Abstract
Background
Medication use is highly prevalent with advanced age, but clinical studies are rarely conducted in the elderly, leading to limited knowledge regarding age-related pharmacokinetic changes.
Objective
The objective of this study was to investigate which pharmacokinetic parameters determine drug exposure changes in the elderly by conducting virtual clinical trials for ten drugs (midazolam, metoprolol, lisinopril, amlodipine, rivaroxaban, repaglinide, atorvastatin, rosuvastatin, clarithromycin and rifampicin) using our physiologically based pharmacokinetic (PBPK) framework.
Methods
PBPK models for all ten drugs were developed in young adults (20–50 years) following the best practice approach, before predicting pharmacokinetics in the elderly (≥ 65 years) without any modification of drug parameters. A descriptive relationship between age and each investigated pharmacokinetic parameter (peak concentration [Cmax], time to Cmax [tmax], area under the curve [AUC], clearance, volume of distribution, elimination-half-life) was derived using the final PBPK models, and verified with independent clinically observed data from 52 drugs.
Results
The age-related changes in drug exposure were successfully simulated for all ten drugs. Pharmacokinetic parameters were predicted within 1.25-fold (70%), 1.5-fold (86%) and 2-fold (100%) of clinical data. AUC increased progressively by 0.9% per year throughout adulthood from the age of 20 years, which was explained by decreased clearance, while Cmax, tmax and volume of distribution were not affected by human aging. Additional clinical data of 52 drugs were contained within the estimated variability of the established age-dependent correlations for each pharmacokinetic parameter.
Conclusion
The progressive decrease in hepatic and renal blood flow, as well as glomerular filtration, rate led to a reduced clearance driving exposure changes in the healthy elderly, independent of the drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The number of people older than 65 years of age is predicted to double in the US and Europe by 2050 [1, 2]. The burden of age-related comorbidities, such as cardiovascular diseases, hypertension, diabetes mellitus and renal impairment, increases in the elderly [3], resulting in twice as high medication use compared with middle-aged adults [4]. Despite the growing population of elderly individuals, clinical trials are generally not conducted in this special population, leading to a knowledge gap regarding the effect of adult age on drug pharmacokinetics [5]. However, advanced age is characterized by anatomical, physiological and biological changes [6], which have the potential to affect the absorption, distribution, metabolism and elimination processes of a drug, resulting in altered pharmacokinetics.
The impact of advanced age on drug absorption processes remains inconclusive due to contradictory findings in the literature. For instance, gastric emptying time is reported to be slower, similar or faster in the elderly compared with young adults. The distribution of drugs is affected by a progressive increase in adipose tissue weight, while total body water declines in the elderly. Hepatic drug metabolism is potentially affected by age-related changes in liver weight and hepatic blood flow, which are decreased by 10% and 18%, respectively, in 70-year-old individuals compared with 30-year-old individuals. Reports regarding hepatic enzyme activity in the elderly are sparse and contradictory. The only well-studied hepatic enzyme is cytochrome P450 (CYP) 3A, which shows age-independent activity [6]. Polasek et al. investigated five different probe substrates for hepatic metabolism, namely caffeine (CYP1A2), warfarin (CYP2C9), phenytoin (CYP2C19), desipramine (CYP2D6) and midazolam (CYP3A), and found a clearance decrease of 30–40% in 70-year-old subjects compared with younger individuals, which was explained by the changes in liver volume and blood flow rather than enzyme activity [7]. Data regarding drug transporter activity are generally sparse, but in a compact meta-analysis, age was tested as a covariate for hepatic drug transporter activity and was found to be non-significant [8]. Lastly, the most significant change with adult aging is the reduction in renal drug clearance, namely because of a reduction in kidney weight caused by a loss of nephrons, decreased renal blood flow, and, consequently, a decline in the glomerular filtration rate during the entire adulthood [6].
Age-dependent anatomical, physiological and biological changes can be incorporated into a physiologically based pharmacokinetic (PBPK) model, which is used to overcome sparse clinical data, offering the possibility to run virtual clinical trials for special populations such as the elderly [6, 9, 10]. A PBPK model describes the absorption, distribution, metabolism and elimination of a drug in a physiologically relevant compartmental structure, where each compartment represents an organ or tissue. Dynamic movement of the drug between compartments is mediated by regional blood flows and described by ordinary differential equations. The PBPK model is informed by a combination of in vitro and in vivo data regarding drug characteristics that are separated from physiological data describing the population of interest [11].
The first aim of the present study was to evaluate the prediction of drug disposition in the elderly, of our previously developed and parameterized PBPK framework [11]. The second aim was to determine pharmacokinetic parameters driving clinically observed drug exposure changes in the elderly through sensitivity analysis on age. We investigated which physiological and drug-specific parameters determined the degree of age-dependent changes in pharmacokinetic parameters in the model, by sensitivity analysis and with clinically observed data for 52 drugs additionally collected. Lastly, we analyzed the age at which pharmacokinetic parameters changed more than expected with respect to interindividual variability in comparison with the youngest studied age group (20–24 years).
2 Methods
2.1 Physiologically Based Pharmacokinetic (PBPK) Model
A whole-body PBPK model constructed in Matlab® 2017a was used. The model structure and code have been published previously [11]. Virtual individuals aged 20–99 years were generated according to our published repository describing age-dependent changes in anatomical, physiological and biological system parameters. Variability was considered for all population parameters by using a normal distribution [6].
2.2 Parameters of Simulated Drugs
A structured literature search was performed to identify drugs with available pharmacokinetic data in elderly individuals in order to clinically verify the PBPK model simulations. Ten drugs were selected: midazolam, metoprolol, lisinopril, amlodipine, rivaroxaban, repaglinide, atorvastatin, rosuvastatin, clarithromycin and rifampicin. Input drug parameters were obtained from verified, published PBPK models [11,12,13,14,15,16,17,18,19], except for lisinopril. The lisinopril PBPK model was developed combining published in vitro data (bottom-up approach) with available clinical clearance data (top-down approach). PBPK models were modified to adequately predict the pharmacokinetics in young adults before scaling the PBPK models to the elderly. Simulations of metoprolol were carried out in extensive metabolizers of CYP2D6 only. Tissue distribution of the amlodipine model has been modified to be used in a whole-body PBPK model based on the observed volume of distribution [20]. Metabolic CYP3A4 clearance of amlodipine was calculated from the fraction of CYP3A4-mediated clearance [21] and clinically obtained intravenous clearance [20]. The in vitro ratio between CYP3A4- and CYP3A5-mediated clearance for amlodipine was implemented [22]. The rest of the missing, observed amlodipine clearance was assigned to the unspecified hepatic intrinsic clearance. Active hepatic drug transport was included in the repaglinide PBPK model based on published in vitro data [19]. The rifampicin clearance after intravenous administration was retrogradely calculated to an unspecified intrinsic hepatic clearance under the consideration of the renal clearance of rifampicin [23, 24]. The parameters of the ten simulated drugs can be found in electronic supplementary Table S-1.
2.3 Workflow for Simulations
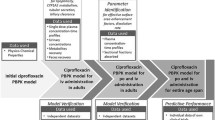
The strategy for building and verifying the PBPK models and subsequently extrapolating the pharmacokinetics to elderly adults is described in Fig. 1. All PBPK models were verified in young adults (20–50 years), following the best practice approach [25], before scaling to elderly adults (≥ 65 years), without any modification of drug parameters. Published clinical study results (electronic supplementary Table S-2) were used to assess the accuracy of the predictions. Observed data were extracted from the literature using GetData Graph digitizer V. 2.26. Pharmacokinetic parameters were not given in six publications [26,27,28,29,30,31] and thus the area under the curve for one dosing interval (AUCt) was calculated using the linear trapezoidal method, the area under the curve extrapolated to infinity (AUC∞) was extrapolated from the last three given concentration–time points, and the clearance was calculated as dose/AUC∞. If more than one published study was available, the weighted mean and standard deviation of reported pharmacokinetic parameters were calculated. Observed data were published in different formats and were converted into arithmetic mean and standard deviation [32, 33].
The simulations were matched as closely as possible to the published observed studies in terms of demographics, dose, dosing regimen and number of subjects (n), with 10 trials × n virtual subjects being simulated in each case. If more than one published study was available for a drug, the dosing regimen was the same, the study participants were summed up and the weighted mean of demographic parameters was used. No adjustment to the PBPK model was carried out when scaling drug pharmacokinetics to the elderly. Calculation of pharmacokinetic parameters in the PBPK framework used has been described previously [11].
2.4 Verification of the PBPK Drug Models
Predicted concentration–time profiles were visually compared with observed clinical data for young and elderly adults (electronic supplementary Table S-2). Furthermore, published pharmacokinetic parameters [peak concentration (Cmax), time to Cmax (tmax), AUC and elimination half-life (t½)] were compared against our simulation results. Simulations were defined as being successful in young adults if the predicted pharmacokinetic parameters were within the twofold interval of the observed data.
2.5 Extrapolation to Aged Individuals
The final PBPK models were utilized to predict age-related changes in pharmacokinetic parameters from 20 to 99 years in 500 virtual individuals split into ten different trials (proportion of women: 0.5) in 5-year steps. The analyzed pharmacokinetic parameters (Cmax, tmax, AUC, clearance, apparent volume of distribution and t½) were normalized to the youngest investigated age group (20–24 years). We examined when age-related differences of investigated pharmacokinetic parameters changed more than expected from interindividual variability defined as the 1.25-fold interval (bioequivalence criterion). Additionally, the normalized pharmacokinetic parameters were fitted to descriptive linear, exponential and monotonic functions containing age as an independent variable. The corrected Akaike’s information criterion was used to select the best-performing function among those tested, which, in contrast to the coefficient of determination, exhibits no bias to higher parameterized models. The analysis was performed for men, women and all virtual subjects, to investigate whether sex has an impact on age-related changes in pharmacokinetic parameters.
2.6 Sensitivity Analysis
Sensitivity analysis was performed to investigate which population- and drug-specific parameters drive the age-related pharmacokinetic parameter changes in the used PBPK model. A fictive drug was generated, informed by the median input parameters of the ten investigated drugs, and it was ensured that age-related pharmacokinetic changes fell within the average predicted rate (data not shown). Sensitivity analysis was run in a single male individual representative of the entire population at ages 20–99 years, in 5-year steps, looking at the:
- (a)
octanol-water partition coefficient (logP) between − 5 and + 5, and its impact on age-related changes in the apparent volume of distribution;
- (b)
fraction unbound in plasma (fup) between 0.01 and 1, and its impact on age-related changes in clearance;
- (c)
hepatic blood flow between 8 and 30% of cardiac output, and its impact on age-related changes in clearance of a fictive drug being exclusively cleared hepatically;
- (d)
renal blood flow between 5 and 25% of cardiac output, and its impact on age-related changes in clearance of a fictive drug being exclusively cleared renally;
- (e)
glomerular filtration rate between 60 and 150 mL/min, and its impact on age-related changes in clearance of a fictive drug being exclusively cleared renally.
Additionally, the total clearance of the fictive drug was either assigned completely to CYP3A4, CYP2D6, CYP2C9, CYP1A2, CYP2C8, UGT1A1, to an unspecified hepatic intrinsic clearance, or to the renal clearance to investigate the impact of different clearance pathways on the age-related changes in clearance.
2.7 Verification of the Extrapolation to Aged Individuals
To verify the derived correlation between age and the investigated pharmacokinetic parameters, a literature search was performed using the MEDLINE database to screen for clinical studies comparing the pharmacokinetics in young and elderly individuals. Keywords used were ‘pharmacokinetic’ plus ‘aging’, ‘young vs. elderly’ or ‘young vs. geriatric’. Inclusion criteria were a direct comparison of the pharmacokinetics between young adults with a mean age ≤ 35 years and adults aged ≥ 40 years. The age bands were chosen to allow inclusion of middle-aged adults. The subject should apparently be healthy, with no disease conditions or medication use that could possibly alter the pharmacokinetics of the drug of interest (the included drugs and references can be found in electronic supplementary Table S-3). Included pharmacokinetic parameters were normalized to young adults and the observed data were visually compared against the prediction of the derived age-dependent functions.
To check the performed sensitivity analysis against clinically observed data, logP, fup and the main metabolizing enzyme were collected for each of the investigated drugs in the additional dataset (electronic supplementary Table S-3). Physiological parameters, such as hepatic and renal blood flow and glomerular filtration rate, important in determining drug clearance, were not usually measured in the published clinical studies and were thus calculated assuming random variability to adequately describe the general aging population [6]. Between-group comparisons of the main route of elimination (hepatic vs. renal) and route of administration (intravenous vs. oral) were performed using the Wilcoxon test after checking normal distribution, by the Shapiro–Wilk test. Analysis of variance was performed for the impact of the main metabolizing enzyme on age-related changes in clearance. Pearson’s correlation was performed for continuous variables (i.e. logP, fup, age, hepatic and renal blood flow, glomerular filtration rate).
3 Results
3.1 Predicting Drug Pharmacokinetics in the Elderly
PBPK models for all drugs were developed and adjusted in young adults (20–50 years) until simulations captured the clinically observed concentrations (the clinical studies used are listed in electronic supplementary Table S-2) visually and the observed pharmacokinetic parameters were predicted within twofold. Afterwards, drug pharmacokinetics were simulated in elderly adults aged ≥ 65 years, without any modification of drug parameters, and the predictions were compared with clinically observed data (Table 1). Ten drugs commonly used in the elderly and for which clinical pharmacokinetic data have been published in study participants older than 65 years, were investigated. The enzymes and transporters involved in the disposition of the ten investigated drugs, as well as references to clinical studies used to verify the simulations, can be found in electronic supplementary Tables S-1 and S-2.
3.1.1 Midazolam
The benzodiazepine midazolam is predominantly metabolized by CYP3A (95.2%) and, to a lesser extent, by UGT1A1 (4.7%). The remaining 0.1% of midazolam is excreted via glomerular filtration [18]. Clinically observed data of a single oral dose of midazolam (4 mg in young adults and 3–4 mg in the elderly) were contained in the 95% prediction interval for the PBPK simulations in both age groups (Fig. 2a, b). Pharmacokinetic parameters were normalized to 4 mg for comparison between both age groups. The predicted AUC was in close agreement with the observed clinical data in young (44.1 ± 23.2 ng*h/mL vs. 50.9 ± 19.1 ng*h/mL) and elderly adults (42.9 ± 17.2 ng*h/mL vs. 54.8 ± 22.5 ng*h/mL), including the observed variability [34, 35]. The ratio of elderly/young for Cmax and tmax was predicted to be within the 1.25-fold interval (predicted:observed ratio of 0.98 and 0.87, respectively), while the t½ of midazolam was overpredicted in both age groups (predicted:observed ratio of 1.30 and 1.89) [34].
Predicted versus observed concentration–time profiles for midazolam (a young, and b elderly), metoprolol (c young, and d elderly), and lisinopril (e young, and f elderly). The red markers show the observed clinical data (mean ± SD), and the solid lines, dashed line, and shaded area represent the mean of each virtual trial, the mean, and the 95% confidence interval, respectively, of all virtual individuals. Green and blue show simulations in young and elderly adults, respectively. Used clinical studies for model verification can be found in electronic supplementary Table S-2. SD standard deviation
3.1.2 Metoprolol
Metoprolol is predominantly metabolized by CYP2D6 (79.2%). Other routes of metoprolol elimination involve CYP3A (13.7%) and renal clearance (7.1%) [13]. The β-blocker metoprolol was studied as a single oral dose of 100 mg. The variability of metoprolol was covered by the PBPK model in young and elderly individuals (Fig. 2c, d). Cmax, AUC and t½ were accurately scaled to elderly individuals by the PBPK model (predicted:observed ratio, for the ratio of elderly/young, of 1.10, 1.06 and 0.97, respectively). The tmax of metoprolol was overpredicted in the elderly by twofold as the model suggested only a slight increase in the elderly compared with young adults, whereas tmax was half in the observed study [36].
3.1.3 Lisinopril
The angiotensin-converting enzyme (ACE) inhibitor lisinopril is filtered by the glomerulus and is not bound to any plasma-binding protein [37]. The predictions of a single oral dose of lisinopril (20 mg) were in close agreement to clinically observed data in young and elderly adults (Fig. 2e, f). The Cmax of lisinopril was well-predicted in young adults (predicted:observed ratio of 0.95), but Cmax was predicted to increase in the elderly by 9% and was increased in the clinical study by 51% [27, 28]. The ratio of elderly/young for tmax and AUC was accurately predicted by the model (predicted:observed ratio of 1.10 and 0.95, respectively).
3.1.4 Amlodipine
Amlodipine is metabolized by CYP3A (49.4%), an unspecified enzymatic pathway (4.0%), and eliminated unchanged by glomerular filtration (6.1%). Additionally, biliary clearance (40.5%) was implemented in the model, representing clearance of amlodipine metabolites [14]. The observed elimination phase of amlodipine (2.5 mg once daily) was well captured by the PBPK model for young and elderly individuals, including variability (Fig. 3a, b). Cmax was well predicted in both age groups (predicted:observed ratio of 0.98 and 0.92), but tmax was reached too early in the simulation (predicted:observed ratio of 0.5 in both age groups). However, the age-related increase in tmax of 14% observed in the clinical study in elderly compared with young study participants [38] was correctly captured by the model (predicted:observed ratio of 1.0). Simulated AUC and t½ were both in close agreement with the observed clinical data (predicted:observed ratio of 1.06 and 1.25 in the young, and 1.01 and 1.14 in the elderly, respectively).
Predicted versus observed concentration–time profiles for amlodipine (a young, and b elderly) and rivaroxaban (c young, and d elderly). The red markers show the observed clinical data (mean ± SD), and the solid lines, dashed line, and shaded area represent the mean of each virtual trial, the mean, and the 95% confidence interval, respectively, of all virtual individuals. Green and blue show simulations in young and elderly adults, respectively. Used clinical studies for model verification can be found in electronic supplementary Table S-2. SD standard deviation
3.1.5 Rivaroxaban
Rivaroxaban is metabolized by CYP3A4 (20.0%), CYP2J2 (15.7%), an unspecified enzymatic pathway (18.3%), and is cleared unchanged by the kidney (46.0%) [11]. A 10 mg single oral dose of the anticoagulant rivaroxaban was studied. The observed terminal elimination phase of rivaroxaban was contained within the 95% confidence interval (CI) of the PBPK model simulations for young and elderly adults (Fig. 3c, d), as were the observed ratios of Cmax, AUC and t½ for elderly/young adults (predicted:observed ratio of 0.89, 0.84, and 0.98, respectively).
3.1.6 Repaglinide
Repaglinide is mainly metabolized by CYP2C8 (75.5%) and, to a minor extent, by CYP3A4 (24.5%) [39]. The uptake of repaglinide into hepatocytes is mediated by OATP1B1 [19]. A 2 mg single oral dose of the antidiabetic drug repaglinide was simulated. The mean prediction of repaglinide was close to the mean observed, but the observed variability of repaglinide pharmacokinetics was not completely captured by the model (Fig. 4a, b). Cmax, tmax and AUC were predicted with good accuracy in young (predicted:observed ratio of 0.84. 0.86 and 1.18, respectively) and elderly adults (predicted:observed ratio of 0.80, 1.00 and 1.06, respectively). t½ was overpredicted in both age groups (predicted:observed ratio of 1.77 and 1.58), but the predicted ratio of elderly/young was in agreement with clinically observed data (predicted:observed ratio of 0.89) [40].
Predicted versus observed concentration–time profiles for repaglinide (a young, and b elderly), atorvastatin (c young, and d elderly), and rosuvastatin (e young, and f elderly). The red markers show the observed clinical data (mean ± SD), and the solid lines, dashed line, and green-shaded area represent the mean of each virtual trial, the mean, and the 95% confidence interval, respectively, of all virtual individuals. Green and blue show simulations in young and elderly adults, respectively. Used clinical studies for model verification can be found in electronic supplementary Table S-2. SD standard deviation
3.1.7 Atorvastatin
Atorvastatin is mainly metabolized by CYP3A (88.9%). Other minor excretion routes for atorvastatin are CYP2C8 (0.2%), UGT1A1 and 1A3 (both 4.9%), and renal elimination (1.1%). Atorvastatin uptake into hepatocytes is mediated by OATP1B1 [16]. The concentration–time profile of atorvastatin (40 mg single dose) was in good agreement with the observed data of young volunteers, also capturing the variability adequately (Fig. 4c). In the elderly, only one clinical pharmacokinetic study was identified, and the terminal elimination phase was underpredicted in adults aged older than 65 years (Fig. 4d). Cmax was shown to increase by 22% in the clinical study [41], but was predicted to be 6% lower by the PBPK model. The AUC was accurately predicted in young and elderly adults (predicted:observed ratio of 1.11 and 1.06). t½ was overpredicted by 42% in the young adults (observed 9.2 ± 3.6 h vs. predicted 13.1 ± 3.7 h), but was predicted adequately by the PBPK model in the elderly (predicted:observed ratio of 0.86).
3.1.8 Rosuvastatin
The uptake of rosuvastatin into hepatocytes is mediated by different uptake transporters. Rosuvastatin is cleared via enzymatic metabolism (1.8%), biliary clearance (74.6%) and renal clearance (23.6%) [17]. A single oral dose of rosuvastatin (40 mg) was captured by the model (Fig. 4e, f). The Cmax of rosuvastatin was underpredicted in young and elderly adults (predicted:observed ratio of 0.78 and 0.74), but the decrease in Cmax with age was accurately predicted for rosuvastatin (predicted:observed ratio, for the ratio of elderly/young, of 0.96). The predicted increase in AUC and t½ with adult age was in accordance with the observed clinical data (predicted:observed ratio of 1.20 and 0.87).
3.1.9 Clarithromycin
The antibiotic clarithromycin is mainly metabolized in the liver (75.6%), predominately by CYP3A and, to a lesser extent, the kidney (24.4%) [42]. Clinically observed data of oral clarithromycin 500 mg twice daily were contained in the 95% prediction interval for the PBPK simulations of young and elderly adults (Fig. 5a, b). All pharmacokinetic parameters were predicted within 1.25-fold of clinically observed data.
Predicted versus observed concentration–time profiles for clarithromycin (a young, and b elderly) and rifampicin (c young, and d elderly). The red markers show the observed clinical data (mean ± SD), and the solid lines, dashed line, and shaded area represent the mean of each virtual trial, the mean, and the 95% confidence interval, respectively, of all virtual individuals. Green and blue show simulations in young and elderly adults, respectively. Used clinical studies for model verification can be found in electronic supplementary Table S-2. SD standard deviation
3.1.10 Rifampicin
Rifampicin, a potent inducer of CYP3A, is mainly metabolized in the liver (92.6%) and only a small fraction is excreted by the kidney (7.4%) [43]. Predictions of rifampicin 300 mg twice daily were comparable with clinically observed data for young and elderly individuals (Fig. 5c, d). The AUC was accurately predicted in young (21,428 ± 7648 ng*h/mL vs. 23,608 ± 15,742 ng*h/mL) and elderly adults (37,342 ± 16,485 ng*h/mL vs. 36,793 ± 20,424 ng*h/mL). The predicted increase in Cmax, tmax and t½ was in accordance with clinically observed data (predicted:observed ratio of 0.81, 0.73 and 0.87, respectively).
3.2 Pharmacokinetic Parameters Driving Age-Related Changes in Drug Exposure
After the successful prediction of drug pharmacokinetics in adults older than 65 years of age, the developed PBPK models of all ten drugs have been used to simulate Cmax, tmax, AUC, clearance, apparent volume of distribution and t½ for individuals aged 20–99 years. The impact of adult age on Cmax was not consistent for the different drugs investigated (Fig. 6). The predicted Cmax of atorvastatin declined with age by 0.42%, whereas the Cmax of amlodipine and clarithromycin increased by 1.1% per year. The Cmax of midazolam, metoprolol and rosuvastatin did not change with advanced age. tmax showed a tendency to increase with age, but was only outside the bioequivalence criterion for lisinopril and rosuvastatin, with a maximum change of 43%. The predicted AUC showed a linear increase of 0.9% per year from the age of 20 years. Atorvastatin showed no changes in AUC with age, whereas repaglinide and lisinopril showed the highest change, with up to a 2.5-fold difference compared with young adults. Clearance decreased with age, with a more than a 1.25-fold change at the age of 55 years. Atorvastatin showed the least change with age (0.06% per year), while lisinopril showed the highest change, with a 0.84% decrease in drug clearance per year, matching the decline in the glomerular filtration rate. The apparent volume of distribution was independent of adult age for all investigated drugs. t½ increased by an average of 0.8% per year. The lowest and highest age-related change in t½ was estimated for clarithromycin (0.32% per year) and lisinopril (1.4% per year). The age-dependent changes for all investigated pharmacokinetic parameters were independent of sex.
Pharmacokinetic parameters normalized to the youngest investigated age group (20–24 years). Circles, triangles, diamonds, squares, plus symbol, cross symbol, left-, right-, and downward-pointing triangles, and dots represent midazolam, metoprolol, lisinopril, amlodipine, rivaroxaban, repaglinide, atorvastatin, rosuvastatin, clarithromycin, and rifampicin, respectively. Blue and red markers show data for men and women, respectively. The solid line and grey-shaded area display the fitted mean relationship with estimated variability between age and the pharmacokinetic parameter of interest. The grey dashed lines represent the 1.25-fold interval (bioequivalence criterion). AUC area under the curve, Cmax peak concentration, CLF clearance, tmax time to Cmax, t½ elimination half-life, VdF apparent volume of distribution
Sensitivity analysis demonstrated that predicted age-related changes in the apparent volume of distribution and drug clearance did not depend on the investigated drug characteristics (logP, fup, route of elimination), but did depend on physiological changes in hepatic and renal blood flow and glomerular filtration rate (Fig. 7).
Results of the conducted sensitivity analysis for drug (a–c) and physiological (d–f) parameters. CLint,hep intrinsic, hepatic clearance, CLF clearance, fup fraction unbound in plasma, GFR glomerular filtration rate, logP octanol-water partition coefficient, QKI renal blood flow, QLI hepatic blood flow, VdF apparent volume of distribution
If changes in pharmacokinetics with advanced age depend only on age-related physiological alterations, as determined by the conducted sensitivity analysis, our derived correlations between altered pharmacokinetics and age (Fig. 6) should hold true for any drug. In order to verify this hypothesis, a literature search was conducted to seek studies having a direct pharmacokinetic comparison between young and elderly adults. Clinically observed data for 52 additional drugs (electronic supplementary Table S-3) were gathered. The observed age-dependent pharmacokinetic alterations of this additional dataset could be appropriately described by our derived relationship (Fig. 8). Variability was underestimated for Cmax and tmax, but clinically observed data for the apparent volume of distribution, AUC, clearance and t½ were mostly contained within the estimated variability of derived age-related changes. There were no significant differences between the main route of elimination (hepatic vs. renal) and route of administration (intravenous vs. oral) for any of the investigated pharmacokinetic parameters. Age was significantly correlated for alterations in AUC, clearance and volume of distribution, but not for Cmax, tmax and t½ (Table 2). Surprisingly, there was no correlation between the lipophilicity of a drug and the clinically observed age-related changes in the volume of distribution (Figs. 7, 9). Age-related changes in drug clearance were not impacted by the extent of protein binding (Table 2) or the main enzyme responsible for drug metabolism (p value 0.31). In contrast, there was a linear correlation between hepatic blood flow and the clearance of mainly hepatically cleared drugs (correlation coefficient 0.27, 95% CI 0.05–0.48, p = 0.02), as well as a correlation between renal blood flow (correlation coefficient 0.60, 95% CI 0.30–0.79, p = 0.006) and glomerular filtration rate (correlation coefficient 0.65, 95% CI 0.38–0.82, p = 0.001) and the clearance of mainly renally cleared drugs (Fig. 9).
Verification of derived pharmacokinetic parameter changes with age against 52 additional drugs. The solid black line, grey-shaded area, and grey dashed lines represent the fitted mean relationship, the estimated variability, and the 1.25-fold interval (bioequivalence criterion), respectively. Red, blue, and green markers show drugs primarily undergoing hepatic, renal, and biliary eliminations, respectively. Triangle and circles represent intravenous and oral drug administration. References of the studied drugs can be found in electronic supplementary Table S-3. AUC area under the curve, Cmax peak concentration, CLF clearance, tmax time to Cmax, t½ elimination half-life, VdF apparent volume of distribution
Correlation between drug (logP, fup) and physiological parameters (Qxx, GFR) against age-related changes in VdF and CLF. Red, blue, and green markers show drugs primarily undergoing hepatic, renal, and biliary eliminations, respectively. Triangle and circles represent intravenous and oral drug administration. References of the studied drugs can be found in electronic supplementary Table S-3. CLF clearance, fup fraction unbound in plasma, GFR glomerular filtration rate, logP octanol-water partition coefficient, Qxx blood flow to respective organ, VdF apparent volume of distribution
4 Discussion
The elderly population is usually excluded from clinical trials, resulting in a knowledge gap regarding the effect of adult age on drug pharmacokinetics. In this study, the pharmacokinetics of ten drugs that are metabolized and excreted through different pathways were successfully predicted in the elderly using a whole-body PBPK model demonstrating the predictive power of the PBPK approach to investigate and predict drug disposition in special populations. The conducted virtual trials across adulthood elucidated that an increase in AUC of 0.9% per year from the age of 20 years is mainly determined by decreased drug clearance, which itself is caused by a progressive decrease in hepatic and renal blood flow, as well as glomerular filtration rate. Those physiological changes drive age-dependent drug exposure changes in the elderly, independent of the drug, as shown by sensitivity analysis and clinically observed data of 52 drugs additionally collected.
Predictions from our developed PBPK models for all investigated drugs compared well with observed clinical data based on visual inspection. In all cases, clinical data in the elderly were quite sparse. The predicted decline in midazolam clearance with age (i.e. − 21% by the age of 85 years, when clearance is corrected by body weight) is in agreement with the results reported by Polasek et al. [7], who investigated the influence of adult age on drugs being metabolized by a dominant hepatic CYP enzyme.
The age-dependent pharmacokinetics of metoprolol have previously been investigated in three different studies, with contradictory findings. Kendall et al. found a decline in clearance of 60% [26], which could be explained through the age-dependent changes in liver weight and hepatic blood flow [6], but two other studies reported a higher clearance in the elderly compared with young study participants [36, 44]. Metoprolol is mainly metabolized by CYP2D6, an enzyme with known genotypes, leading to distinct drug metabolizing phenotypes, which could explain the observed variability [13]. It would be of interest to conduct a clinical pharmacokinetic study for metoprolol in the elderly, using CYP2D6 genotyping, to adequately analyze the impact of age on metoprolol clearance.
We found lisinopril had the highest age-dependent impact on drug clearance, due to the pronounced age-dependent decline in the glomerular filtration rate [6]. Tubular secretion was not modelled, and it is worthwhile mentioning that tubular secretion cannot be excluded for lisinopril, although its renal clearance matches the glomerular filtration rate [45]. The rate of absorption was predicted too rapidly for amlodipine and rivaroxaban; however, the observed Cmax were well predicted and the observed terminal elimination phases were contained in the 95% prediction interval of the PBPK model simulations for both drugs. The reason for the slower absorption rate observed in clinical studies could be the formulation, and more sophisticated absorption models [46] might improve the prediction of tmax. Nevertheless, the tmax ratio for the elderly/young was exactly predicted by the PBPK model (predicted:observed ratio of 1.0 for both drugs) and was thus judged to be sufficient for age-dependent analysis. Across the ten drugs studied, the drug clearance of atorvastatin was least impacted by age, which is likely explained by the fact that metabolism in the intestine is least impacted by adult age and extensive intestinal metabolism is a key characteristic of atorvastatin [47].
Overall, the pharmacokinetics of the ten drugs selected for model qualification were adequately predicted in the elderly, confirming the predictive power of the PBPK approach in special populations, which was previously shown for seven model drugs of CYP enzymes [10], as well as for morphine and furosemide administered intravenously [9]. The AUC was generally predicted to increase progressively, with an average rate of 0.9% per year from the age of 20 years. Despite the higher drug exposure in the elderly, dose adjustment based on age alone is not recommended in the labelling of any of the ten studied drugs. The age-related changes in AUC can be explained by the linear decrease in drug clearance as a result of reduced hepatic and renal blood flow and glomerular filtration rate [6], rather than drug characteristics (i.e. logP, fup, main metabolizing enzyme, main route of elimination), as shown by our conducted sensitivity analysis (Fig. 7) and statistical analysis of clinically observed age-dependent pharmacokinetic alterations of 52 drugs additionally collected (Fig. 9). These findings are contrary to conventional thinking. It is believed that volume of distribution changes with advanced age because of altered body composition [6], which would suggest a higher volume of distribution for lipophilic drugs and a lower volume of distribution for hydrophilic drugs. Indeed, there are contradictory findings in the literature regarding altered [48,49,50] and unaltered volume of distributions [51,52,53] with advanced age.
Cusack et al. investigated digoxin pharmacokinetics in the elderly and found that the volume of distribution was not different when corrected for body weight [54], which was used in our study. Contradictory findings were also found for the main metabolizing enzyme of a drug. Age was found to be a significant covariate for CYP2C9 only in a compact meta-analysis looking at hepatic CYP abundance [55]. However, in a study investigating probe substrates, age dependency was detected for CYP1A2, CYP2D6 and CYP2E1, but not CYP2C9 [56]. In contrast, in a third study, CYP1A2 activity was reported to be independent of aging [57]. Taken together, the small sample sizes generally used for analysis, and the high biological variability (e.g. for hepatic CYP abundance), prevented a meaningful investigation regarding the impact of drug characteristics on age-related changes in drug pharmacokinetics. In our compact analysis, we combined clinically observed data and PBPK modelling to demonstrate that the described drug characteristics do not significantly impact age-related pharmacokinetic changes.
Blood flows were not usually measured in the clinical studies of the 52 drugs additionally collected and hence were estimated based on observed data of reduced hepatic and renal blood flow with advanced age [6], and thus was a limitation of this study. However, Vestal et al. measured age-related changes in propranolol clearance, a purely passively distributed drug, and determined hepatic blood flow alterations in the same subjects, showing a linear correlation and supporting the results of our study [58]. The glomerular filtration rate is often determined by measuring creatinine clearance, which is not an ideal marker in the elderly due to senile sarcopenia in aging subjects [59], but linear correlations between the rate of glomerular filtration alterations and age-related changes in clearance of mainly renally excreted drugs were shown in numerous different studies [53, 60, 61], supporting the findings of the present work.
Importantly, the impact of age was independent of sex for all investigated drugs, which is in line with clinically observed data [34, 41, 62]. However, the pharmacokinetics can differ between females and males [63], and therefore more research is needed to investigate the impact of sex in combination with aging.
A clear limitation of our study is that clinical pharmacokinetic data for individuals older than 85 years of age are sparse and thus the simulation results in this age group need to be interpreted with caution. Furthermore, clinical data, as well as the virtual population used in this study, represent healthy elderly individuals. It is known that certain comorbidities, for instance renal impairment [64], liver cirrhosis [65] or heart disease [66], can change physiology and thus impact drug pharmacokinetics. Whether these physiological changes are more pronounced in elderly adults needs to be determined.
It should be emphasized that age-related changes in physiology and biology do not just impact pharmacokinetics but also modify the pharmacodynamics of drugs, resulting in altered drug effects. Differences in the effects of drugs in the elderly can be explained by changes in the number of receptors, the affinity of the drugs to its receptor, and changes in physiological and homeostatic processes with aging [67]. Elderly adults are more sensitive to the sedative effects of benzodiazepines such as midazolam. The dose of midazolam required to reach comparable sedation, such as in young adults below the age of 50 years, was shown to be halved in the elderly [68]. Conversely, the sensitivity to the β-adrenergic receptor, and therefore the pharmacodynamic effect of β-blockers such as metoprolol, is reduced in the elderly as a result of receptor downregulation or alterations in binding affinity [67]. Age-related differences in the antihypertensive effect of lisinopril appear to not be clinically relevant [69]. Conversely, the decrease in systolic blood pressure after amlodipine administration was shown to be greater in the elderly compared with young patients, while the decrease in diastolic blood pressure was similar in both groups [38, 70]. Rivaroxaban has a well-established correlation between plasma concentration and FXa activity and prothrombin time prolongation [71]. Thus, the higher plasma concentration in the elderly may lead to a more pronounced pharmacodynamic effect compared with young adults [72]. Statins appear to have the same pharmacodynamic effect in the elderly compared with young adults [73]. Taken together, clinical management of elderly individuals should consider not only age-related pharmacokinetic changes but also age-related pharmacodynamic changes and the presence of comorbidities, all of which predispose elderly individuals to inappropriate prescribing.
5 Conclusions
The PBPK approach demonstrated its usefulness to predict drug disposition in special populations such as elderly individuals. Furthermore, conducting virtual clinical trials across the entire adult population in combination with clinically observed data in young and older subjects elucidated that drug elimination rather than absorption or distribution is likely responsible for age-related drug exposure changes in the elderly. When treating elderly individuals, it is assumed that a dose reduction of 25–50% is appropriate, independent of the drug [74]. This empirical rule of thumb was shown to be justified through the PBPK simulations and a meta-analysis of existing literature data. Drug clearance decreased with age, with no more than a threefold difference between the youngest and oldest studied age group. Our PBPK model, in combination with the statistical analysis of the clinically observed pharmacokinetic data of 52 drugs additionally collected, showed that the age-related physiological decrease in hepatic and renal blood flow and glomerular filtration rate, rather than drug characteristics, are responsible for drug exposure changes in the elderly. Furthermore, age-related differences in drug exposure were outside of the expected interindividual variability (defined as the 1.25-fold interval) at 55 years of age compared with the youngest studied age group. Importantly, this finding can inform future clinical trials aiming to understand the pharmacokinetic differences in older versus younger adults. Additionally, pharmacodynamic alterations and the presence of comorbidities should be considered when prescribing treatments in the elderly.
References
Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. Washington, DC: United States Census Bureau, Economics and Statistics Administration; 2014.
European Union—Eurostats. People in the EU—population projections; 2017. https://ec.europa.eu/eurostat/statistics-explained/index.php/People_in_the_EU_-_population_projections#Age_dependency_ratios. Cited 6 Feb 2019.
Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. 2017;5:335–41.
Eurostat. Medicine use statistics; 2014. https://ec.europa.eu/eurostat/statistics-explained/index.php/Medicine_use_statistics. Cited 15 Nov 2018.
US Food and Drug Administration. Diversity in clinical trials; 2018. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm535306.htm. Cited 15 Nov 2018.
Stader F, Siccardi M, Battegay M, Kinvig H, Penny MA, Marzolini C. Repository describing an aging population to inform physiologically based pharmacokinetic models considering anatomical, physiological, and biological age-dependent changes. Clin Pharmacokinet. 2019;58(4):483–501.
Polasek TM, Patel F, Jensen BP, Sorich MJ, Wiese MD, Doogue MP. Predicted metabolic drug clearance with increasing adult age. Br J Clin Pharmacol. 2013;75(4):1019–28.
Burt HJ, Riedmaier AE, Harwood MD, Crewe HK, Gill KL, Neuhoff S. Abundance of hepatic transporters in Caucasians: a meta-analysis. Drug Metab Dispos. 2016;44(10):1550–61.
Schlender J-F, Meyer M, Thelen K, Krauss M, Willmann S, Eissing T, et al. Development of a whole-body physiologically based pharmacokinetic approach to assess the pharmacokinetics of drugs in elderly individuals. Clin Pharmacokinet. 2016;55(12):1573–89.
Chetty M, Johnson TN, Polak S, Salem F, Doki K, Rostami-Hodjegan A. Physiologically based pharmacokinetic modelling to guide drug delivery in older people. Adv Drug Deliv Rev. 2018;135:85–96.
Stader F, Penny MA, Siccardi M, Marzolini C. A comprehensive framework for physiologically based pharmacokinetic modelling in Matlab®. CPT Pharmacomet Syst Pharmacol. 2019;8(7):444–59.
Rowland-Yeo K, Jamei M, Rostami-Hodjegan A. Predicting drug–drug interactions: application of physiologically based pharmacokinetic models under a systems biology approach. Expert Rev Clin Pharmacol. 2013;6(2):143–57.
Chetty M, Rose RH, Abduljalil K, Patel N, Lu G, Cain T, et al. Applications of linking PBPK and PD models to predict the impact of genotypic variability, formulation differences, differences in target binding capacity and target site drug concentrations on drug responses and variability. Front Pharmacol. 2014;5(258):1–14.
Mukherjee D, Zha J, Menon RM, Shebley M. Guiding dose adjustment of amlodipine after co-administration with ritonavir containing regimens using a physiologically-based pharmacokinetic/pharmacodynamic model. J Pharmacokinet Pharmacodyn. 2018;45(3):443–56.
Marzolini C, Rajoli R, Battegay M, Elzi L, Back D, Siccardi M. Physiologically based pharmacokinetic modeling to predict drug–drug interactions with efavirenz involving simultaneous inducing and inhibitory effects on cytochromes. Clin Pharmacokinet. 2017;56(4):409–20.
Zhang T. Physiologically based pharmacokinetic modeling of disposition and drug–drug interactions for atorvastatin and its metabolites. Eur J Pharm Sci. 2015;77:216–29.
Jamei M, Bajot F, Neuhoff S, Barter Z, Yang J, Rostami-Hodjegan A, et al. A mechanistic framework for in vitro–in vivo extrapolation of liver membrane transporters: prediction of drug–drug interaction between rosuvastatin and cyclosporine. Clin Pharmacokinet. 2014;53(1):73–87.
Rowland-Yeo K, Walsky R, Jamei M, Rostami-Hodjegan A, Tucker G. Prediction of time-dependent CYP3A4 drug–drug interactions by physiologically based pharmacokinetic modelling: impact of inactivation parameters and enzyme turnover. Eur J Pharm Sci. 2011;43(3):160–73.
Varma MV, Lai Y, Kimoto E, Goosen TC, El-Kattan AF, Kumar V. Mechanistic modeling to predict the transporter-and enzyme-mediated drug–drug interactions of repaglinide. Pharm Res. 2013;30(4):1188–99.
Faulkner J, McGibney D, Chasseaud L, Perry J, Taylor I. The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily. Br J Clin Pharmacol. 1986;22(1):21–5.
Stader F, Kinvig H, Battegay M, Khoo S, Owen A, Siccardi M, et al. Analysis of clinical drug–drug interaction data to predict uncharacterized interaction magnitudes between antiretroviral drugs and co-medications. Antimicrob Agents Chemother. 2018;62(7):1–12.
Zhu Y, Wang F, Li Q, Zhu M, Du A, Tang W, et al. Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation. Drug Metab Dispos. 2014;42(2):245–9.
Varma MV, Lai Y, Feng B, Litchfield J, Goosen TC, Bergman A. Physiologically based modeling of pravastatin transporter-mediated hepatobiliary disposition and drug–drug interactions. Pharm Res. 2012;29(10):2860–73.
Almond LM, Mukadam S, Gardner I, Okialda K, Wong S, Hatley O, et al. Prediction of drug–drug interactions arising from CYP3A induction using a physiologically based dynamic model. Drug Metab Dispos. 2016;44(6):821–32.
Ke A, Barter Z, Rowland-Yeo K, Almond L. Towards a best practice approach in PBPK modeling: case example of developing a unified efavirenz model accounting for induction of CYPs 3A4 and 2B6. CPT Pharmacomet Syst Pharmacol. 2016;5(7):367–76.
Kendall M, Brown D, Yates R. Plasma metoprolol concentrations in young, old and hypertensive subjects. Br J Clin Pharmacol. 1977;4(4):497–9.
Gautam P, Vargas E, Lye M. Pharmacokinetics of lisinopril (MK521) in healthy young and elderly subjects and in elderly patients with cardiac failure. J Pharm Pharmacol. 1987;39(11):929–31.
Gomez HJ, Cirillo VJ, Moncloa F. The clinical pharmacology of lisinopril. J Cardiovasc Pharmacol. 1987;9:S27–34.
Ulm E, Hichens M, Gomez H, Till A, Hand E, Vassil T, et al. Enalapril maleate and a lysine analogue (MK-521): disposition in man. Br J Clin Pharmacol. 1982;14(3):357–62.
Sagirli O, Ersoy L. An HPLC method for the determination of lisinopril in human plasma and urine with fluorescence detection. J Chromatogr B. 2004;809(1):159–65.
Acocella G, Pagani V, Marchetti M, Baroni G, Nicolis F. Kinetic studies on rifampicin. I. Serum concentration analysis in subjects treated with different oral doses over a period of two weeks. Chemotherapy. 1971;16(6):356–70.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(13):1–10.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135–59.
Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology. 1984;61(1):27–35.
Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of HIV protease inhibitors 1: inactivation, induction and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39(6):1070–8.
Quarterman C, Kendall M, Jack D. The effect of age on the pharmacokinetics of metoprolol and its metabolites. Br J Clin Pharmacol. 1981;11(3):287–94.
Obach RS, Lombardo F, Waters NJ. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab Dispos. 2008;36(7):1385–405.
Abernethy DR, Gutkowska J, Lambert MD. Amlodipine in elderly hypertensive patients: pharmacokinetics and pharmacodynamics. J Cardiovasc Pharmacol. 1988;12:S67–71.
Kudo T, Goda H, Yokosuka Y, Tanaka R, Komatsu S, Ito K. Estimation of the contribution of CYP2C8 and CYP3A4 in repaglinide metabolism by human liver microsomes under various buffer conditions. J Pharm Sci. 2017;106(9):2847–52.
Hatorp V, Huang W-C, Strange P. Repaglinide pharmacokinetics in healthy young adult and elderly subjects. Clin Ther. 1999;21(4):702–10.
Gibson DM, Bron NJ, Richens MA, Hounslow NJ, Sedman AJ, Whitfield LR. Effect of age and gender on pharmacokinetics of atorvastatin in humans. J Clin Pharmacol. 1996;36(3):242–6.
Rodvold KA. Clinical pharmacokinetics of clarithromycin. Clin Pharmacokinet. 1999;37(5):385–98.
Acocella G. Clinical pharmacokinetics of rifampicin. Clin Pharmacokinet. 1978;3(2):108–27.
Regårdh C, Landahl S, Larsson M, Lundborg P, Steen B, Hoffmann K-J, et al. Pharmacokinetics of metoprolol and its metabolite α-OH-metoprolol in healthy, non-smoking, elderly individuals. Eur J Clin Pharmacol. 1983;24(2):221–6.
Beermann B. Pharmacokinetics of lisinopril. Am J Med. 1988;85(3):25–30.
Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab Pharmacokinet. 2009;24(1):53–75.
Lennernäs H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42(13):1141–60.
Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, Mezey E. Aging and ethanol metabolism. Clin Pharmacol Ther. 1977;21(3):343–54.
Redolfi A, Borgogelli E, Lodola E. Blood level of cimetidine in relation to age. Eur J Clin Pharmacol. 1979;15(4):257–61.
Abernethy D, Greenblatt D, Shader R. Imipramine and desipramine disposition in the elderly. J Pharmacol Exp Ther. 1985;232(1):183–8.
Castleden CM, George CF. The effect of ageing on the hepatic clearance of propranolol. Br J Clin Pharmacol. 1979;7(1):49–54.
Jaillon P, Gardin M, Lecocq B, Richard M, Meignan S, Blondel Y, et al. Pharmacokinetics of nalbuphine in infants, young healthy volunteers, and elderly patients. Clin Pharmacol Ther. 1989;46(2):226–33.
Rho JP, Jones A, Woo M, Castle S, Smith K, Bawdon RE, et al. Single-dose pharmacokinetics of intravenous ampicillin plus sulbactam in healthy elderly and young adult subjects. J Antimicrob Chemother. 1989;24(4):573–80.
Cusack B, Kelly J, O’Malley K, Noel J, Lavan J, Horgan J. Digoxin in the elderly: pharmacokinetic consequences of old age. Clin Pharmacol Ther. 1979;25(6):772–6.
Achour B, Russell MR, Barber J, Rostami-Hodjegan A. Simultaneous quantification of the abundance of several cytochrome P450 and uridine 5′-diphospho-glucuronosyltransferase enzymes in human liver microsomes using multiplexed targeted proteomics. Drug Metab Dispos. 2014;42(4):500–10.
Parkinson A, Mudra D, Johnson C, Dwyer A, Carroll K. The effects of gender, age, ethnicity, and liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol. 2004;199(3):193–209.
Simon T, Becquemont L, Hamon B, Nouyrigat E, Chodjania Y, Poirier J, et al. Variability of cytochrome P450 1A2 activity over time in young and elderly healthy volunteers. Br J Clin Pharmacol. 2001;52(5):601–4.
Vestal R, Wood A, Branch R, Shand D, Wilkinson G. Effects of age and cigarette smoking on propranolol disposition. Clin Pharmacol Ther. 1979;26(1):8–15.
Musso CG, Oreopoulos DG. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011;119(Suppl. 1):1–5.
Hockings N, Ajayi A, Reid J. Age and the pharmacokinetics of angiotensin converting enzyme inhibitors enalapril and enalaprilat. Br J Clin Pharmacol. 1986;21(4):341–8.
Faulkner R, Bohaychuk W, Lanc R, Haynes J, Desjardins R, Yacobi A, et al. Pharmacokinetics of cefixime in the young and elderly. J Antimicrob Chemother. 1988;21(6):787–94.
Kubitza D, Becka M, Roth A, Mueck W. The influence of age and gender on the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor. J Clin Pharmacol. 2013;53(3):249–55.
Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–57.
Rowland Yeo K, Aarabi M, Jamei M, Rostami-Hodjegan A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev Clin Pharmacol. 2011;4(2):261–74.
Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49(3):189–206.
Rodighiero V. Effects of cardiovascular disease on pharmacokinetics. Cardiovasc Drugs Ther. 1989;3(5):711–30.
Wooten JM. Pharmacotherapy considerations in elderly adults. South Med J. 2012;105(8):437–45.
Albrecht S, Ihmsen H, Hering W, Geisslinger G, Dingemanse J, Schwilden H, et al. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther. 1999;65(6):630–9.
Goa KL, Balfour JA, Zuanetti G. Lisinopril. A review of its pharmacology and clinical efficacy in the early management of acute myocardial infarction. Drugs. 1996;52(4):564–88.
Leenen FH, Coletta E. Pharmacokinetic and antihypertensive profile of amlodipine and felodipine-ER in younger versus older patients with hypertension. J Cardiovasc Pharmacol. 2010;56(6):669–75.
Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78(4):412–21.
Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin. 2008;24(10):2757–65.
Szadkowska I, Stanczyk A, Aronow WS, Kowalski J, Pawlicki L, Ahmed A, et al. Statin therapy in the elderly: a review. Arch Gerontol Geriatr. 2010;50(1):114–8.
Vass M, Hendriksen C. Medication for older people. Z Gerontol Geriatr. 2005;38(3):190–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Swiss National Foundation (Grant number 166204), the OPO Foundation, and the Isaac Dreyfus Foundation. Melissa A. Penny was additionally supported by the Swiss National Foundation Professorship (PP00P3 170702).
Conflict of interest
Felix Stader, Hannah Kinvig, Melissa A. Penny, Manuel Battegay, Marco Siccardi, and Catia Marzolini have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stader, F., Kinvig, H., Penny, M.A. et al. Physiologically Based Pharmacokinetic Modelling to Identify Pharmacokinetic Parameters Driving Drug Exposure Changes in the Elderly. Clin Pharmacokinet 59, 383–401 (2020). https://doi.org/10.1007/s40262-019-00822-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00822-9