 60 mg ACT-709478 o.d.) and cohort 4 (10, 30, 60, and 100 mg ACT-709478 o.d.), the tolerability considerably improved. The overall number of AEs in subjects taking active treatment in these cohorts were 187 and 126 (including AEs upon co-administration of midazolam) reported in 11 (100%) and nine (90%) subjects. In view of the long study duration and the increased number of subjects as compared with cohorts 1 and 2, the number of AEs decreased in cohorts 3 and 4 (numbers normalized per study day and number of subjects are shown in Fig. 4).
Number of adverse events (AEs) by cohort and by intensity reported by subjects after administration of ACT-709478 or placebo (different number of subjects and study duration per cohort in [a] and normalized to number of subjects and study duration in [b]). Cohort 1, 30 mg; cohort 2, 10 mg; cohort 3, 10–30–60 mg; cohort 4, 10-30-60-100 mg. N = number of subjects in the corresponding cohort
A summary of AEs across cohorts is given in Table 3. The most frequent AEs were dizziness, somnolence, and headache. Different paresthesia symptoms (localized most frequently in the face, hands, and finger tips) were reported in 11 out of 46 subjects, all taking active treatment and mainly reported in cohort 1. Furthermore, four subjects experienced AEs leading to discontinuation of the study drug.
There were no serious AEs reported during study conduct and no clinically relevant effects of ACT-709478 on vital signs, heart rate telemetry, clinical laboratory variables, and physical examination. Moreover, with the exception of one finding (right bundle branch block), no clinically relevant ECG findings were observed.
3.5 Holter Electrocardiogram Evaluation
Mean placebo-corrected ΔQTcF (ΔΔQTcF) varied between −17.6 and 5.0 ms across cohorts, without an indication of dose dependency. The estimated population slope of the concentration–QTc relationship was −0.009 ms per ng/mL (90% CI −0.016 to −0.002) with a treatment effect-specific intercept of 0.1 ms (90% CI −4.0 to 4.1). The mean ∆ΔQTcF was predicted to be −7.58 ms (90% CI −13.08 to −2.08) and −10.2 ms (90% CI −17.5 to −2.9) for 60 mg and 100 mg of ACT-709478 o.d., respectively. Based on this analysis, a QT effect above 10 ms was excluded up to the highest attained concentration in this study and is not expected above because of the negative slope, as depicted in Fig. 5. The central tendency and the categorical outliers’ analyses did not indicate a treatment-related effect on any ECG variables.
4 Discussion
Previously, the selective triple T-type Ca2+ channel blocker ACT-709478 was administered to healthy male subjects as a single-dose administration up to 400 mg showing a good tolerability and PK profile [15]. In the present study, the tolerability, safety, PK, and PD of multiple-dose administrations of ACT-709478 were studied for the first time in healthy male and female subjects.
Overall, the PK profile observed after multiple-dose administrations of ACT-709478 in fed conditions was in line with the SAD study in which the study drug was given in fasted conditions with the exception of the food effect group at 60 mg (i.e., administration in fasted and fed conditions) [15]. Following multiple-dose administrations of 10, 30, 60, and 100 mg ACT-709478, a median tmax of approximately 4 h was determined. In the SAD study, a similar median tmax was observed up to the 60 mg dose. For higher doses, i.e., 120–400 mg, multiple plasma concentration peaks were observed, determining the median tmax at 24 h post-dose [15]. Multiple peaks were not observed anymore in the present study. This observation corresponds to the results of the SAD study, in which the appearance of a second peak was marginal under fed conditions [15]. The geometric mean t½ was comparable among cohorts, ranging from 45 to 53 h, and similar to the t½ of approximately 40 h observed after single-dose administration of ACT-709478 [15]. As expected, ACT-709478 accumulated approximately three fold in cohorts 1 and 2, which is in line with the long t½. Because of the accumulation, higher plasma concentrations were reached in the MAD study, even though lower doses were administered as compared with the SAD study. In contrast to the present study, a less than dose-proportional increase in exposure parameters was observed in the SAD study. Solubility limitations of ACT-709478 might have contributed to the non-proportional increase by an incomplete dissolution of the study drug in the gastrointestinal tract. This hypothesis was underlined by a 60% increase in Cmax after administration in fed conditions (i.e., high-fat, high-calorie breakfast) compared with fasted conditions. The light breakfast administered in the present study and the subsequently improved solubilization of the lipophilic compound ACT-709478 in the gastrointestinal tract probably resulted in an enhanced absorption and, therefore, a dose-dependent increase in Cmax and AUCτ. Steady state was reached by the majority of the subjects after 7 days of dosing, the trough plasma concentrations fluctuated slightly up to the last day of dosing only during the last up-titration step at 100 mg.
Baseline midazolam PK parameters were comparable to those reported previously [18, 29,30,31]. Maximum plasma concentration and AUC0–24 values of midazolam were decreased by 7 and 9% and 21 and 30% when administered concomitantly with 60 and 100 mg ACT-709478, respectively, compared with midazolam alone. In contrast, tmax and t½ remained unchanged. An investigational drug can be classified as a weak, moderate, or strong inducer based on its effect on a probe substrate [16]. Accordingly, the decrease of midazolam AUC between 20 and 50% would classify ACT-709478 as a weak inducer of CYP3A4. However, because of the unchanged t½, an induction of intestinal rather than hepatic CYP3A4 is assumed [32, 33]. Moreover, the absence of a considerable difference in the change of Cmax of midazolam from 60 to 100 mg ACT-709478 and no effect of ACT-709478 on 1-hydroxymidazolam PK parameters further indicate a low relevance of the induction.
The PD battery was performed to assess if there were any sedative treatment effects of ACT-709478 and to broadly characterize any effects on the central nervous system. In line with the results of the SAD study [15], no clear sign of treatment-emergent reduced vigilance or psychedelic effects was observed. The isolated significant values in the subjective tests in cohort 1 were not supported by the objective saccadic eye movement test.
After multiple-dose administrations of 30 mg o.d., a tolerability signal was observed with subjects showing central nervous system-related AEs. Therefore, the dose was reduced in the second cohort to 10 mg, which was well tolerated and subsequently used as a starting dose for an up-titration regimen. In cohorts 3 and 4, doses up to 60 and 100 mg o.d., respectively, were well tolerated. With the exception of three severe AEs in cohort 1, all AEs were either of mild or moderate intensity. Of the three severe AEs, only headache was assessed as drug related by the investigator. The deep vein thrombosis was considered as not drug related owing to the long bed rest of a subject and the vasovagal syncope was experienced by a subject taking placebo. No serious AE was reported during study conduct. The most frequently reported AEs across the cohorts were dizziness, somnolence, and headache. Moreover, different paresthesia symptoms were reported in 11 subjects, all taking active treatment and mainly in the first cohort. Considering the longer treatment duration, the higher dose, and the fewer paresthesia symptoms that were reported in later cohorts, the finding considerably improved with up-titration. In general, the observed AEs were similar to those reported in the SAD study [15].
Potential effects of ACT-709478 on ECG variables or vital signs were closely monitored because T-type Ca2+ channels located in heart cells and renal tissue are known to be implicated in the pacemaker current and in the regulation of aldosterone, respectively [34, 35]. In line with the SAD data, no clinically relevant treatment-emergent findings in vital signs, ECGs, or heart rate telemetry were observed, except for one clinically significant ECG finding of a right bundle branch block. The ECG finding was reported in a subject in cohort 4 after seven doses of 10 mg ACT-709478. The subject was discontinued from the study and the finding spontaneously resolved a few days afterwards. The collected Holter ECG data did not indicate any clinically relevant effect of ACT-709478 on ECG variables.
The tolerability signal detected in cohort 1 led to adaptations of the study design. Instead of increasing the dose in cohort 2, the dose was decreased. Additionally, an up-titration regimen was implemented in cohorts 3 and 4. These changes resulted in different study durations in cohorts 1–2 (12 days of dosing), cohort 3 (21 days of dosing), and cohort 4 (29 days of dosing of ACT-709478). As a limitation of this study design, the number of AEs and other safety parameters between cohorts need to be compared with caution. Another limitation of the study was the rather small number of subjects (n = 8) for the CYP3A4 DDI study part. However, the inter-subject variability of PK parameters was low and the midazolam results after co-administration of 60 and 100 mg of ACT-709478 were consistent. Therefore, these data are a good indication of the impact of multiple doses of ACT-709478 on the CYP3A4 metabolism of midazolam.
Selective blockade of T-type Ca2+ channels is a promising mechanism of action for the pharmacological treatment of certain types of epilepsy. The good tolerability and PK profile observed in this study support the continuation of compound development with clinical studies in patients with epilepsy to evaluate its clinical benefit.
5 Conclusions
In this study, multiple oral doses of ACT-709478 were administered. A tolerability signal was detected in cohort 1; therefore, the dose was decreased in cohort 2. The subsequently established up-titration regimen considerably improved tolerability. Multiple doses up to 100 mg o.d. were well tolerated. A dose-proportional exposure was determined across cohorts. Upon co-administration of ACT-709478, a weak induction of gastrointestinal CYP3A4 activity was observed, unlikely to be of clinical relevance.
References
Behr C, Goltzene MA, Kosmalski G, Hirsch E, Ryvlin P. Epidemiology of epilepsy. Rev Neurol (Paris). 2016;172(1):27–36.
Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005;46(Suppl. 9):10–4.
Szaflarski JP, Lindsell CJ, Zakaria T, Banks C, Privitera MD. Seizure control in patients with idiopathic generalized epilepsies: eEG determinants of medication response. Epilepsy Behav. 2010;17(4):525–30.
Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365(10):919–26.
Ventola CL. Epilepsy management: newer agents, unmet needs, and future treatment strategies. PT. 2014;39(11):776–92.
French JA, White HS, Klitgaard H, Holmes GL, Privitera MD, Cole AJ, et al. Development of new treatment approaches for epilepsy: unmet needs and opportunities. Epilepsia. 2013;54(Suppl. 4):3–12.
Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klockner U, et al. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci. 1999;19(6):1912–21.
Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–61.
Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19(6):1895–911.
Weiss N, Zamponi GW. T-type calcium channels: from molecule to therapeutic opportunities. Int J Biochem Cell Biol. 2019;108:34–9.
Khosravani H, Zamponi GW. Voltage-gated calcium channels and idiopathic generalized epilepsies. Physiol Rev. 2006;86(3):941–66.
Zamponi GW, Lory P, Perez-Reyes E. Role of voltage-gated calcium channels in epilepsy. Pflug Arch. 2010;460(2):395–403.
Cheong E, Shin HS. T-type Ca2 + channels in absence epilepsy. Pflug Arch. 2014;466(4):719–34.
Bezencon O, Heidmann B, Siegrist R, Stamm S, Richard S, Pozzi D, et al. Discovery of a potent, selective T-type calcium channel blocker as a drug candidate for the treatment of generalized epilepsies. J Med Chem. 2017;60(23):9769–89.
Richard M, Kaufmann P, Kornberger R, Dingemanse J. First-in-man study of ACT-709478, a novel selective triple T-type calcium channel blocker. Epilepsia. 2019;60(5):968–78.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Draft guidance for industry: clinical drug interaction studies: study design, data analysis, and clinical implications. October 2017. Available from: https://www.fda.gov/media/82734/download. Accessed 3 Jan 2020.
Wandel C, Bocker R, Bohrer H, Browne A, Rugheimer E, Martin E. Midazolam is metabolized by at least three different cytochrome P450 enzymes. Br J Anaesth. 1994;73(5):658–61.
Juif PE, Boehler M, Donazzolo Y, Bruderer S, Dingemanse J. A pharmacokinetic drug-drug interaction study between selexipag and midazolam, a CYP3A4 substrate, in healthy male subjects. Eur J Clin Pharmacol. 2017;73(9):1121–8.
Baloh RW, Sills AW, Kumley WE, Honrubia V. Quantitative measurement of saccade amplitude, duration, and velocity. Neurology. 1975;25(11):1065–70.
van Steveninck AL, Schoemaker HC, Pieters MS, Kroon R, Breimer DD, Cohen AF. A comparison of the sensitivities of adaptive tracking, eye movement analysis and visual analog lines to the effects of incremental doses of temazepam in healthy volunteers. Clin Pharmacol Ther. 1991;50(2):172–80.
van Steveninck AL, Mandema JW, Tuk B, van Dijk JG, Schoemaker HC, Danhof M, et al. A comparison of the concentration-effect relationships of midazolam for EEG-derived parameters and saccadic peak velocity. Br J Clin Pharmacol. 1993;36(2):109–15.
Hoever P, de Haas S, Winkler J, Schoemaker RC, Chiossi E, van Gerven J, et al. Orexin receptor antagonism, a new sleep-promoting paradigm: an ascending single-dose study with almorexant. Clin Pharmacol Ther. 2010;87(5):593–600.
de Visser SJ, van der Post J, Pieters MS, Cohen AF, van Gerven JM. Biomarkers for the effects of antipsychotic drugs in healthy volunteers. Br J Clin Pharmacol. 2001;51(2):119–32.
Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88(1):82–8.
Hoever P, de Haas SL, Dorffner G, Chiossi E, van Gerven JM, Dingemanse J. Orexin receptor antagonism: an ascending multiple-dose study with almorexant. J Psychopharmacol. 2012;26(8):1071–80.
Liem-Moolenaar M, de Boer P, Timmers M, Schoemaker RC, van Hasselt JG, Schmidt S, et al. Pharmacokinetic-pharmacodynamic relationships of central nervous system effects of scopolamine in healthy subjects. Br J Clin Pharmacol. 2011;71(6):886–98.
Kaida K, Takahashi M, Akerstedt T, Nakata A, Otsuka Y, Haratani T, et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006;117(7):1574–81.
Gough K, Hutchison M, Keene O, Byrom B, Ellis S, Lacey I, et al. Assessment of dose proportionality: report from the statisticians in the pharmaceutical industry/Pharmacokinetics UK Joint Working Party. Ther Innov Regul Sci. 1995;29(3):1039–48.
Boof ML, Alatrach A, Ufer M, Dingemanse J. Interaction potential of the dual orexin receptor antagonist ACT-541468 with CYP3A4 and food: results from two interaction studies. Eur J Clin Pharmacol. 2019;75(2):195–205.
Dingemanse J, Nicolas L, Binkert C. Clinical pharmacology of single- and multiple-ascending doses of ACT-178882, a new direct renin inhibitor, and its pharmacokinetic interaction with food and midazolam. Fundam Clin Pharmacol. 2013;27(6):698–710.
Hoch M, Hoever P, Alessi F, Theodor R, Dingemanse J. Pharmacokinetic interactions of almorexant with midazolam and simvastatin, two CYP3A4 model substrates, in healthy male subjects. Eur J Clin Pharmacol. 2013;69(3):523–32.
Davis J, Langdon G, Layton G, Chong CL, Ndongo MN, Vourvahis M. The effect of lersivirine, a next-generation NNRTI, on the pharmacokinetics of midazolam and oral contraceptives in healthy subjects. Eur J Clin Pharmacol. 2012;68(11):1567–72.
Tu JH, He YJ, Chen Y, Fan L, Zhang W, Tan ZR, et al. Effect of glycyrrhizin on the activity of CYP3A enzyme in humans. Eur J Clin Pharmacol. 2010;66(8):805–10.
Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25(3):533–5.
Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3(8):a003947.
Acknowledgements
We thank Anne‐Helène Clugery and Beya Khouildi for project management; Adil Aouboukdir, Zsofia Molnar, and Antonella Santilli for monitoring; Margaux Boehler for scientific support; Swiss Bioquant, Reinach, Switzerland and ACC GmbH Analytical Clinical Concepts, Leidersbach, Germany, for bioanalytical assessments; ERT Inc., Philadelphia, PA, USA, for handling and analysis of the Holter ECG data; and Aixial, Brno, Czech Republic, for the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Actelion Pharmaceuticals Ltd, the predecessor of Idorsia Pharmaceuticals Ltd, and Idorsia Pharmaceuticals Ltd.
Conflict of Interest
At the time of study conduct, Muriel Richard, Priska Kaufmann, and Jasper Dingemanse were employees at Actelion Pharmaceuticals Ltd (predecessor of Idorsia Pharmaceuticals Ltd) and Idorsia Pharmaceuticals Ltd. Marion Ort was an employee at Idorsia Pharmaceuticals Ltd. Rüdiger Kornberger was an investigator of this study that was funded by Actelion Pharmaceuticals Ltd and Idorsia Pharmaceuticals Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (ethics committee of Berlin) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
All subjects gave written informed consent prior to any study-mandated procedure.
Rights and permissions
About this article
Cite this article
Richard, M., Kaufmann, P., Ort, M. et al. Multiple-Ascending Dose Study in Healthy Subjects to Assess the Pharmacokinetics, Tolerability, and CYP3A4 Interaction Potential of the T-Type Calcium Channel Blocker ACT-709478, A Potential New Antiepileptic Drug. CNS Drugs 34, 311–323 (2020). https://doi.org/10.1007/s40263-019-00697-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00697-1