Abstract
Background
The pharmacokinetic (PK) profile of a drug is influenced by several factors, which can lead to a suboptimal dosing regimen in specific patient populations. As obesity becomes increasingly prevalent, it is important that optimized dosing schemes are available for these patients. To set up such dosing schemes, PK studies should be performed in this population. Regarding paracetamol (acetaminophen [APAP]), obese patients would benefit from a tailored dosing scheme, as both the volume of distribution and metabolism are increased compared with non-obese patients. This includes metabolism by cytochrome P450 2E1, which is involved in APAP-associated hepatotoxicity. To decrease the burden for patients in these PK studies, finger-prick sampling could be used.
Objective
The aim of this study was to compare the quantitative determination of APAP and four metabolites in different blood-based matrices and to determine if capillary dried blood samples, collected directly following finger-prick, could serve as a tool to investigate APAP PK in obese and non-obese patients.
Methods
In this study, we performed a clinical validation of methods for the determination of APAP and four of its metabolites (APAP-glucuronide, APAP-sulfate, APAP-mercapturate, and APAP-cysteine) in blood, plasma, and dried blood. The latter was obtained by volumetric absorptive microsampling (VAMS), either starting from the venous blood or collected directly following a finger-prick. Results were compared between the different matrices and, in addition, blood:plasma (B:P) ratios were determined for the different analytes.
Results
Liquid and dried venous blood results were in good agreement. Furthermore, differences between capillary (finger-prick) and venous VAMS blood samples remained limited for most analytes. However, for APAP-cysteine, caution should be paid to the interpretation of concentrations in (dried) blood. With the exception of APAP, concentrations were higher in plasma compared with blood, with B:P ratios ranging between 0.52 and 0.65. A time-dependent change in median B:P ratio was observed for APAP and APAP-cysteine. Additionally, a time-dependent trend was seen for APAP, as well as for APAP-glucuronide and APAP-mercapturate, for the distribution between capillary and venous blood.
Conclusions
We demonstrated that finger-prick sampling is a viable alternative to conventional venous blood sampling to investigate the PK of APAP and its metabolites in obese and non-obese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
First study to quantify paracetamol (APAP) and its four main metabolites in venous blood and corresponding dried venous and capillary samples in the context of a pharmacokinetic (PK) study. |
Comparative evaluation of concentrations of APAP and its metabolites in plasma, blood, and dried venous and capillary volumetric absorptive microsampling (VAMS) samples. |
First report of blood:plasma (B:P) ratios for the APAP metabolites. |
Demonstration of suitability of finger-prick sampling to conduct PK studies for APAP in obese and non-obese patients. |
1 Introduction
Dosing recommendations for most drugs are developed based on pharmacokinetic (PK) and pharmacodynamic (PD) studies in an average adult population. However, these recommended doses may not lead to the desired therapeutic effect in certain specific patient populations, who may benefit from a more tailored dosing scheme. To allow the set-up of such specific dosing schemes, it is essential to understand the PK and PD profile of therapeutic drugs in the targeted population. Different patient characteristics can influence the PK profile of a drug, such as age and weight [1,2,3,4].
Worldwide, obesity, defined as a body mass index (BMI) ≥30 kg/m2, is becoming more prevalent, and hence the need for adapted drug dosing guidelines for this population becomes increasingly important [5,6,7]. The PK profile of a drug in obese patients is influenced by its distribution between fat and lean tissues, but also the metabolism and clearance of the drug can be altered compared with non-obese patients [3, 4].
Paracetamol (acetaminophen [APAP]) is one of the drugs for which the PK profile in obese patients has already been investigated [8,9,10,11]. To date, the available data indicate that both the volume of distribution and the total clearance for APAP are increased in obese patients compared with non-obese patients [10]. Based on these two findings, an increased dose to attain therapeutic concentrations could be anticipated. However, when looking at the different metabolism pathways, there is evidence that cytochrome P450 (CYP) 2E1-mediated metabolites are formed earlier and to a greater extent in obese patients [9]. This pathway is involved in the development of APAP-induced hepatotoxicity and hence caution should be paid when considering to increase APAP dosing [12]. These findings demonstrate that adapting drug dosages to meet the needs of specific patient populations is not always straightforward. Therefore, further studies are needed to (1) investigate whether APAP dosing in obese patients should be adapted, taking into account the aforementioned findings, and (2) set up dosage guidelines for this specific patient population.
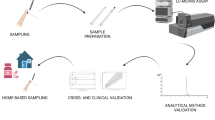
PK studies are a significant burden to patients due to the frequent sampling of (usually) venous blood. This burden can be decreased by performing population PK studies with less sampling time points per patient or by following a more patient-centric approach, e.g. via the use of finger-prick sampling as an alternative for conventional venous blood sampling. Finger-prick sampling is not only less invasive for the patient but also allows the collection of samples from patients outside the clinic, e.g. in the home environment. Besides dried blood spots collected on conventional cards, more recently, several approaches have been introduced that allow the volumetric collection of a drop of blood, directly from the fingertip [13]. One of these approaches is volumetric absorptive microsampling (VAMS), in which a polymeric tip wicks up a fixed amount of blood, irrespective of the hematocrit [14].
In this study, we aimed at investigating VAMS-assisted finger-prick sampling as a tool for APAP PK studies. APAP and four of its metabolites, paracetamol-glucuronide (APAP-Gluc), paracetamol-sulfate (APAP-Sulf), paracetamol-mercapturate (APAP-Merc), and paracetamol-cysteine (APAP-Cys), were quantified in plasma, venous (liquid and dried) and dried capillary blood samples from both obese and non-obese patients. Results obtained in the different blood-based matrices were compared to determine if capillary dried blood samples could be used as an alternative matrix to investigate APAP PK. As such, this study is the first to quantify APAP and its four main metabolites in both liquid and dried blood (micro)samples, in the context of a PK study. Moreover, blood:plasma (B:P) ratios were determined for all of the analytes, which, except for APAP, have not yet been reported elsewhere. Last, as far as we are aware, this study is the first to extensively compare all different blood-based matrices for an analyte and its metabolites in a time-dependent manner. Hence, the framework of this study may serve as a basis for other PK studies aiming at comprehensively evaluating the suitability of finger-prick sampling as an alternative to conventional venous sampling.
2 Methods
2.1 Participants
Severely obese patients undergoing bariatric surgery (BMI > 35 kg/m2) and non-obese patients (BMI between 18.5 and 30 kg/m2) undergoing elective laparoscopic procedures (Nissen fundoplication, cholecystectomy, bowel surgery, or hernial repair surgery) were eligible for inclusion in this study. Patients were excluded if they were pregnant, suffered from renal insufficiency (estimated glomerular filtration rate [eGFR] < 30 mL/min), had a pre-existing liver condition or a liver disease detected by liver function tests (aspartate aminotransferase or alanine aminotransferase > 3 times the upper limit of normal values), Gilbert–Meulengracht syndrome, or if they were allergic to APAP. Furthermore, patients taking medication known to affect CYP2E1 or uridine 5′-diphospho-glucuronosyltransferase (UDP) activity, with chronic alcohol intake or alcohol consumption < 72 h before surgery, or reporting chronic APAP use or intake within the previous 24 h were excluded from the study. Patients were recruited during their pre-operational consult at Ghent University Hospital and provided written informed consent. As is common practice, all patients had refrained from eating prior to the surgery, which also limited the differences in the sample matrix (e.g., postprandially more lipids may be present). This study was approved by the Ghent University Hospital Ethics Committee (BC-07469).
2.2 Sample Collection
Venous lithium-heparin anticoagulated blood (4.0 mL BD Vacutainer tubes; BD Benelux, Erembodegem, Belgium), and 10 µL capillary VAMS samples (cVAMS; brand name Mitra®, Neoteryx, Torrance, USA), obtained via finger-prick, were collected from 21 patients (16 obese, 5 non-obese). Samples were collected during and after surgery, over a window of 30 h, as indicated in Fig. 1. Venous blood samples and corresponding cVAMS samples were collected within 2 min of each other. For administration of APAP, the standard peri- and postoperative pain management protocol was followed: 2 g APAP after induction of anesthesia, followed by 1 g APAP every 6 h until 30 h after the first dose. APAP was administered intravenously over 15 min with a volumetric pump. Patients also received ibuprofen (600 mg/12 h) and piritramide as needed. For patients completing the entire trajectory, 16 venous blood samples and 7 cVAMS samples were collected.
After sample collection, samples were stored at 4 °C, followed by transportation at ambient conditions to the Laboratory of Toxicology within 24 h after collection (covered by stability data [15]). Either immediately or within a timeframe of 12 h (samples were stored at 4 °C, also covered by stability data [15]), plasma was derived from a fraction of the venous blood by centrifugation (5 min at 5000 g). 10 µL VAMS samples were also prepared from the venous blood (vVAMS), at time points where a corresponding cVAMS sample was collected. VAMS samples were dried for a minimum of 2 h, and all samples were stored at – 80 °C until analysis.
2.3 Sample Analysis
Previously developed and validated methods were used for the analysis of the samples. APAP and four metabolites (APAP-Gluc, APAP-Sulf, APAP-Merc, and APAP-Cys) were quantified in venous plasma, venous blood, and in venous or capillary VAMS samples via liquid chromatography-tandem mass spectrometry (LC-MS/MS), with an individual LC-MS/MS method, and hence individual calibrators and QCs for each of the different matrices [15]. In short, starting from 100 µL plasma or blood, the analytes were extracted (10 min, 23 °C) via a protein precipitation step. For the VAMS samples, a 30 min extraction at 60 °C with acetonitrile-water (80:20, v/v) was performed. Subsequently, the supernatant was evaporated and reconstituted in 100 µL water (0.01% formic acid). The compounds were chromatographically separated in a 4 min gradient run. The assay ranges from 0.10 to 50.0 µg/mL for APAP, APAP-Gluc, and APAP-Sulf, and from 0.01 to 5.00 µg/mL for APAP-Merc and APAP-Cys. The method validation demonstrated that accurate and precise results are obtained, with accuracies ranging from 88–112%, and intra- and interday coefficient of variation (CV) ≤ 12.5% and 18.8%, respectively, at the lower limit of quantification (LLOQ), and ≤ 9.9% and 12.4%, respectively, for the other QC levels [15]. Furthermore, method validation included in-depth evaluation of stability, (hematocrit-independent) recovery and (hematocrit-independent) matrix effects [15]. The effect of a lipemic sample matrix was not included as part of method validation based on reasons detailed in electronic supplementary material (ESM) Sect. 1.
2.4 Comparison of the Different Matrices
For all analytes, four comparisons were made between the different matrices: (1) blood versus plasma; (2) vVAMS versus blood; (3) cVAMS versus vVAMS; and (4) cVAMS versus blood. Method agreement was evaluated via the generation of Bland–Altman (BA) plots, and time-dependent differences between the matrices were evaluated via the generation of boxplots per sampling time point. MedCalc (version 19.7.2; Medcalc Software, Oostende, Belgium) was used to generate BA plots and to perform Passing–Bablok regression analysis. Boxplots were generated using Graphpad Prism 9 (version 9.0.2; Graphpad Software, San Diego, CA, USA). When comparing vVAMS and blood concentrations, an acceptance criterion of < 20% mean difference for two-thirds of the samples was taken for all analytes, as recommended by Capiau et al. [16]. For the other comparisons, no specific preset acceptance criteria can be applied, as there may be intrinsic differences between blood and plasma concentrations on the one hand and venous and capillary concentrations on the other hand. A detailed description regarding these different comparisons can be found in ESM Sect. 2.
3 Results and Discussion
3.1 Paracetamol
An overview of the results for all analytes per comparison can be found in Table 1.
For APAP, a negative slope of − 0.26 (95% confidence interval [CI] − 0.31 to − 0.21) was found when comparing blood and plasma results (Fig. 2a; n = 278), indicating that the bias changes with the concentration. Overall, a mean bias of 5.5% (95% CI 4.5–6.5%) was found. The limits of agreement (LoA) lay at − 11.4% and 22.4% (Table 1), with the span between the upper and lower LoA being within the anticipated differences between both methods based on the method validation [15]. A median B:P ratio of 1.07 (95% CI 1.06–1.09) was found, which is in alignment with previous reports [17, 18]. When looking at the B:P ratio per sampling time point (Fig. 2b), the median B:P ratio at t0.25 was significantly lower than the ratio at time points longer after APAP administration (t3.00 to t30.00). This time-dependent change in B:P ratio was also seen when plotting the median B:P ratio in function of the sampling time (ESM Fig. S1a), where the median B:P ratio increased until an equilibrium was reached after approximately 3 h. Moreover, a negative bias was observed in the samples with high APAP concentrations (i.e., the samples collected at the early sampling time points), which also indicates that the B:P equilibrium was not yet attained in these samples.
Bland–Altman plots for the comparison of a blood and plasma concentrations; c vVAMS and blood concentrations; e cVAMS and vVAMS concentrations; and g cVAMS and blood concentrations of paracetamol. The green area indicates the 20% acceptance criterion. Boxplots of b paracetamol B:P ratios and f paracetamol cVAMS/vVAMS concentration ratios per sampling time point. The boxes indicate the 25th and 75th percentile and the median, and the flags show the minimum and maximum values. The inverted black triangles indicate the time points at which intravenous administration of paracetamol over 15 min was finished. Single and double asterisks (* and **) indicate significant differences from the median of b t0.25 or f t0.50 and t24.25, respectively (Dunn’s multiple comparison test, α = 0.05). d Passing–Bablok regression analysis of paracetamol concentrations in vVAMS plotted against the blood concentrations. VAMS volumetric absorptive microsampling, vVAMS venous VAMS, cVAMS capillary VAMS, B:P blood:plasma, CI confidence interval
For the comparison of vVAMS and blood (Fig. 2c; n = 132), a slight positive trend was observed over the concentration range (slope 0.13; 95% CI 0.02–0.25). The mean bias was 5.9% (95% CI 4.4–7.3%) and the LoA lay at − 10.5% and 22.2% (Table 1). As recommended by Capiau et al., an acceptance criterion of < 20% mean difference for two-thirds of the samples was taken when comparing vVAMS and blood concentrations [16]. For APAP, this acceptance criterion was fulfilled, with 128/132 of the differences being < 20%, demonstrating that the determination in dried blood (starting from VAMS samples) resulted in overall limited and acceptable differences, compared with the determination in liquid blood. Passing–Bablok regression demonstrated that a significant, but limited, proportional error was present, whereas no systematic error could be detected (Fig. 2d).
When comparing vVAMS and cVAMS concentrations (Fig. 2e; n = 131), a mean difference of 4.0% was observed (95% CI 1.6–6.4%). The LoA lay at − 23.3% and 31.3% (Table 1). When looking at the ratio cVAMS/vVAMS per time point (Fig. 2f), the median ratio for the time points shortest after APAP administration (t0.50 and t24.25) was significantly higher than at the time points longer after APAP administration (t1.00 to t30.00). This points to a time-dependent change of the capillary/venous APAP concentration ratio. This was also previously reported by Mohammed et al. and Spooner et al., who reported higher APAP concentrations in capillary blood than in venous blood up to 60 min after dosing [19, 20]. It should be noted that these studies involved oral dosing of APAP, whereas here, APAP was administered intravenously. As can be seen in Fig. 2e, the span between the LoA for the comparison of cVAMS versus vVAMS is wider than for the comparison of vVAMS versus liquid blood (54.6% vs. 32.7%, respectively). When the results obtained at t0.50 and t24.25 for the comparison of cVAMS versus vVAMS were excluded from the BA plot, the span between the LoA decreased to 38.6% (ESM Fig. S2a). Hence, the wider span in Fig. 2e can primarily be explained by the aforementioned altering cVAMS/vVAMS ratio over the different time points. Therefore, an equilibration between capillary and venous APAP concentrations should be taken into account when evaluating APAP PK, which also applies to the following metabolites showing a time-dependent difference in capillary and venous concentrations. To some extent, the wider span can also be explained by a somewhat larger imprecision for the analysis of cVAMS samples, when compared with vVAMS samples generated in the laboratory. The latter obviously applies for all following analytes. Two previous studies, conducted by our own group, have found that VAMS sampling from a finger-prick added an additional imprecision of 5% and 9% compared with VAMS, which were sampled in the laboratory from venous blood [21, 22].
For the comparison of cVAMS and blood concentrations (Fig. 2g; n = 131), a mean difference of 9.1% (95% CI 6.8–11.5%) was found, and the LoA were − 17.4% and 35.7% (Table 1). Remarkably, the mean bias here was higher than that observed for the comparison of vVAMS and blood (5.9%) and cVAMS and vVAMS (4.0%). However, when comparing cVAMS and blood results, two variables are evaluated and should be taken into account: (1) the effect of the use of VAMS as a sampling technique, and (2) the effect of capillary versus venous blood sampling. Hence, the results obtained for the cVAMS-blood comparison represent ‘a combination’ of the results obtained for the vVAMS-blood comparison and the cVAMS-vVAMS comparison. Obviously, this also applies to the following analytes. Additionally, considering the time-dependent change in the capillary/venous APAP concentration ratio, results obtained at t0.50 and t24.25 were excluded from the BA plot (ESM Fig. S3a). The span covered by the LoA decreased from 53.1 to 45.9%, however to a lesser extent than observed when comparing cVAMS and vVAMS results.
3.2 Paracetamol-Glucuronide
For APAP-Gluc, a negative bias was observed when comparing blood with plasma concentrations (Fig. 3a; n = 280), indicating a B:P ratio < 1. The median B:P ratio observed was 0.52 (95% CI 0.51–0.53). As far as we are aware, this is the first time B:P ratios are reported for any of the APAP metabolites. The mean difference was − 61.3% (95% CI − 62.4 to − 60.1%) and the LoA lay at − 81.0% and − 41.5% (Table 1). A negative slope was observed for the regression line (− 0.24; 95% CI − 0.35 to − 0.13), indicating that the bias changes with the concentration. No significant differences in the median B:P ratio were observed among the different time points (Fig. 3b).
Bland–Altman plots for the comparison of a blood and plasma concentrations; c vVAMS and blood concentrations; e cVAMS and vVAMS concentrations; and g cVAMS and blood concentrations of paracetamol-glucuronide. The green area indicates the 20% acceptance criterion. Boxplots of b paracetamol-glucuronide B:P ratios and f paracetamol-glucuronide cVAMS/vVAMS concentration ratios per sampling time point. The boxes indicate the 25th and 75th percentile and the median, and the flags show the minimum and maximum values. The inverted black triangles indicate the time points at which intravenous administration of paracetamol over 15 min was finished. Single and double asterisks (* and **) indicate significant differences from the median of b t0.25 or f t0.50 and t24.25, respectively (Dunn’s multiple comparison test, α = 0.05). d Passing–Bablok regression analysis of paracetamol-glucuronide concentrations in vVAMS plotted against the blood concentrations. VAMS volumetric absorptive microsampling, vVAMS venous VAMS, cVAMS capillary VAMS, B:P blood:plasma, CI confidence interval
For the comparison of concentrations in vVAMS and liquid blood samples (Fig. 3c; n = 132), a mean difference of 1.1% (95% CI − 0.5 to 2.8%) was found, and the LoA lay within the ± 20% interval (Table 1). Consequently, this comparison met the preset acceptance criterion, with 128/132 of the differences laying within the ± 20% interval. A positive slope of 0.36 (95% CI 0.11–0.60) was observed. Here, a slight proportional error as well as a systematic error were observed (Fig. 3d), as 1 and 0 were (just) not included in the 95% CI of the slope and intercept, respectively.
For the comparison of cVAMS and vVAMS concentrations (Fig. 3e; n = 132), a mean difference of 6.5% (95% CI 4.3–8.7%) was found, and the LoA were − 18.1% and 31.2% (Table 1). Evaluation of the cVAMS/vVAMS ratios for each time point (Fig. 3f) revealed significant differences between the median ratio of t0.50 and time points t2.00 to t30.00. When excluding the results obtained at t0.50 and t24.25 from the BA plot (ESM Fig. S2b), the span between the LoA only slightly decreased (from 49.3 to 42.6%), with this decrease being much less pronounced than that observed for APAP (16%, from 54.6 to 38.6%) [ESM Fig. S2a]. This could be expected, since for APAP-Gluc, contrarily to APAP, the ratio obtained at t24.25 was not significantly different from the ratio obtained at the other time points.
When comparing cVAMS with blood results (Fig. 3g; n = 131), a mean difference of 7.5% (95% CI 5.1–9.8%) was observed and the LoA lay at − 18.6% and 33.5% (Table 1). When excluding the results obtained at the first time points after APAP administration (t0.50 and t24.25) [ESM Fig. S3b], the span covered by the LoA essentially remained the same (decrease from 52.1 to 50.9%).
3.3 Paracetamol-Sulfate
For APAP-Sulf, an overall negative bias of 41.9% (95% CI − 43.1 to − 40.7%) was found when comparing blood and plasma concentrations (Fig. 4a; n = 280), as was also the case for APAP-Gluc, although the overall bias here was less negative. The median B:P ratio determined was 0.65 (95% CI 0.64–0.66). The LoA lay at − 61.3% and − 22.5% (Table 1). Similarly as for APAP-Gluc, the trend line showed a negative slope of − 0.51, however the 95% CI was wider (− 0.86 to − 0.16). Furthermore, there were no significant differences between the time points in terms of median B:P ratio (Fig. 4b).
Bland–Altman plots for the comparison of a blood and plasma concentrations; c vVAMS and blood concentrations; e cVAMS and vVAMS concentrations; and g cVAMS and blood concentrations of paracetamol-sulfate. The green area indicates the 20% acceptance criterion. Boxplots of b paracetamol-sulfate B:P ratios and f paracetamol-sulfate cVAMS/vVAMS concentration ratios per sampling time point. The boxes indicate the 25th and 75th percentile and the median, and the flags show the minimum and maximum values. The inverted black triangles indicate the time points at which intravenous administration of paracetamol over 15 min was finished. Single and double asterisks (* and **) indicate significant differences from the median of b t0.25 or f t0.50 and t24.25, respectively (Dunn’s multiple comparison test, α = 0.05). d Passing–Bablok regression analysis of paracetamol-sulfate concentrations in vVAMS plotted against the blood concentrations. VAMS volumetric absorptive microsampling, vVAMS venous VAMS, cVAMS capillary VAMS, B:P blood:plasma, CI confidence interval
For the comparison of APAP-Sulf concentrations in vVAMS and blood samples (Fig. 4c; n = 133), a mean bias of 0.2% (95% CI − 1.5 to 2.0%) was found. Again, the acceptance criterion was met, with 126/133 of the differences within ± 20%. The LoA lay at − 19.7% and 20.2% (Table 1). For this comparison, the slope (− 0.56) did not significantly differ from 0 (95% CI − 1.23 to 0.12). Passing–Bablok regression showed no significant deviation from 1, and from 0 for the slope and the intercept, respectively (Fig. 4d).
When comparing cVAMS and vVAMS concentrations (Fig. 4e; n = 132), a mean difference of 1.0% (95% CI − 1.0 to 3.0%) was noted, and the LoA lay at − 22.0% and 24.0% (Table 1). When looking at the ratio cVAMS/vVAMS (Fig. 4f), no significant differences in the median could be deduced amongst the different time points. Consequently, contrarily to APAP and APAP-Gluc, the span between the LoA only slightly changed when the results obtained at the first time points after APAP administration (t0.50 and t24.25) were excluded from the BA plot (ESM Fig. S2c).
A mean difference of 1.3% (95% CI − 1.1 to 3.6%) was found for the comparison of cVAMS and blood results (Fig. 4g; n = 132). The LoA lay at − 25.6% and 28.1% (Table 1). In line with the cVAMS-vVAMS comparison and similar to what was observed for APAP-Gluc, the range covered by the LoA hardly changed when results obtained at t0.50 and t24.25 were excluded from the BA plot (ESM Fig. S3c).
3.4 Paracetamol-Mercapturate
As was the case for the other APAP metabolites, APAP-Merc concentrations were also lower in blood than in plasma (Fig. 5a; n = 242). The mean bias was − 63.4% (95% CI − 64.8 to − 62.1%) and the LoA was − 84.3% and − 42.5% (Table 1). Only a slight negative slope was found (− 0.89; 95% CI − 16.3 to 14.5), indicating that the differences were evenly spread around the mean over the complete concentration range. The median B:P ratio found was 0.52 (95% CI 0.51–0.53). While for the other analytes, the vast majority of the samples had concentrations above the LLOQ (except for t0 samples, collected before the administration of any APAP), for APAP-Merc, the samples collected shortly after the first APAP dose (t0.25 and t0.50) frequently had undetectable or low signals for APAP-Merc. For blood and plasma samples collected at t0.25 and t0.50, only three and four samples had a quantifiable concentration of APAP-Merc, respectively. Therefore, the B:P ratios at t0.25 and t0.50 were not included when evaluating the B:P ratio per sampling time point. Overall, for 249/280 (89%) plasma and 243/280 (87%) blood samples a result could be obtained, with 242 paired samples being available for method comparison. APAP-Merc is the last one being formed in the metabolism pathway of the metabolites included in this study (ESM Fig. S4). Hence, it could be expected that at the early time points, the analyte is not yet formed or the concentration is still too low to be detected using the current method, as was also the case in the study by Flint et al. [23]. When looking at the B:P ratio per sampling time point, no time-dependent change in median B:P ratio was observed (Fig. 5b).
Bland–Altman plots for the comparison of a blood and plasma concentrations; c vVAMS and blood concentrations; e cVAMS and vVAMS concentrations; and g cVAMS and blood concentrations of paracetamol-mercapturate. The green area indicates the 20% acceptance criterion. Boxplots of b paracetamol-mercapturate B:P ratios and f paracetamol-mercapturate cVAMS/vVAMS concentration ratios per sampling time point. The boxes indicate the 25th and 75th percentile and the median, and the flags show the minimum and maximum values. The inverted black triangles indicate the time points at which intravenous administration of paracetamol over 15 min was finished. Single and double asterisks (* and **) indicate significant differences from the median of b t0.75 or f t1.00 and t24.25, respectively (Dunn’s multiple comparison test, α = 0.05). d Passing–Bablok regression analysis of paracetamol-mercapturate concentrations in vVAMS plotted against the blood concentrations. VAMS volumetric absorptive microsampling, vVAMS venous VAMS, cVAMS capillary VAMS, B:P blood:plasma, CI confidence interval
The comparison of vVAMS and blood concentrations (Fig. 5c; n = 117) showed that 111/117 of the differences were within ± 20%, again fulfilling the acceptance criterion. At t0.50, only four blood samples and the corresponding vVAMS samples had a concentration above the LLOQ, which could be compared. The mean difference was 2.0% (95% CI − 0.2 to 4.1%) and the LoA lay at − 21.0% and 24.9% (Table 1). Likewise as for the blood-plasma comparison, a negative slope was found (− 44.9; 95% CI − 84.6 to − 5.3), although this does not seem to affect the determination of APAP-Merc in VAMS samples compared with blood in a relevant manner, as evidenced by the relatively narrow span of the LoA. Moreover, Passing–Bablok regression showed no proportional and systematic error (Fig. 5d).
A mean difference of 6.7% (95% CI 4.2–9.2%) was found when comparing cVAMS and vVAMS samples (Fig. 5e; n = 116). The LoA lay at − 19.9% and 33.3% (Table 1). Here, in only three of the cVAMS-vVAMS sample pairs collected at t0.50 a quantitative result was obtained. Therefore, no cVAMS/vVAMS ratio was included for t0.50 in this comparison. The median cVAMS/vVAMS ratio found at t1.00 was higher than the ratio obtained for the other time points (Fig. 5f). When the results obtained at the first time points after APAP administration were excluded from the BA plot, the span between the LoA decreased to a similar extent as was seen for APAP-Gluc (ESM Fig. S2d).
When comparing cVAMS and blood results (Fig. 5g; n = 116), a mean difference of 8.7% (95% CI 5.7–11.6%) was noted and the LoA lay at − 23.0% and 40.3% (Table 1). The span covered by the LoA decreased when excluding the results obtained at t1.00 and t24.25 (from 63.3 to 54.8%) [ESM Fig. S3d].
3.5 Paracetamol-Cysteine
When comparing blood and plasma concentrations of APAP-Cys (Fig. 6a; n = 278), an overall negative bias of 47.0% was found (95% CI − 51.3 to − 42.7%). Again, a trend could be discerned over the concentration range, with a more negative bias with increasing concentrations, indicated by the slope of the regression line (slope − 40.2; 95% CI − 51.6 to − 28.9). The LoA lay at − 118.0% and 24.0% (Table 1). The range covered by these LoA is very broad (142%), and hence we can say that the agreement between blood and plasma concentrations for APAP-Cys is poor. When looking at the data in more detail (ESM Fig. S5a), mainly the first two sampling time points after the first APAP dose (t0.25 and t0.50) led to the differences with a positive bias. As discussed in our previous report, we anticipate that in blood samples where APAP is present, there will be higher concentrations of APAP-glutathione (APAP-GSH) in blood compared with plasma [15]. It is also known that APAP-GSH degrades to APAP-Cys. As in this comparison, the first time points (t0.25 and t0.50) also correspond to the samples with the highest APAP concentrations (see Sect. 3.1), it can be anticipated that in these blood samples, high APAP-GSH levels may have been present, which could have degraded to APAP-Cys. This would cause a falsely high concentration of APAP-Cys in those blood samples. Since we assume that this happens to a much lesser extent in plasma because of the lower concentration of APAP-GSH in plasma, this could cause a positive bias in whole blood. When the results obtained at t0.25 and t0.50 were excluded from the BA plot (ESM Fig. S5b), the span between the LoA nearly halved (from 142 to 76%), which supports our hypothesis that the agreement between APAP-Cys blood and plasma concentrations is mainly poor at the first sampling time points, when APAP concentrations are high. APAP-GSH was not included in the current assessment of patient samples, as it was not included in the analytical methods for quantitative determination in the different matrices. As discussed in our previous report, this decision was made based on preliminary analysis of plasma samples from patients included in the PK study receiving therapeutic doses of APAP. In these samples, all analytes were detected, except for APAP-GSH, which was undetectable or only present at levels below the proposed LLOQ of 0.10 µg/mL in most of the samples [15]. As no data are available on the stability of APAP-GSH in blood, we cannot estimate to what extent APAP-GSH contributes to the bias and concentration-dependent trend. The positive bias at the first two sampling time points could also be detected when evaluating the median B:P ratio per sampling time point (Fig. 6b). The median B:P ratio obtained at t0.25 was significantly higher than the B:P ratios obtained at all other time points, except t0.50. The median B:P ratio of the latter was in turn significantly higher than the B:P ratio obtained at t24.25. For APAP-Cys, a median B:P ratio of 0.56 was found (95% CI 0.55–0.58).
Bland–Altman plots for the comparison of a blood and plasma concentrations; c vVAMS and blood concentrations; e cVAMS and vVAMS concentrations; and g cVAMS and blood concentrations of paracetamol-cysteine. The green area indicates the 20% acceptance criterion. Boxplots of b paracetamol-cysteine B:P ratios and f paracetamol-cysteine cVAMS/vVAMS concentration ratios per sampling time point. The boxes indicate the 25th and 75th percentile and the median, and the flags show the minimum and maximum values. The inverted black triangles indicate the time points at which intravenous administration of paracetamol over 15 min was finished. Single and double asterisks (* and **) indicate significant differences from the median of b t0.25 or f t0.50 and t24.25, respectively (Dunn’s multiple comparison test, α = 0.05). d Passing–Bablok regression analysis of paracetamol-cysteine concentrations in vVAMS plotted against the blood concentrations. VAMS volumetric absorptive microsampling, vVAMS venous VAMS, cVAMS capillary VAMS, B:P blood:plasma, CI confidence interval
For the comparison of APAP-Cys concentrations in vVAMS and blood samples (Fig. 6c; n = 131), a mean bias of 10.4% was found (95% CI 6.4–14.4%). Likewise, as for the blood-plasma comparison, the trend line shows a negative slope (− 52.6; 95% CI − 72.2 to − 33.1), and the LoA lay at − 34.5% and 55.3% (Table 1). Overall, 40/131 (30%) of the differences were larger than 20%. While formally this is still borderline within the acceptance criterion (33%), we consider this too large to conclude equivalence. There seems to be a concentration-dependent difference in bias, and mainly for the concentrations < 0.15 µg/mL, mostly corresponding to the earliest sampling time points, the bias is unacceptable. A hypothesis for this positive bias could again be related to the presence of falsely elevated concentrations of APAP-Cys in these samples, owing to the degradation of APAP-GSH in these samples. Upon drying of the vVAMS, for a minimum of 2 h, it is possible that APAP-GSH further degrades to APAP-Cys, while the corresponding blood samples were almost immediately frozen at − 80 °C after having been used to generate vVAMS samples. Although the method validation, where stability was evaluated using samples spiked with a mix of all analytes, did not reveal stability issues for the evaluated analytes [15], it can be expected that APAP-GSH (not monitored here owing to stability issues) is formed in samples taken shortly after administration, when APAP concentrations are much higher than those of the metabolites. As we know that APAP-GSH may degrade to APAP-Cys, this may lead to falsely elevated APAP-Cys concentrations in the patient samples collected shortly after APAP administration. During the method validation, APAP concentrations (and hence also possibly in vitro formed APAP-GSH) were low when APAP-Cys concentrations were low. Therefore, if during method validation any APAP-Cys would have been formed due to the degradation of APAP-GSH, it may not have significantly increased the concentration of APAP-Cys, also readily present in the validation set. Here, however, APAP (and hence also APAP-GSH formed thereof) was high when APAP-Cys concentrations were low. Therefore, the relative contribution of APAP-Cys formed by degradation of APAP-GSH could be relevant and yield a falsely elevated result for APAP-Cys if APAP-GSH was not stable during drying of the vVAMS samples. Furthermore, the range covered by the LoA decreased when excluding the results obtained at the first sampling time point (t0.50) [ESM Fig. S6], however this decrease was less pronounced when compared with the blood-plasma comparison. Both a proportional and systematic error were found (Fig. 6d).
For the comparison of vVAMS and cVAMS concentrations (Fig. 6e; n = 132), the LoA span was narrower than in the vVAMS-blood comparison (Table 1). The mean difference was 1.4% (95% CI − 0.9 to 3.7%). There was also no significant difference between the time points in terms of the cVAMS/vVAMS median ratio (Fig. 6f) and, consequently, removal of the results obtained at t0.50 only slightly decreased the span covered by the LoA (ESM Fig. S2e).
A mean difference of 11.8% (95% CI 8.0–15.5%) was found and the LoA lay at − 30.6% and 54.1% (Table 1) for the comparison of cVAMS and blood results (Fig. 6g; n = 131). Here, the mean bias and the range covered by the LoA was similar to that seen for the comparison of vVAMS and blood results (84.7% and 89.8% for the cVAMS-blood comparison and vVAMS-blood comparison, respectively) (Fig. 6c). Furthermore, when excluding the results obtained at t0.50 from the BA plot (ESM Fig. S7), the span covered by the LoA decreased to a similar extent as was seen for the vVAMS-blood comparison (ESM Fig. S6) [i.e., a decrease of 13.7% and 10.6% for the cVAMS-blood comparison and vVAMS-blood comparison, respectively]. This could be expected, as it can be assumed that the degradation of APAP-GSH to APAP-Cys will occur to a similar extent in both vVAMS and cVAMS samples during the drying of the VAMS samples. The latter was also substantiated by the relatively good agreement between APAP-Cys cVAMS and vVAMS concentrations (Fig. 6e).
For the purpose of PK evaluations, it is also valuable to look at the summed concentrations of APAP-Merc and APAP-Cys, as these two analytes represent the CYP2E1 metabolism pathway (ESM Fig. S4). Therefore, the results obtained for APAP-Merc and APAP-Cys were also summed and analyzed similarly as the other analytes. Overall, similar results as for the comparison based only on APAP-Cys were found. This could be expected, since, at all sampling time points, APAP-Cys concentrations were higher than APAP-Merc concentrations (with APAP-Cys contributing to ≥ 76% of the summed concentrations of the metabolites) [ESM Fig. S8]. A detailed overview of these results can be found in ESM Sect. 3.
There were some limitations of this study. First, an intrinsic limitation is the monitoring of APAP-Cys in both liquid and dried blood samples. Due to the possible post-sampling degradation of APAP-GSH to APAP-Cys, APAP-Cys concentrations in (dried) blood should be interpreted with caution. Hence, as discussed in the previous paragraph, for the CYP2E1 metabolism pathway, the concentrations of APAP-Cys and APAP-Merc will be summed in the PK analysis. If any APAP-GSH would be present and degrade to APAP-Cys during sample analysis and storage, the concentration of APAP-Cys will reflect the sum of APAP-GSH and APAP-Cys at the time of sample collection. Second, although the number of patients included in this study was relatively low, the many sampling time points did allow us to conduct an in-depth comparative evaluation, based on a sufficiently high number of data points. Last, at this point, no PK evaluation has been conducted, owing to the limited number of patients. The ongoing inclusion of more obese and non-obese patients in this study will allow us to make comparative statements on potential differences in PK parameters between these populations in the future.
4 Conclusion
In this study, we compared the concentrations of APAP and four of its metabolites in different matrices, namely plasma, blood, vVAMS, and cVAMS, the latter obtained via finger-prick, in obese and non-obese patients.
For APAP, a median B:P ratio of 1.07 was found. Overall, the differences between APAP concentrations in blood and plasma remained limited. For the metabolites, a strongly negative mean bias was seen in the comparison of blood versus plasma concentrations, with B:P ratios ranging from 0.52 to 0.65. Furthermore, the differences remained limited, except for APAP-Cys, for which the range covered by the LoA was very broad. Additionally, a time-dependent change in B:P ratio was found for APAP and APAP-Cys.
Importantly, the comparison of vVAMS and blood concentrations met the preset acceptance criteria, demonstrating that the use of VAMS devices did not relevantly affect the quantification of the analytes compared with their determination in liquid venous blood. Only for APAP-Cys, caution should be paid to the interpretation of concentrations in dried blood, as we also stated previously.
Furthermore, the differences between capillary and venous concentrations of all analytes remained limited, demonstrating that finger-prick sampling is suitable to conduct PK studies for APAP. A time-dependent difference was observed for APAP, APAP-Gluc, and APAP-Merc, with higher capillary than venous concentrations shortly after dosing. Hence, an equilibration between capillary and venous concentrations should be taken into account when evaluating the PK profile of these analytes. The agreement of the PK parameters determined from the different matrices, as well as potential differences between obese and non-obese patients, is to be evaluated and requires further inclusion of patients, which is ongoing.
To conclude, the set-up described in this study can be applied as a framework for future PK studies for other analytes, allowing extensive comparison of different matrices and evaluation of time-dependent differences between these matrices.
References
Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol. 2015;79(3):395–404. https://doi.org/10.1111/bcp.12267.
Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76. https://doi.org/10.1080/03602530902722679.
Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–31. https://doi.org/10.2165/00003088-200039030-00004.
Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87. https://doi.org/10.2165/11318100-000000000-00000.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. https://doi.org/10.1016/S0140-6736(14)60460-8.
NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42. https://doi.org/10.1016/S0140-6736(17)32129-3
Jain R, Chung SM, Jain L, Khurana M, Lau SW, Lee JE, et al. Implications of obesity for drug therapy: limitations and challenges. Clin Pharmacol Ther. 2011;90(1):77–89. https://doi.org/10.1038/clpt.2011.104.
Goday Arno A, Farre M, Rodriguez-Morato J, Ramon JM, Perez-Mana C, Papaseit E, et al. Pharmacokinetics in morbid obesity: influence of two bariatric surgery techniques on paracetamol and caffeine metabolism. Obes Surg. 2017;27(12):3194–201. https://doi.org/10.1007/s11695-017-2745-z.
van Rongen A, Valitalo PAJ, Peeters MYM, Boerma D, Huisman FW, van Ramshorst B, et al. Morbidly obese patients exhibit increased CYP2E1-mediated oxidation of acetaminophen. Clin Pharmacokinet. 2016;55(7):833–47. https://doi.org/10.1007/s40262-015-0357-0.
Abernethy DR, Divoll M, Greenblatt DJ, Ameer B. Obesity, sex, and acetaminophen disposition. Clin Pharmacol Ther. 1982;31(6):783–90. https://doi.org/10.1038/clpt.1982.111.
Hakim M, Anderson BJ, Walia H, Tumin D, Michalsky MP, Syed A, et al. Acetaminophen pharmacokinetics in severely obese adolescents and young adults. Paediatr Anaesth. 2019;29(1):20–6. https://doi.org/10.1111/pan.13525.
James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31(12):1499–506. https://doi.org/10.1124/dmd.31.12.1499.
Delahaye L, Veenhof H, Koch BCP, Alffenaar JC, Linden R, Stove C. Alternative sampling devices to collect dried blood microsamples: state-of-the-art. Ther Drug Monit. 2021;43(3):310–21. https://doi.org/10.1097/FTD.0000000000000864.
Denniff P, Spooner N. Volumetric absorptive microsampling: a dried sample collection technique for quantitative bioanalysis. Anal Chem. 2014;86(16):8489–95. https://doi.org/10.1021/ac5022562.
Delahaye L, Baerdemaeker L, Stove CP. Determination of paracetamol and its metabolites via LC-MS/MS in dried blood volumetric absorptive microsamples: a tool for pharmacokinetic studies. J Pharm Biomed Anal. 2021;206: 114361. https://doi.org/10.1016/j.jpba.2021.114361.
Capiau S, Veenhof H, Koster RA, Bergqvist Y, Boettcher M, Halmingh O, et al. Official international association for therapeutic drug monitoring and clinical toxicology guideline: development and validation of dried blood spot-based methods for therapeutic drug monitoring. Ther Drug Monit. 2019;41(4):409–30. https://doi.org/10.1097/FTD.0000000000000643.
Gwilt JR, Robertson A, McChesney EW. Determination of blood and other tissue concentrations of paracetamol in dog and man. J Pharm Pharmacol. 1963;15:440–4. https://doi.org/10.1111/j.2042-7158.1963.tb12811.x.
Naritomi Y, Terashita S, Kagayama A, Sugiyama Y. Utility of hepatocytes in predicting drug metabolism: comparison of hepatic intrinsic clearance in rats and humans in vivo and in vitro. Drug Metabol Dispos Biol Fate Chem. 2003;31(5):580–8. https://doi.org/10.1124/dmd.31.5.580.
Mohammed BS, Cameron GA, Cameron L, Hawksworth GH, Helms PJ, McLay JS. Can finger-prick sampling replace venous sampling to determine the pharmacokinetic profile of oral paracetamol? Br J Clin Pharmacol. 2010;70(1):52–6. https://doi.org/10.1111/j.1365-2125.2010.03668.x.
Spooner N, Lad R, Barfield M. Dried blood spots as a sample collection technique for the determination of pharmacokinetics in clinical studies: considerations for the validation of a quantitative bioanalytical method. Anal Chem. 2009;81(4):1557–63. https://doi.org/10.1021/ac8022839.
Van Uytfanghe K, Heughebaert L, Stove CP. Self-sampling at home using volumetric absorptive microsampling: coupling analytical evaluation to volunteers’ perception in the context of a large scale study. Clin Chem Lab Med. 2021;59(5):e185–7. https://doi.org/10.1515/cclm-2020-1180.
Verstraete J, Stove C. Volumetric absorptive microsampling (VAMS) as a reliable tool to assess thiamine status in dried blood microsamples: a comparative study. Am J Clin Nutr. 2021;114(3):1200–7. https://doi.org/10.1093/ajcn/nqab146.
Flint RB, Mian P, van der Nagel B, Slijkhuis N, Koch BCP. Quantification of acetaminophen and its metabolites in plasma using UPLC-MS: doors open to therapeutic drug monitoring in special patient populations. Ther Drug Monit. 2017;39(2):164–71. https://doi.org/10.1097/Ftd.0000000000000379.
Acknowledgments
The authors would like to thank Emiel Bogaert and Ann De Bruyne for the collection of the patient samples, as well as the patients who participated in this study.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: LD, CS. Performed the experiments: LB, LD. Analyzed the data: LB, LD. Wrote the paper: LB, LD, CS, LDB. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Funding
Laura Boffel would like to thank the Research Foundation Flanders (FWO) for granting her a PhD fellowship (11M2322N).
Conflicts of interest/competing interests
Laura Boffel, Lisa Delahaye, Luc De Baerdemaeker, and Christophe P. Stove have no conflicts of interest to declare.
Ethics approval
This study was approved by Ghent University Hospital Ethical Committee (BC-07469).
Consent to participate
All participants provided written informed consent agreeing to participate in this study.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boffel, L., Delahaye, L., De Baerdemaeker, L. et al. Application of a Volumetric Absorptive Microsampling (VAMS)-Based Method for the Determination of Paracetamol and Four of its Metabolites as a Tool for Pharmacokinetic Studies in Obese and Non-Obese Patients. Clin Pharmacokinet 61, 1719–1733 (2022). https://doi.org/10.1007/s40262-022-01187-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01187-2