Abstract
Background and Objective
Ankylosing spondylitis is a chronic, progressive, inflammatory, multidimensional, musculoskeletal disease primarily involving the axial skeleton. In addition, ankylosing spondylitis is associated with increased morbidity and mortality, significantly affecting productivity and overall quality of life. The aim of the present study was to evaluate the cost effectiveness of tofacitinib compared to currently marketed biologic treatment in patients with active ankylosing spondylitis who have responded inadequately to conventional therapy (biologic-naïve population) or previous biologic therapy (biologic-experienced population) in Greece.
Methods
A published model comprising a decision tree and a three-state Markov model was adapted from a public payer perspective over a lifetime horizon. Adalimumab and secukinumab, having the highest market shares among biologics for the treatment of ankylosing spondylitis in Greece (standard practice), were selected as comparators in the analysis. Clinical parameters captured treatment response defined per Assessment of Spondyloarthritis International Society 20 response, short-term and long-term changes in Bath Ankylosing Spondylitis Disease Activity Index and Bath Ankylosing Spondylitis Functional Index scores, long-term biologic treatment discontinuation, and adverse events. Efficacy, safety data, and utility values were elicited from the published literature. Direct costs pertaining to drug acquisition, monitoring, adverse events, and disease management costs were considered in the analysis (€2022). Model outcomes were patients’ quality-adjusted life-years, total costs, and incremental cost-effectiveness ratios. All future outcomes were discounted at 3.5% per annum. A probabilistic sensitivity analysis was conducted to account for model uncertainty.
Results
In a biologic-naïve population, compared with adalimumab, tofacitinib produced an estimated 0.06 additional quality-adjusted life-years [QALYs] (10.67 vs 10.73), at additional costs of €2403 (€147,096 vs €149,500) resulting in an incremental cost-effectiveness ratio of €41,378 per QALY gained. In a biologic-experienced population, the total cost per patient for tofacitinib and secukinumab was estimated to be €151,371 and €145,757, respectively. In terms of health outcomes, tofacitinib was associated with a 0.13 increment in QALYs compared with secukinumab resulting in an incremental cost-effectiveness ratio of €42,784 per QALY gained. The probabilistic sensitivity analysis confirmed the deterministic results for both populations.
Conclusions
Tofacitinib was estimated to be a cost-effective option for the treatment of active ankylosing spondylitis in Greece for both biologic-naive and biologic-experienced patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ankylosing spondylitis is a chronic, progressive, inflammatory, multidimensional, musculoskeletal disease primarily involving the axial skeleton. |
The present study evaluates the cost effectiveness of tofacitinib compared to currently marketed biologic treatment in Greek patients with active ankylosing spondylitis. |
Tofacitinib was estimated to be a cost-effective option for the treatment of active ankylosing spondylitis in Greece for both biologic-naive and biologic-experienced patients. |
1 Introduction
Ankylosing spondylitis (AS) is a chronic, progressive, inflammatory, multidimensional, musculoskeletal disease primarily involving the axial skeleton [1]. Similarly, to other chronic diseases, AS is associated with increased morbidity and mortality, significantly affecting productivity and overall quality of life [2,3,4].
In Greece, the prevalence rate of AS ranges between 0.24% [5] and 0.29% [6] and is found to be more common among male than female individuals, with a mean age of AS onset at 25.83 (± 6.5) years [5]. Moreover, real-world evidence using the country-wide electronic prescription database on 42,815 Greek patients with inflammatory rheumatic disease showed a higher burden of depression and anxiety among patients with AS compared with patients with psoriatic arthritis and rheumatoid arthritis [7].
The ultimate goal of AS treatment is to achieve clinical remission/inactive disease [8, 9]. Following the Greek therapeutic protocol, in line with the European League Against Rheumatism recommendations, the current treatment option for AS is non-steroidal anti-inflammatory drugs (NSAIDs), as first-line therapy that can be effective for the treatment of pain and stiffness. However, a significant proportion of patients do not achieve substantial clinical improvements with NSAIDs, and most require biological treatment [8, 9], including tumor necrosis factor and interleukin-17 inhibitors. Nevertheless, many patients do not achieve disease activity/treatment targets (remission/inactive disease), and efficacy is not sustained over time; consequently, more effective therapies are needed to maximize patient outcomes [10,11,12,13]. Evidence from Greece also points out the limited efficacy of current treatment options as well as high treatment discontinuation rates among patients with AS, primarily because of treatment inefficacy and adverse events (AEs).
Despite the progress in the treatment of AS, there is an unmet clinical need in terms of achieving and maintaining treatment goals in real-world settings. Taking into consideration this unmet need, new Janus kinase inhibitors have exhibited promising results for the treatment of AS in a number of phase III trials [14, 15].
More recently, tofacitinib citrate (Xeljanz®), an oral Janus kinase inhibitor with functional selectivity for cytokine receptors associated with Janus kinase 1 and/or Janus kinase 3 over receptors that signal through pairs of Janus kinase 2, has been approved by the European Medicines Agency for adult patients with AS who have responded inadequately to conventional therapy.
Two clinical trials (phase III A3921120 [NCT03502616] [16] and phase II A3921119 [NCT01786668]) [17] demonstrated the efficacy and safety of tofacitinib compared with conventional care (CC). The A3921120 study met its primary endpoint, showing that the percentage of patients achieving an Assessment of SpondyloArthritis International Society 20 (ASAS20) response at week 16 was significantly greater with tofacitinib (56.4%) versus placebo (29.4%) [p < 0.0001]. In addition, the percentage of ASAS40 responses was significantly greater with tofacitinib (40.6%) versus placebo (12.5%) [p < 0.0001], a key secondary endpoint of the study.
Although tofacitinib has an established clinical profile and represents a promising oral advanced therapy for the management of AS, it also imposes a tangible cost to the healthcare system and payers. In this light, the objective of the present study was to evaluate the cost effectiveness of tofacitinib compared to currently marketed biologic treatment in patients with active AS who have responded inadequately to conventional therapy in Greece.
2 Methods
A cohort modeling approach combining a decision tree model in the first 16 weeks and a three-state Markov model for the remainder of modeled time horizon, with a cycle length of 16 weeks was locally adapted from a Greek public payer perspective. Model-extrapolated outcomes included patients’ patient quality-adjusted life-years (QALYs), total costs per patient, and the incremental cost-effectiveness ratio (ICER) per QALY gained. An annual discounting of 3.5% was applied for both effectiveness and cost estimations as often used in such studies [18, 19]. Moreover, the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) was followed in reporting the cost-effectiveness analysis results of tofacitinib compared with available comparators.
2.1 Patient Population, Interventions, and Comparators
The target population was adult patients with active AS based on the modified New York Criteria for AS despite NSAID therapy or adult patients who are intolerant to NSAIDs, including both biologic disease-modifying anti-rheumatic drugs (bDMARD-naïve) and bDMARD/tumor necrosis factor-alfa inhibitor-inadequate response (IR) populations. The definition of the target population was in line with that of the intention-to-treat population of the phase III A3921120 clinical trial for tofacitinib (including a mix of bDMARD-naïve [approximately 77%] and bDMARD/tumor necrosis factor-alfa inhibitor-IR [23%] populations) [16]. The economic model considered two distinct subpopulations of patients with active AS: (1) bDMARD-naïve patients and (2) bDMARD/tumor necrosis factor-alfa inhibitor-IR patients.
The relevant comparators to tofacitinib in the bDMARD-naïve and bDMARD/tumor necrosis factor-alfa inhibitor-IR populations were reflective of local clinical practice, taking into consideration the availability of clinical data to allow a robust economic evaluation. More specifically, for the bDMARD-naïve population, the comparator was adalimumab, while for the bDMARD/tumor necrosis factor-alfa inhibitor-IR population, the comparator was secukinumab. These comparisons were chosen on the grounds that adalimumab and secukinumab are highly effective and widely tested therapies in routine clinical practice, representing the most marketed biological therapies for the treatment of AS in Greece (standard practice). Moreover, the same approach has been used in similar cost-effectiveness studies conducted in Greece in other disease areas [20,21,22,23,24]. Tofacitinib, adalimumab, and secukinumab dose and frequency of administration were modeled according to European Medicines Agency licensed dosing schedules that are commonly followed by Greek clinical practice.
2.2 Model Description
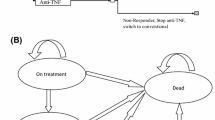
The current model is an adapted version of the York model in AS and similar to prior models used to demonstrate the cost effectiveness of treatment interventions in the National Institute for Health and Care Excellence (NICE) submission [25, 26]. A cohort was modeled to initially enter a decision-tree model to determine response to treatment at 16 weeks, followed by entry into a Markov state-transition model comprising three health states for on biologic treatment, CC, and death (Fig. 1).
Treatment response was assessed at 16 weeks, when these patients were categorized as responders or non-responders. The 16-week timeframe was in line with the definition of the primary endpoint in the phase III A3921120 trial for tofacitinib (i.e., percentage of patients achieving a ASAS20 response at week 16) [16].
Non-responders to biologic treatment at 16 weeks would then enter the ‘CC’ health state of the Markov model, assuming that these patients would discontinue the initial biologic treatment upon treatment failure and would not switch to another biologic treatment. In contrast to non-responders who would enter the ‘CC’ health state at 16 weeks, responders to biologic treatment would enter the ‘on biologic therapy’ health state. These patients would then be at the risk of treatment discontinuation and would enter the ‘CC’ health state upon treatment discontinuation, assuming no sequential treatment (Fig. 1).
It is important to note that patients withdrawing from their initial biologic therapy may switch to a sequential biologic treatment [9]. However, despite the relevance of treatment sequences from a real-world clinical practice perspective, there was a paucity of clinical evidence in the bDMARD/tumor necrosis factor-alfa inhibitor-IR population and a lack of clinical evidence for sequential treatment strategies. As a result, modeling sequential treatment strategies would have required extra assumptions without clear evidence for or against, which would have introduced more uncertainties. Therefore, sequential treatment strategies were not considered in the model, owing to the difficulty of interpretation. This assumption was also used in similar published cost-effectiveness studies [27, 28].
The model tracked the progression of AS by modeling both the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Bath Ankylosing Spondylitis Disease Activity Index (BASFI) scores over time, which are commonly used to capture disease activity and physical functioning, respectively, in patients with AS. The modeled BASDAI and BASFI scores in each model cycle were then used to predict the costs and utilities in that model cycle. A half-cycle correction was applied.
2.3 Model Inputs and Parameters
2.3.1 Short-Term and Long-Term Health Outcomes and Treatment Discontinuation
Short-term health effects over the initial 16-week treatment period were captured by ASAS20 and used to define responder’s versus non-responders at 16 weeks, in line with the definition of the primary endpoint for the tofacitinib phase III A3921120 study (i.e., percentage of patients achieving an ASAS20 response at week 16) [16] (Table 1). In the absence of head-to-head clinical trials of tofacitinib compared to adalimumab and secukinumab, a network meta-analysis was conducted to examine the relative treatment effect on response rates for the comparators using the CC (placebo) arm as the reference treatment arm (Electronic Supplementary Material [ESM]).
Disease progression was demonstrated by changes in BASDAI and BASFI scores. Baseline BASDAI and BASFI scores were based on baseline scores from the A3921120 clinical trial [16] and were applied in the model separately for the bDMARD-naïve and bDMARD/tumor necrosis factor-alfa inhibitor-IR populations. In the first 16 weeks of the model, patients would experience improvements in both BASDAI and BASFI scores, conditional on the response status at 16 weeks, regardless of the treatment arm. Responders would experience larger improvements in both BASDAI and BASFI scores compared with non-responders, in line with NICE Technology Appraisal (TA) 407 [26] and NICE TA383 [25].
In addition to the short-term effects on BASDAI and BASFI scores, the model captured the impact of treatment on long-term disease progression. For BASDAI scores, conditional changes from baseline at week 16 were assumed to be maintained while patients remained in the ‘on biologic therapy’ health state. Upon discontinuation of biologic therapy, the BASDAI score was assumed to rebound to baseline.
Patients with AS experience progressive deterioration of function over time, dependent on the extent of disease activity and radiographic progression. This was captured as long-term progression in the BASFI score. As the BASDAI score was assumed constant in the long term, natural progression of BASFI score was modeled as dependent on radiographic progression measured by the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) using the following equation. The annual linear change of the BASFI score for biologic treatment (0.034) = annual linear change of mSASSS change (1.440×0.42) × BASFI change with 1 unit change in mSASSS (0.057). More specifically, the annual linear change of BASFI score was calculated as a product of the annual linear change of mSASSS and the independent effect of a 1-unit change in mSASSS on the BASFI score. In line with NICE TA407 [26] and NICE TA383 [25], the independent effect of a 1-unit change in mSASSS on the BASFI score (0.057) was based on the coefficient in the multivariate model reported in Landewé et al. [29] Assuming that patients receiving biologic treatment were associated with a slower rate of mSASSS change compared with patients receiving CC, a relative rate (0.42) from Haroon et al. [30] was applied to the annual change in mSASSS for CC (i.e., 1.440) to derive the annual change in mSASSS in patients receiving biologic treatment, regardless of the biologic type (Table 1).
The change in BASDAI scores beyond 16 weeks was specific for patients who responded to the initial biologic treatment at 16 weeks and therefore remained on the biologic treatment. These patients were at risk of biologic discontinuation beyond 16 weeks and therefore a rebound in BASFI scores upon discontinuation.
Moreover, the annual withdrawal rate was also considered in the model. More specially for patients initially treated with any biologic treatment, patients who were defined as responders at 16 weeks were assumed to remain on that treatment until discontinuation. Consistent with NICE TA383 [25] and preferred by the evidence review group (ERG) in NICE TA407 [26], the same long-term discontinuation probability (11% per year) was applied to all the biologic treatment arms, regardless of drug class.
2.3.2 Safety
Adverse events were included in the model in terms of the associated impact on the costs. In line with NICE TA407 [26] and NICE TA383 [25], only serious infections (categorized as tuberculosis reactivation and other serious infections) were included in the model. These AEs were selected because of the increased risk of infection with biologic therapies [31]. The distribution of serious infections was assumed to be 5% tuberculosis and 95% other serious infections, in line with the assumption adopted in NICE TA407 [26]. Moreover, the AE rates (per 100 patient-years) reported in the trial publications were converted to per model-cycle probabilities of AE, which were then applied for as long as patients remained taking specific treatment.
2.3.3 Mortality
Patients were at risk of AS mortality at any timepoint in the model. Age- and sex-adjusted AS mortality estimates were derived by applying the sex-specific relative risks of death in the AS population (male 1.63; female 1.38) [32] versus the general population to the age- and sex-dependent mortality estimates of the general Greek population (World Health Organization National Life Tables for Greece [33]).
2.3.4 Utility Inputs
To estimate the total QALYs per patient over time, the proportion of patients alive in each model cycle was multiplied by the utility at that timepoint. In line with NICE TA407 [26] and NICE TA383 [25], to account for the negative impact of progressed disease activity and physical functioning on patients’ quality of life, the utility value in each model cycle was typically modeled based on a multivariable regression model. More specifically, BASDAI, BASFI, sex, and age were typically included as covariates in the regression models for utilities in the prior NICE TAs. Moreover, consistent with NICE TA407 [26] and NICE TA383 [25], it was assumed that AEs were associated with no utility decrement in the base-case analysis.
2.3.5 Resource Use and Cost Data
Healthcare resource use and cost inputs relating to drug acquisition, administration, monitoring, AEs, and disease management were considered in the model. Because the analysis was conducted from the public payer perspective, only direct medical costs that are reimbursed in the context of the public sector were accounted for. The cost estimation was performed by applying local unit costs to the volume of resources needed for patients’ care. All unit costs correspond to the year of analysis, namely 2022 in €.
More specifically, drug acquisition costs were calculated by combining the dose of each agent with the reimbursed drug unit cost, as derived following current legislation, and publicly available price data in Greece. The reimbursed drug costs were calculated on the grounds of the ex-factory prices, as they were published in the latest price bulletin issued by the Greek Ministry of Health [34] and after also applying the relevant mandatory minimum discounts provided in the corresponding legislation (Table 2). The drugs’ dose and frequency of administration were based on the Summary of Product Characteristics reported by the European Medicines Agency.
Moreover, following local clinical practice, it was assumed that all patients were able to self-administer subcutaneous injections and oral tablets and, hence, no administration costs were accrued for adalimumab, tofacitinib, and secukinumab. Disease-related costs including accounting for AS disease management costs were estimated using the exponential BASFI regression mode for annual direct medical costs taken from the literature [25, 35] (Table 2).
Monitoring services included routine outpatient visits and laboratory tests. Unit costs for each resource were obtained from the Government Gazette and EOPYY official website [36] (Table 2).
The model also included costs for treating and managing AEs such as tuberculosis and other serious infections. Costs related to tuberculosis and other serious infections were sourced from the list of Diagnosis Related Groups issued by the Greek Ministry of Health [37] (Table 2).
3 Model Analyses
The aforementioned approach and data were used to calculate mean estimates of life-time costs and QALYs for each comparator. The cost effectiveness of tofacitinib versus other treatment comparators was evaluated by calculating the incremental cost per QALY gained (ICER).
One-way sensitivity analyses were conducted to determine the key drivers of cost-effectiveness results separately for the bDMARD-naïve and bDMARD/tumor necrosis factor-alfa inhibitor-IR populations. Each parameter has been allocated a lower value and an upper value that correspond to the lower and upper bounds of the 95% confidence interval. When each parameter is varied independently, the sensitivity of the model results to that parameter can be estimated. The one-way sensitivity analyses results were presented as tornado plots presenting the parameters for which the associated uncertainty has the greatest impact on the relevant model outcomes.
Moreover, a probabilistic sensitivity analysis (PSA) was conducted to assess the parametric uncertainty associated with the base-case model results. In each iteration, the PSA sets all parameters with uncertainty in the model to a value that is randomly sampled from its assigned probability distribution and records model results. Distributions built in the PSA were beta (for utilities and probabilities), gamma (for cost data), lognormal (for relative risk data), and per convention in economic analyses (ESM). This process is repeated multiple times to provide an estimation of the uncertainty surrounding the cost effectiveness of interventions. Hence, the model used simulation modeling to run 1000 analyses, in order to be able to construct cost-effectiveness acceptability curves, which indicate the likelihood of the incremental cost per QALY to fall below specified willingness-to-pay (WTP) thresholds. Moreover, different time horizons (10, 20, and 40 years) and discount rates for both effectiveness and cost estimations (0% and 6% instead of 3.5% in the base case) were tested and compared to the base-case analysis.
4 Results
4.1 Base-Case Analyses
In the bDMARD-naive population, the analysis indicated that over a lifetime horizon that the total cost per patient for tofacitinib and adalimumab was estimated to be €149,500 and €147,096, respectively. With respect to effectiveness in terms of QALYs, tofacitinib was found to be associated with 10.730 QALYs, while the QALY for adalimumab was 10.672. The incremental analysis of tofacitinib versus adalimumab resulted in an ICER of €41,378 per QALY gained (Table 3).
The analysis in the bDMARD-tumor necrosis factor-alfa inhibitor-IR population showed that over a lifetime horizon, tofacitinib was associated with a 0.13 increment in QALYs compared with secukinumab, at an additional cost of €5614. The corresponding ICER of tofacitinib compared to secukinumab was €42,784, per QALY gained (Table 3).
4.2 Sensitivity Analysis Results
In the bDMARD-naive population, the results of one-way sensitivity analyses for the comparison of tofacitinib versus adalimumab indicated that the most influential parameters on the model results were the response-dependent BASFI change from baseline and the coefficient for BASFI score in the utility equation (Fig. 2). While in the bDMARD-tumor necrosis factor-alfa inhibitor-IR population, the results of one-way sensitivity analysis results for the comparison of tofacitinib versus secukinumab reported that the response-dependent BASDAI and BASFI changes from baseline by ASAS20 were the parameters with the greatest effects on the base-case results (Fig. 3).
One-way sensitivity analysis results of tofacitinib versus adalimumab in a biologic disease-modifying antirheumatic drug (bDMARD)-naïve population. AE adverse event, ASAS Assessment of Spondyloarthritis International Society, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Disease Activity Index, btwn between, CC conventional care, CFB change from baseline, coeff. coefficient, mgmt management, mSASSS modified Stoke Ankylosing Spondylitis Spinal Score, PY patient-years, QALY quality-adjusted life-year, SE standard error, tx treatment
One-way sensitivity analysis results of tofacitinib versus secukinumab in a biologic disease-modifying antirheumatic drug/tumor necrosis factor-alfa inhibitor inadequate response (bDMARD/TNFi-IR) population. AE adverse event, ASAS Assessment of Spondyloarthritis International Society, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Disease Activity Index, btwn between, CC conventional care, CFB change from baseline, coeff. coefficient, disc. discontinuation, mgmt. management, mSASSS modified Stoke Ankylosing Spondylitis Spinal Score, prob. probability, PY patient-years, QALY quality-adjusted life-year, SE standard error, tx treatment
It is noteworthy that tofacitinib maintained its cost-effective profile in both populations, when the model time horizon was set at 10, 20, 30, and 40 years as well as in all tested sensitivity analyses, exhibiting ICERs below the WTP threshold of €60,000 per QALY gained. The PSA indicated that the total costs of each intervention and QALY yielded were comparable to the base-case analyses. The ICER on the PSA was €46,167 of tofacitinib compared to adalimumab in the bDMARD-naive population, and €51,651 of tofacitinib compared to secukinumab in the bDMARD/tumor necrosis factor-alfa inhibitor-IR population. Moreover, the PSA showed that at the WTP threshold of €60,000 per QALY gained, treatment with tofacitinib had a 69% and 75% (Fig. S1 of the ESM) probability of being a cost-effective option compared with adalimumab and secukinumab, respectively. The results of the PSA confirmed the robustness of the base-case results in both populations.
5 Discussion
The results of the present study suggest that tofacitinib is a cost-effective treatment strategy compared with adalimumab or secukinumab from a public payer perspective in Greece and, therefore, tofacitinib represents a valuable treatment option for patients with AS who have responded inadequately to conventional therapy or previous biologic therapy. As the model predicted, tofacitinib is associated with improvements in quality-adjusted life expectancy than adalimumab or secukinumab. Nonetheless, the health gains of tofacitinib gains were associated with slightly higher direct medical costs, generating ICERs from €41,378 to €42,784 per QALY gained that fall well below the defined WTP of €60,000 in Greece.
While currently there is no officially established WTP threshold in Greece, for a health intervention to be considered cost effective, a WTP threshold of €60,000 per QALY gained was used in the current analysis. This assumption was based on published studies recommendation based on which a health intervention should be considered as cost effective if the ICER is between one and three times the GDP per capita of that country [38,39,40]. The Greek GDP per capita was taken from the International Monetary Fund, which estimated it at €20,000 using current prices [41] at the time of the analysis.
Sensitivity analyses indicated that the base-case results were relatively insensitive to variations in input parameters and assumptions. Furthermore, the stability of base-case findings was further endorsed by the PSA, and the PSA revealed that the probability of tofacitinib being a cost-effective option at the WTP threshold of €60,000 per QALY gained was higher than 69% versus all available comparators in both populations.
To the best of our knowledge, this is the first study aiming to evaluate the cost effectiveness of tofacitinib compared to adalimumab or secukinumab in adult patients with AS who have responded inadequately to conventional therapy or previous biologic therapy. Nevertheless, previous similar studies that were caried out in Greece from a public payer perspective have recorded the cost-effective profile of tofacitinib in patients with moderate-to-severe active ulcerative colitis [42] and rheumatoid arthritis [22]. Moreover, similar findings were obtained in a recent Greek study conducted from a public payer perspective, which showed that tofacitinib was likely to provide similar or greater health benefits because of lower cost administration method compared with adalimumab for the treatment of patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis [23].
Ankylosing spondylitis is a chronic systemic rheumatic disease with major consequences for a patient’s health and well-being. Despite the availability of several treatment options, the heterogeneity of the disease makes it difficult to achieve satisfactory outcomes for all patients, with the unmet need for new pharmacologic treatments remaining. Therefore, it is of key importance to include new treatments involving innovative mechanisms of action and convenient routes of administration in the therapeutic arsenal to optimize patients’ quality of care and quality of life.
In terms of limitations, there was a lack of direct clinical evidence of relative responder rates for the comparison of tofacitinib and comparators. In the absence of such a head-to-head clinical trial, a network meta-analysis was undertaken based on data reported by clinical trials. Nonetheless, a network meta-analysis is considered as a valid method provided direct comparative trials are lacking. Moreover, the selection of the most appropriate comparators was the first priority in the analysis, and the use of evidence synthesis using recommended methodologies is becoming increasingly important and accepted for health technology assessment globally [43]. Second, given a lack of clinical evidence for tumor necrosis factor-alfa inhibitors in the bDMARD/tumor necrosis factor-alfa inhibitor-IR population and for treatment sequences in AS in general, no exploratory sequencing analysis was conducted for or included in the model. Even though in real-world clinical practice patients might switch to another biologic upon failure of the initial biologic treatment, there was a paucity of clinical evidence in the bDMARD/tumor necrosis factor-alfa inhibitor-IR population. Even if clinical evidence existed for tumor necrosis factor-alfa inhibitors for the bDMARD/tumor necrosis factor-alfa inhibitor-IR population, it would remain questionable whether the efficacy of tumor necrosis factor-alfa inhibitors in the bDMARD/tumor necrosis factor-alfa inhibitor-IR population might be dependent on or independent of the prior bDMARD, as there was no clinical study that compared the efficacy of different treatment sequences. Despite the uncertainty around these assumptions, a series of sensitivity analyses indicated that model outcomes are robust, as the main results remained unchanged in a wide range of parameter values.
6 Conclusions
To conclude, tofacitinib was estimated to be a cost-effective therapy versus adalimumab and secukinumab in the treatment of active AS in Greece for both biologic-naive and biologic-experienced patients. Important to note is that these favorable results for tofacitinib were found against adalimumab and secukinumab, the most marketed biological therapies for the treatment of AS in Greece (standard practice).
References
Zhu W, He X, Cheng K, et al. Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res. 2019;7:22.
Ariza-Ariza R, Hernández-Cruz B, Navarro-Sarabia F. Physical function and health-related quality of life of Spanish patients with ankylosing spondylitis. Arthritis Rheum. 2003;49(4):483–7.
Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl. 2006;78:4–11.
Ward MM. Health-related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis Care Res. 1999;12(4):247–55.
Andrianakos A, Trontzas P, Christoyannis F, et al. Prevalence of rheumatic diseases in Greece: a cross-sectional population based epidemiological study. The ESORDIG Study. J Rheumatol. 2003;30(7):1589–601.
Anagnostopoulos I, Zinzaras E, Alexiou I, et al. The prevalence of rheumatic diseases in central Greece: a population survey. BMC Musculoskelet Disord. 2010;11:98.
Bournia VK, Tektonidou MG, Vassilopoulos D, et al. Introduction and switching of biologic agents are associated with antidepressant and anxiolytic medication use: data on 42 815 real-world patients with inflammatory rheumatic disease. RMD Open. 2020;6(3): e001303.
Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19–34.
Ministry of Health. Greek therapeutic protocol for axial spondyloarthritis. 2018. https://www.moh.gov.gr/articles/health/domes-kai-draseis-gia-thn-ygeia/kwdikopoihseis/therapeytika-prwtokolla-syntagografhshs/diagnwstika-kai-therapeytika-prwtokolla-syntagografhshs/5627-diagnwstika-kai-therapeytika-prwtokolla-syntagografhshs-reymatologikwn-noshmatwn. Accessed 7 Jul 2022.
Deodhar A, Strand V, Conaghan PG, et al. Unmet needs in ankylosing spondylitis patients receiving tumour necrosis factor inhibitor therapy; results from a large multinational real-world study. BMC Rheumatol. 2020;4:19.
Navarro-Compán V, Plasencia-Rodríguez C, de Miguel E, Diaz Del Campo P, Balsa A, Gratacós J. Switching biological disease-modifying antirheumatic drugs in patients with axial spondyloarthritis: results from a systematic literature review. RMD Open. 2017;3(2): e000524.
Spadaro A, Lubrano E, Marchesoni A, et al. Remission in ankylosing spondylitis treated with anti-TNF-α drugs: a national multicentre study. Rheumatology (Oxford). 2013;52(10):1914–9.
Hunter T, Schroeder K, Sandoval D, Deodhar A. Persistence, discontinuation, and switching patterns of newly initiated TNF inhibitor therapy in ankylosing spondylitis patients in the United States. Rheumatol Ther. 2019;6(2):207–15.
Tahir H. Therapies in ankylosing spondylitis-from clinical trials to clinical practice. Rheumatology (Oxford). 2018;57(Suppl._6):vi3-8.
Veale DJ, McGonagle D, McInnes IB, et al. The rationale for Janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatology (Oxford). 2019;58(2):197–205.
Deodhar A, Sliwinska-Stanczyk P, Xu H, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80(8):1004–13.
van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2017;76(8):1340–7.
Gourzoulidis G, Koulentaki M, Koumarianou A, et al. Cost-effectiveness of trifluridine/tipiracil as a third-line treatment of metastatic gastric cancer, including adenocarcinoma of the gastrohesophageal junction, among patients previously treated in Greece. Expert Rev Pharmacoecon Outcomes Res. 2022;22(2):259–69.
Gourzoulidis G, Barmpouni M, Kossyvaki V, Vietri J, Tzanetakos C. Health and economic outcomes of 20-valent pneumococcal conjugate vaccine compared to 15-valent pneumococcal conjugate vaccine strategies for adults in Greece. Front Public Health. 2023;11:1229524.
Koulentaki M, Tzanetakos C, Stratigos A, et al. EE106 Cost-effectiveness analysis of bimekizumab in patients with moderate-to-severe plaque psoriasis in Greece. Value Health. 2022;25(12 Suppl.):S74.
Gourzoulidis G, Rigopoulos D, Kountouris V, Christou P, Kiri S, Tzanetakos C. PBI28 Cost-effectiveness of certolizumab pegol for the treatment of moderate to severe plaque psoriasis in Greece. Value Health. 2019;22:S422.
Kourkoulas N, Athanasakis K, Boubouchairopoulou N, Chouchouli K, Kyriopoulos J. PMS41 Cost-effectiveness analysis of tofacitinib in combination with methotrezate in the treatment of moderate to severe rheumatoid arthritis in Greece. Value Health. 2018;21:S294.
Tzanetakos C, Solakidi A, Psarra M, Nikitopoulou E, Gourzoulidis G. Economic evaluation of tofacitinib for the treatment of active polyarticular juvenile idiopathic arthritis in Greece. Value Health. 2023;26(11).
Tzanetakos C, Kotsis I, Gourzoulidis G. Cost-effectiveness analysis of upadacitinib in patients with active non-radiographic axial spondyloarthritis in Greece. Value Health. 2023;26(11).
Corbett M, Soares M, Jhuti G, et al. Tumour necrosis factor-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluation. Health Technol Assess. 2016;20(9):1–334 (v–vi).
National Institute for Health and Care Excellence. Secukinumab for active ankylosing spondylitis after treatment with non-steroidal anti-inflammatory drugs or TNF-alpha inhibitors. 2016; https://www.nice.org.uk/guidance/ta407. Accessed 7 Jul 2022.
Emery P, Van Keep M, Beard S, et al. Cost Effectiveness of secukinumab for the treatment of active ankylosing spondylitis in the UK. Pharmacoeconomics. 2018;36(8):1015–27.
Borse RH, Brown C, Muszbek N, Chaudhary MA, Kachroo S. Cost-effectiveness of golimumab in ankylosing spondylitis from the UK payer perspective. Rheumatol Ther. 2017;4(2):427–43.
Landewé R, Dougados M, Mielants H, van der Tempel H, van der Heijde D. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis. 2009;68(6):863–7.
Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65(10):2645–54.
Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta‐analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011(2):CD008794. https://doi.org/10.1002/14651858.CD008794.pub2
Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis. 2011;70(11):1921–5.
World Health Organisation (WHO). Greece life tables. https://apps.who.int/gho/data/view.main.60640?lang=en. Accessed 1 Jul 2022.
Greek Ministry of Health. Drug price bulletin. 2022. www.moh.gov.gr/. Accessed 3 Jul 2022.
Tzanetakos C, Tzioufas A, Goules A, et al. Cost-utility analysis of certolizumab pegol in combination with methotrexate in patients with moderate-to-severe active rheumatoid arthritis in Greece. Rheumatol Int. 2017;37(9):1441–52.
National Organisation for Healthcare Services Provision. EOPYY. 2022. www.eopyy.gov.gr/Home/StartPage?a_HomePage=Index. Accessed 20 Jun 2022.
Greek Ministry of Health. Diagnosis related groups (DRG) list. 2012. www.moh.gov.gr. Accessed 2 Jul 2022.
Thokala P, Ochalek J, Leech AA, Tong T. Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics. 2018;36(5):509–22.
Cameron D, Ubels J. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11(1):1447828.
Tzanetakos C, Gourzoulidis G. Does a standard cost-effectiveness threshold exist? The case of Greece. Value Health Reg Issues. 2023;36:18–26.
International Monetary Fund. World economic outlook database. 2022. Available from: www.imf.org/external/pubs/ft/weo/2019/02/weodata/index.aspx. Accessed 12 Jun 2022.
Vellopoulou K, Stefanou G, Tzanetakos C, et al. Cost-effectiveness of tofacitinib for the treatment of moderate to severe active ulcerative colitis in Greece. Eur J Gastroenterol Hepatol. 2021;33(3):325–33.
European Network for Health Technology (EUnetHTA). Guidelines: comparators & comparisons: direct and indirect comparisons. 2015. www.eunethta.eu/wp-content/uploads/2018/01/Comparators-Comparisons-Direct-and-indirect-comparisons_Amended-JA1-Guideline_Final-Nov-2015.pdf. Accessed 1 Sep 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Pfizer Hellas.
Conflicts of Interest
Argyro Solakidi and Eleni Nikitopoulou are employees of Pfizer Hellas. George Gourzoulidis, Argyro Solakidi, Marina Psarra, Eleni Nikitopoulou, and Charalampos Tzanetakos have no conflicts of interest that are directly relevant to the content of this article.
Authors’ Contributions
Conceived the study: GG, CT, AS; designed the study: GG, CT, AS; analyzed the data: GG, CT; interpreted the data: GG, CT, AS, MP, EN; contributed to writing and review of the manuscript: GG, CT, AS, MP, EN. All authors approved the final version of the completed manuscript and agreed to be accountable for the entirety of this study.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
All data used to conduct this study are included in this article.
Code Availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gourzoulidis, G., Solakidi, A., Psarra, M. et al. Cost Effectiveness of Tofacitinib for the Treatment of Active Ankylosing Spondylitis in Greece. Clin Drug Investig 44, 59–69 (2024). https://doi.org/10.1007/s40261-023-01333-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01333-z