Abstract
We aimed to evaluate the cost-effectiveness of certolizumab pegol (CZP), a pegylated fc-free anti-TNF, as add-on therapy to methotrexate (MTX) versus etanercept, adalimumab, or golimumab in patients with moderate-to-severe active rheumatoid arthritis (RA) not responding to the conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs). A Markov model (6-month cycle length) assessed health and cost outcomes of CZP versus other anti-TNFs recommended for RA in Greece over a patient’s lifetime. Following discontinuation of first-line anti-TNF, patients switched to second anti-TNF and then to a biologic with another mode of action. Sequential use of csDMARDs followed third biologic. Clinical data and utilities were extracted from published literature. Analysis was conducted from third-party payer perspective in Greece. Costs (drug acquisition, administration, monitoring, and patient management) were considered for 2014. Results presented are incremental cost-effectiveness ratios (ICERs) per quality-adjusted life year (QALY). Probabilistic sensitivity analysis (PSA) ascertained robustness of base-case findings. Base-case analysis indicated that CZP+MTX was more costly and more effective compared with Etanercept+MTX (base-case ICER: €3,177 per QALY), whilst versus adalimumab/golimumab, CZP was dominant (less costly, more effective). For all comparisons, CZP treatment resulted in greater improvements in life expectancy and QALYs. PSA indicated that at the willingness-to-pay threshold of €34,000/QALY, CZP+MTX was associated with a 71.6, 97.9, or 99.2% probability of being cost-effective versus etanercept, golimumab, or adalimumab, respectively, in combination with MTX. This analysis demonstrates CZP+MTX to be a cost-effective alternative over Etanercept+MTX and a dominant option over Adalimumab+MTX and Golimumab+MTX for management of RA in Greece.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune disease of unknown etiology that affects approximately 1% of the world’s population and can lead to impaired functioning and mobility as well as premature mortality [1]. The progressive nature of the disease is well known, with 20–30% of untreated patients having permanent work disability within 3 years of diagnosis [2]. Similarly, it has been estimated that patients with RA have a 20–30% reduction in quality of life (QoL) when compared to age-matched individuals from the general population [3]. Overall, long-term morbidity and functional disability have considerable socioeconomic implications for patients and society, posing major challenges to national healthcare systems.
It has been demonstrated that patients with RA are associated with disproportionately high consumption of health services and other indirect costs, with productivity losses being the predominant economic burden of the disease [4]. In the UK, one recent study estimated productivity losses of £1.8 billion per year, in addition to approximately £560 million of direct healthcare costs incurred by the National Health Service (NHS) [5].
The prevalence of RA in the adult population of Greece has been estimated at 0.68%, similar to that observed in many European countries [6]. Although there is paucity of data on the clinical and economic burden of RA in Greece, it is not expected to deviate widely from that observed in other countries.
Therapeutic guidelines from the Greek National Organization for Medicines (EOF) [7], in line with the latest European League against Rheumatism (EULAR) recommendations [8], suggest that RA treatment be initiated with the conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), with methotrexate (MTX) serving as an anchor drug. In case of failing csDMARDs, a biologic agent (bDMARDs) is added to the existing therapy for patients with moderate-to-severe RA. Tumor necrosis factor alpha (TNF-a) inhibitors are usually the first bDMARDs to be prescribed [certolizumab pegol (CZP), etanercept, adalimumab, golimumab, and infliximab]. Following an inadequate response to a first anti-TNF agent, the TNF-inadequate responders (IR) can then either switch to treatment with another anti-TNF agent or with a bDMARD with another mode of action (rituximab, abatacept, and tocilizumab) [7, 8].
Although anti-TNF agents have demonstrated greater efficacy than csDMARDs for the treatment of RA, they are also associated with a four- to sixfold increase in the direct costs of treatment [9, 10]. This marked increase in costs, driven by the elevated price of anti-TNF treatments, in conjunction with the increased clinical benefits associated with their use, has resulted in numerous cost-effectiveness studies investigating bDMARDs and csDMARDs [11]. Overall, the use of anti-TNF agents was found to be a cost-effective strategy in patients, whose disease activity was poorly controlled with the conventional treatment (csDMARDs). However, in the absence of head-to-head trials between these agents, it is difficult to determine which agent is optimal. Indirect economic evaluation of multiple biologics is scarce, and currently, there are none published in Greece.
CZP is the first PEGylated Fc-free anti-TNF agent that has been shown to rapidly and significantly improve signs and symptoms of RA and physical function and inhibit radiographic progression [12,13,14]. It is a relatively novel TNF-a inhibitor with an established clinical profile which gained its marketing authorization in European Union in 2009 [15]. However, under the recent climate of major financial crisis and healthcare budget restrictions in Greece, there is a growing need to use treatments which are both clinically effective and economically efficient.
To this end, the objective of this analysis was to investigate the cost-effectiveness of CZP versus the other subcutaneously administered anti-TNF agents, etanercept, adalimumab, or golimumab, as adjunct treatments to MTX, in patients with moderate-to-severe active RA who had failed treatment with at least one csDMARD (including MTX) in a Greek setting.
Methods
In the present study, a Markov model, previously submitted to the National Institute of Health and Clinical Excellence (NICE) [16] by the manufacturer of CZP as part of the single technology appraisal (STA) process, was locally adapted. Based on this model’s time horizon (base-case: 45 years), the lifetime direct medical costs, life years (LYs), and quality-adjusted life years (QALYs) accrued for RA patients were evaluated in a Greek setting. The cost-utility analysis was conducted from the Greek third-party payer perspective (EOPYY) and an annual discounting of 3.5% was applied to both effectiveness and cost estimations [17].
Model structure
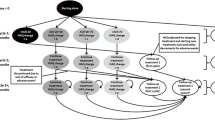
The model structure is depicted in Fig. 1. Patients with moderate-to-severe active RA who had an inadequate response to at least one csDMARD, including MTX, entered the model and began treatment with CZP, etanercept, adalimumab, or golimumab, in combination with MTX. At the end of the first cycle, patients were assigned to one of four response groups based on estimated response rates for the relevant treatment. Response was defined using the American College of Rheumatology (ACR) outcome measures (ACR20, ACR50, and ACR70). Patients not achieving an ACR20 response discontinued treatment and were switched to the next sequential treatment. Patients achieving an ACR20 response remained on their current treatment, in the same Markov state. At the end of each cycle, patients either continued in the same Markov state (and, therefore, treatment), switched treatment due to a lack of efficacy or an adverse event (at the mid-point of the cycle), or died. There were no state transitions other than treatment discontinuation or death. In the first year of the model, treatment response was evaluated at 6 months, after which there were two subsequent time frames, each of 3 months. The cycle length for the remainder of the model was 6 months, which reflects the period of time allowed for a patient to achieve the treatment target (clinical remission or low disease activity) [8].
Model structure. *Follow-up (FU) treatment states: duplicated for each follow-up treatment. Patients not responding in first 6 months of follow-up treatment will move to the next in the sequence. **Reason for discontinuation governed by probabilities after leaving treatment health state. HAQ Health assessment Questionnaire, ACR American College of Rheumatology
Treatment algorithm
In the model, RA patients are allocated to either CZP, etanercept, adalimumab, or golimumab as their first biologic treatment. The choice of comparators was based on the latest EULAR recommendations for the management of RA [8] and includes those anti-TNF agents that follow the same administration route (subcutaneous injection) as CZP and are licensed and recommended for use in Greece. It was assumed that patients would follow the same treatment pathway irrespective of initial treatment. The treatment pathway was based on current clinical practice in Greece, as indicated by local expert rheumatologists. It was assumed that after failing their first anti-TNF, patients would be treated with another anti-TNF with a different molecular structure (first follow-up biologic treatment). Therefore, treatment failure with a monoclonal antibody anti-TNF (i.e., CZP, adalimumab, and golimumab) led to a switch to the human soluble TNF receptor fusion protein, etanercept. Similarly, treatment failure with etanercept led to a switch to a monoclonal anti-TNF; for the base-case comparison of CZP versus etanercept, golimumab was the second anti-TNF therapy. The other biologic monoclonal antibody, adalimumab, was also tested independently in the univariate sensitivity analysis. Failing treatment with a second anti-TNF agent, patients were switched to a third bDMARD, this time with a different mode of action (second follow-up biologic treatment) and rituximab in the base-case analysis. The use of tocilizumab or abatacept instead of rituximab was also considered in the univariate sensitivity analysis. On discontinuation of the first and second follow-up biologic treatments, patients received a sequence of further follow-up therapies, as suggested by local experts (csDMARDs: leflunomide, then cyclosporine, azathioprine, and sulfasalazine). After failing the last treatment in the sequence, patients continued palliative therapy until death.
Model inputs
Study population
The baseline characteristics of the hypothetical cohort (10,000) of RA patients are based on pooled data from the patient populations who participated in the CZP clinical trials: RAPID1, RAPID2, and FAST4WARD [12,13,14]. Demographics were comparable in all studies. The study population was 82.7% female with a mean baseline age of 52.2 years [standard error (SE) 0.27], a mean number of previous csDMARDs of 2.26 (0.05), a mean disease duration of 6.56 (0.21) years, and baseline Health Assessment Questionnaire (HAQ) scores of 1.62 (0.01).
Transition probabilities
The relative effectiveness of the comparators in the first cycle of the model was estimated via an indirect analysis based on ACR20 response data obtained from a published Bayesian random-effects meta-analysis [18]. In a random-effects model, the between-trial variability of the measured effect caused by between-trial heterogeneity is also taken into account. Applying the indirect statistical method proposed by Bucher et al. [19], the relative effectiveness of CZP plus MTX compared to etanercept, adalimumab, or golimumab plus MTX was derived by dividing the estimated odds ratio (OR) of each comparator versus placebo, as extracted by the aforementioned meta-analysis, with the estimated OR of CZP versus placebo. This method relies on the fact that the log of the effect size measure for drug A versus drug B is equal to the difference of the log effect size measures for drug A versus drug C and drug B versus drug C. Next, the absolute effectiveness (odds) in terms of ACR20 response of each treatment comparator at 6 months was calculated by combining the corresponding relative effectiveness with the absolute effectiveness of CZP, as estimated by aggregating ACR20 response data from the CZP plus MTX arms in the RAPID1 and RAPID2 clinical trials [12, 13]. The response rate of CZP was computed as the relative frequency of patients on CZP plus MTX who achieved an ACR20 response for which the corresponding odds were calculated [odds = risk/(1 − risk)]. The odds for etanercept, adalimumab, or golimumab plus MTX were computed by multiplying the OR derived from the indirect analysis, by the odds of CZP plus MTX (Table 1). The authors of the reference meta-analysis used here stressed that the ORs of etanercept and golimumab on ACR70 response versus placebo yielded very wide confidence intervals due to the insufficient numbers of patients, and thus were considered unreliable. Nevertheless, for the purposes of these analyses, we encompassed the corresponding raw results of ORs as first determined by a classical frequentist method followed in the meta-analysis.
In the second and subsequent cycles, discontinuation probabilities were calculated based on assumptions relating to the time on treatment (Table 1) and an exponential “survival” distribution for continuation with treatment. In particular, the time spent on first bDMARD was based on estimates from the Du Pan et al. study [20]. Treatment duration of the first and second follow-up bDMARDs was extracted from another study [21] that dealt with the differential drug retention between alternative anti-TNF agents and biologic agents with another mode of action for TNF-IR. The treatment durations for the csDMARDs were based on the Edwards et al. study [22]. The choice of these studies was relied upon the fact that their estimates were derived from national registries containing medical records of thousands of RA patients followed over long periods of time in general clinical practice. In the absence of relative Greek data, the studies estimates were reviewed by local clinical experts and, where considered appropriate, were tested in sensitivity analysis.
The probability for all-cause mortality for the general population was considered and incorporated into the model. Data were taken from the age and gender-specific mortality rates (2011) for Greece posted on the National Statistical Service official website [23]. These rates were also adjusted with an RA risk multiplier associated with patients’ HAQ score (1.33 per HAQ unit; 95% CI 1.099–1.610) derived from Wolfe et al. [24].
Health states and utility values
The economic model captured the effects on patient QoL as measured by the HAQ and EuroQol Group 5 Dimension (EQ-5D) questionnaire in the CZP RAPID1 and RAPID2 trials [12, 13]. Patient health state changed based on initial treatment, continuation of treatment, and discontinuation of treatment (Table 2). The HAQ disease severity measure was used to assess disease progression. To map utilities from HAQ scores, the Bansback conversion factor (ΔEQ-5D utility = −0.2102 ΔHAQ) was incorporated in the model [25]. The assumption that QoL is linearly associated with HAQ score is the standard practice in most models published in RA [26,27,28].
On entry into the model, patient populations were assigned a mean pre-treatment EQ-5D utility of 0.38. Over the first 6 months of treatment with initial anti-TNF therapy, patients were assigned a change in QoL dependent on their ACR response category. The magnitude of change in EQ-5D utilities was estimated from the patient-level data from the CZP trials [12, 13] by ANCOVA regression analysis. The effect was assumed to be the same for all comparators. Regression models were fitted with age, gender, baseline EQ-5D utilities, disease duration, number of previous csDMARDs, and anti-CCP antibody status as covariates. Regression coefficients were then used to calculate the change in utility.
In the absence of data from long-term clinical trials, the mean change in HAQ on initiation of the first or subsequent follow-up treatments, or during continuation of treatment was assumed to follow estimates taken from Chen et al. [29] and NICE TA126 guidance [30], respectively. This was done to be consistent with NICE recommendations following TA126 and the Health Technology Assessment (HTA) report of Chen et al. conducted as part of the HTA Programme [29]. The research findings of this Programme, which is the largest single national research programme for the UK’s NHS, directly influence key decision-making bodies such as the NICE who rely on HTA outputs to help raise standards of care.
Once a treatment was discontinued, the base-case analysis assumed a change equal to that applied for the initial response to treatment (rebound assumption: 100% loss of initial benefit).
Cost calculations
The total reimbursement cost assigned to each treatment in the model incorporates all resource use resulting from the care of patients within the healthcare system during each cycle of the model [including physician visits, drug consumption, lab tests, and management of RA (dependent on HAQ score)] (Table 3). Resource use associated with each treatment was recorded during the first subsequent cycles of that treatment, based on expert opinion. Drug acquisition costs were calculated by combining the dose of each agent with the reimbursed drug unit cost, as derived from the price bulletin issued by the Greek Ministry of Health [31] (Table 3). Dose schedules for all drugs were obtained from the European Medicine Agency (EMA) official website [15] and validated by clinical experts. For drugs where dose is adjusted for patient weight (abatacept, infliximab, tocilizumab, azathioprine, and cyclosporine), a mean weight of 81.4 kg [12,13,14] was assumed. In the base-case, a per drug unit costing method was considered (unused drug wastage). Administration costs were only generated for bDMARDs requiring infusion [32]. For subcutaneous injections, patients were assumed to be able to self-administer the drug at home without assistance. Monitoring costs involved physician visits and lab tests, the unit costs of which were extracted from Government Gazette and EOPYY official website [33, 34]. The number of visits and lab tests depends on bDMARD and csDMARD and was obtained from local experts to reflect the common clinical practice (Table 3). Other direct costs pertaining to RA management by HAQ score were also considered based on Kobelt et al. [35] [these costs were obtained in US dollars (from 2001), and were inflated to 2014 values [36] and converted to euros [37] before inclusion in the model] (Table 3).
Data analysis
The comparative cost-effectiveness of CZP plus MTX was evaluated by calculating the incremental cost-effectiveness ratios (ICER) per QALY gained. To accommodate variation into the model parameters, one-way sensitivity analyses were performed. Sensitivity tests were consistent with previous analyses presented in the manufacturer’s submission to NICE [16]. Probabilistic sensitivity analyses (PSA) were also performed by attaching probability distributions to input parameters [38, 39]. Specifically, normal distributions were assigned to the absolute clinical effectiveness (transformed to a log odds scale), mean age, baseline mean EQ-5D utility, number of previous csDMARDs, and disease duration. Beta distributions were assigned to gender and a CZP-related cumulative distribution function was assigned to patient weight. Lognormal distributions were assigned to direct costs of RA management and a mortality risk multiplier was associated with patients’ HAQ score. All other parameters were constant.
For each comparison, 1000 trial simulations were performed. Results from these simulations were used to construct cost-effectiveness planes and cost-effectiveness acceptability curves. Although there is no official willingness-to-pay threshold for Greece, a treatment was considered to be cost-effective at a threshold of €34,000 per QALY gained. This was based on the World Health Organization (WHO) guidelines that state a treatment should be considered cost-effective if the ICER is between one and three times the Gross Domestic Product (GDP) per capita of that country, and highly cost-effective at less than one times the GDP per capita [40]. Using the current prices, the International Monetary Fund (IMF) estimated Greek GDP per capita at €17,000 [41].
Results
Base-case analysis
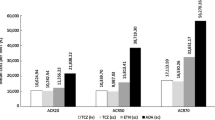
CZP is associated with a greater life expectancy and quality-adjusted life expectancy versus all comparators (Table 4). When compared to etanercept, CZP treatment resulted in a mean increase in survival of 0.02 years, and after adjusting survival for QoL estimates, the model predicted a mean increase in QALYs of 0.12. When compared with golimumab and adalimumab, CZP was associated with a mean increase in life expectancy of 0.08 and 0.07 years and in QALYs of 0.43 and 0.42, respectively.
Analyses of treatment costs revealed CZP to be less costly than golimumab and adalimumab (CZP: €105,041; golimumab: €105,273; adalimumab: €106,006), but more expensive than etanercept (CZP: €105,319; etanercept: €104,939).
Based on these findings, treatment with CZP was estimated to be the dominant strategy when compared to golimumab or adalimumab (less costly and more effective). Compared to etanercept, CZP was associated with an ICER (€3,177 per QALY gained) much lower than the defined willingness-to-pay threshold of €34,000 per QALY gained.
Sensitivity analysis
A number of one-way sensitivity analyses were performed and no considerable variation to the base-case ICER estimates was observed (Table 5). However, some changes were seen in the CZP ICERs dependent on assumptions related to time horizon, baseline HAQ score, and utility progression rate for first-line treatments. The highest ICER estimates were observed when a worse utility progression rate for first biologic treatments (-0.01 per year) was considered, whereas the lowest ICERs were observed when the rebound assumption was reduced from 100 to 50%. The choice of tocilizumab or abatacept for second follow-up biologic therapy had a small impact on the base-case results, as well as the choice of adalimumab or infliximab for first follow-up biologic therapy in the comparison to CZP versus etanercept. With respect to the PSA results, the model estimated that, when using the €34,000 per QALY gained threshold, CZP plus MTX was associated with a 71.6, 97.9, or 99.2% probability of being cost-effective versus etanercept, golimumab, or adalimumab plus MTX, respectively (Fig. 2).
Discussion
Based on clinical findings from clinical trials of CZP plus MTX [12, 13] and a multiple-treatment meta-analysis [18], long-term projections suggest that treatment with CZP would result in greater improvements in life expectancy and quality-adjusted life expectancy when compared with etanercept, adalimumab, or golimumab.
It is important to note that any long-term differences in health outcomes between CZP and comparators were driven solely by the relative differences in ACR response rates applied in the model. The subsequent HAQ progression rates and time using treatments for responding patients were assumed to be equivalent. From a third-party perspective (EOPYY), CZP plus MTX treatment was associated with higher medical costs during first biologic therapy, mainly due to higher drug acquisition costs. These elevated costs could be attributed to the fact that CZP plus MTX is associated with the highest ACR20 response (primary clinical endpoint) among the comparator treatments, and thus, a greater number of patients received that treatment. Although treatment with CZP was associated with higher initial costs, these were attenuated by reductions in follow-up treatment costs related to drug acquisition, administration, monitoring, and patient management. The largest contributing factor to treatment costs was the drug purchasing cost, followed by patient management costs. Combining cost and health outcomes through ICER tool, the model estimated that CZP is likely a dominant strategy compared to golimumab or adalimumab and is highly cost-effective versus etanercept (ICER: €3,177/QALY gained).
The sensitivity analysis performed here indicated that the conclusions drawn from the base-case analysis were robust when the uncertainty surrounding model parameters were tested (Table 5). In almost all univariate sensitivity assumptions, the treatment strategy with CZP was assessed to be either a cost-effective (ICERs below the defined threshold) or a dominant option. Likewise, the probabilistic ICERs of CZP versus comparator treatments were in accordance with the deterministic ICERs from the base-case analysis.
Several potential limitations to this cost-effectiveness study should be considered. First, there is lack of data on the long-term efficacy of bDMARDs or csDMARDs in patients failing treatment with their first bDMARDs, given the short-term nature of most clinical trials. To compensate for this, assumptions relevant to disease progression were made based on previous literature (Table 2). As these assumptions were followed in all treatment arms, no bias has been caused in calculation of the ICER. Moreover, the literature on the safety of bDMARDs is reported in different ways making reliable direct comparison between agents impossible. As such, the costs and outcomes associated with adverse events were not included in the model simulations. Although this is consistent with the approach adopted in a NICE authored model in early RA [42], the consideration of adverse events on cost and health measures may have altered the results of present analysis. Noteworthy is the fact that based on the latest EULAR recommendations [8], no preference of one over another biological agent should be expressed in terms of safety.
Furthermore, the use of HAQ as a measure to assess disease progression suffers the same criticisms levelled at previous models submitted to NICE: that HAQ may be an inadequate measure of QoL and that mapping results from HAQ to utilities may be an inadequate substitute for the direct measurement of EQ-5D utility. Nevertheless, HAQ was used as it was the most well-recorded measure of physical function in rheumatoid arthritis clinical trials. Importantly, the majority of published cost-utility models have used a similar approach [30]. As the single rate of HAQ progression was used for all the first biologic interventions, it is unlikely that any directional bias occurred in the ICER estimations.
With regard to the treatment sequence, there is no consensus about the most appropriate therapy in patients failing first biologic treatment, predominantly due to the lack of available data. Nonetheless, to best reflect current clinical practice in Greece, two expert rheumatologists with extensive clinical experience indicated a common treatment algorithm. The switching of the first anti-TNF agent from soluble receptor to a monoclonal antibody or vice versa is also supported by the literature [43,44,45,46], but switching a second time appears much less effective [44]. In the treatment path nodes where multiple biologic treatments could be chosen, one-way sensitivity analyses were performed.
To calculate the effectiveness of treatments, data from direct comparisons would be preferable to combined data from differing sources. However, due to the scarcity of head-to-head clinical trials between biologic treatments, this was not feasible. Instead, data from a published random-effects meta-analysis were used, the validity of which have been previously discussed [18], and indirectly compared adjusting for clinical effectiveness of the baseline treatment, CZP plus MTX.
The present findings have to be considered in the strict Greek setting and on the basis of the resource and drug prices, as well as clinical data available during the year of analysis. If any of the underlying parameters change, so may the results and the conclusions of the analysis.
In conclusion, the derived ICERs suggest that, in this model CZP plus MTX is likely a cost-effective alternative compared to etanercept and is a dominant option compared to adalimumab or golimumab, in combination with MTX, for the management of RA in Greece.
References
Pollard L, Choy EH, Scott DL (2005) The consequences of rheumatoid arthritis: quality of life measures in the individual patient. Clin Exp Rheumatol 23(5 Suppl 39):S43–S52
Rindfleisch JA, Muller D (2005) Diagnosis and management of rheumatoid arthritis. Am Fam Physician 72(6):1037–1047
Kobelt G, Woronoff AS, Richard B, Peeters P, Sany J (2008) Disease status, costs and quality of life of patients with rheumatoid arthritis in France: the ECO-PR Study. Joint Bone Spine 75(4):408–415. doi:10.1016/j.jbspin.2007.07.015
Boonen A, Severens JL (2011) The burden of illness of rheumatoid arthritis. Clin Rheumatol 30(Suppl 1):S3–S8. doi:10.1007/s10067-010-1634-9
National Audit Office (2009) Services for people with rheumatoid arthritis. Report by the comptroller and auditor general, HC 823 session 2008–2009. National Audit Office, 15 July 2009
Andrianakos A, Trontzas P, Christoyannis F, Kaskani E, Nikolia Z, Tavaniotou E, Georgountzos A, Krachtis P, Group ES (2006) Prevalence and management of rheumatoid arthritis in the general population of Greece—the ESORDIG study. Rheumatology (Oxford) 45(12):1549–1554. doi:10.1093/rheumatology/kel140
National Organization for Medicines (EOF) (2000–2016) Official website. http://www.eof.gr/web/guest. Accessed 30 March 2014
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, Emery P, Gaujoux-Viala C, Gossec L, Nam J, Ramiro S, Winthrop K, de Wit M, Aletaha D, Betteridge N, Bijlsma JW, Boers M, Buttgereit F, Combe B, Cutolo M, Damjanov N, Hazes JM, Kouloumas M, Kvien TK, Mariette X, Pavelka K, van Riel PL, Rubbert-Roth A, Scholte-Voshaar M, Scott DL, Sokka-Isler T, Wong JB, van der Heijde D (2013) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. doi:10.1136/annrheumdis-2013-204573
Fautrel B, Verstappen SM, Boonen A (2011) Economic consequences and potential benefits. Best Pract Res Clin Rheumatol 25(4):607–624. doi:10.1016/j.berh.2011.10.001
van den Hout WB, Goekoop-Ruiterman YP, Allaart CF, de Vries-Bouwstra JK, Hazes JM, Kerstens PJ, van Zeben D, Hulsmans HM, de Jonge-Bok JM, de Sonnaville PB, Dijkmans BA, Breedveld FC (2009) Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 61(3):291–299. doi:10.1002/art.24169
van der Velde G, Pham B, Machado M, Ieraci L, Witteman W, Bombardier C, Krahn M (2011) Cost-effectiveness of biologic response modifiers compared to disease-modifying antirheumatic drugs for rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken) 63(1):65–78. doi:10.1002/acr.20338
Keystone E, Heijde D, Mason D Jr, Landewe R, Vollenhoven RV, Combe B, Emery P, Strand V, Mease P, Desai C, Pavelka K (2008) Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 58(11):3319–3329. doi:10.1002/art.23964
Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, van Vollenhoven RF, Kavanaugh A, Schiff M, Burmester GR, Strand V, Vencovsky J, van der Heijde D (2009) Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 68(6):797–804. doi:10.1136/ard.2008.101659
Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, Goel N, Brezinschek HP, Innes A, Strand V (2009) Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis 68(6):805–811. doi:10.1136/ard.2008.099291
European Medicines Agency (EMA) (1995–2016) Οfficial website. http://www.ema.europa.eu/ema/. Αccessed 30 March 2014
NICE (2010) Certolizumab pegol for the treatment of rheumatoid arthritis (Guidance TA186). http://www.nice.org.uk/guidance/ta186. Accessed 30 March 2014
NICE (2012) Process and methods guides: the guidelines manual. http://www.nice.org.uk/article/pmg6. Accessed 15 December 2013
Launois R, Avouac B, Berenbaum F, Blin O, Bru I, Fautrel B, Joubert JM, Sibilia J, Combe B (2011) Comparison of certolizumab pegol with other anticytokine agents for treatment of rheumatoid arthritis: a multiple-treatment Bayesian metaanalysis. J Rheumatol 38(5):835–845. doi:10.3899/jrheum.100665
Bucher HC, Guyatt GH, Griffith LE, Walter SD (1997) The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 50(6):683–691
Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A, Swiss Clinical Quality Management P (2009) Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 61(5):560–568. doi:10.1002/art.24463
Du Pan SM, Scherer A, Gabay C, Finckh A (2012) Differential drug retention between anti-TNF agents and alternative biological agents after inadequate response to an anti-TNF agent in rheumatoid arthritis patients. Ann Rheum Dis 71(6):997–999. doi:10.1136/annrheumdis-2011-200882
Edwards CJ, Arden NK, Fisher D, Saperia JC, Reading I, Van Staa TP, Cooper C (2005) The changing use of disease-modifying anti-rheumatic drugs in individuals with rheumatoid arthritis from the United Kingdom General Practice Research Database. Rheumatology (Oxford) 44(11):1394–1398. doi:10.1093/rheumatology/kei024
Hellenic Statistical Authority (EL.STAT.) Οfficial website. http://www.statistics.gr/. Αccessed 10 December 2013
Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, Spitz PW, Haga M, Kleinheksel SM, Cathey MA (1994) The mortality of rheumatoid arthritis. Arthritis Rheum 37(4):481–494
Bansback N, Marra C, Tsuchiya A, Anis A, Guh D, Hammond T, Brazier J (2007) Using the health assessment questionnaire to estimate preference-based single indices in patients with rheumatoid arthritis. Arthritis Rheum 57(6):963–971. doi:10.1002/art.22885
Barton P, Jobanputra P, Wilson J, Bryan S, Burls A (2004) The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess 8(11):3
Bansback NJ, Brennan A, Ghatnekar O (2005) Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis 64(7):995–1002. doi:10.1136/ard.2004.027565
Kobelt G, Eberhardt K, Jonsson L, Jonsson B (1999) Economic consequences of the progression of rheumatoid arthritis in Sweden. Arthritis Rheum 42(2):347–356. doi:10.1002/1529-0131(199902)42:2<347:AID-ANR18>3.0.CO;2-P
Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, Fry-Smith A, Burls A (2006) A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess 10(42):iii–iv–xi–xiii
NICE (2007) Rituximab for the treatment of rheumatoid arthritis (Guidance TA126). http://www.nice.org.uk/guidance/ta126. Accessed 30 March 2014
Greek Ministry of Health Drug Price Bulletin. http://www.yyka.gov.gr/. Accessed 30 March 2014
Government Gazzette (2011) Ministerial Decision (FEK 2150B’/27-09-2011). Reimbursment from EOPYY for a day-case healthcare setting
Social Security Sickness Fund (EOPYY) (2012-2016) Official website. http://www.eopyy.gov.gr/Home/StartPage?a_HomePage=Index. Accessed 30 April 2014
Government Gazzette. (2011) Ministerial Decision (FEK 262A’/16-12-2011). Reimbursment from EOPYY for a physician office visit
Kobelt G, Jonsson L, Lindgren P, Young A, Eberhardt K (2002) Modeling the progression of rheumatoid arthritis: a two-country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum 46(9):2310–2319. doi:10.1002/art.10471
Bureau of Labor Statistics CPI Inflation Calculator. http://www.bls.gov/data/inflation_calculator.htm. Accessed 30 March 2014
Organisation for Economic Co-operation and Development (OECD) official website. http://www.oecd.org/. Last accessed 15 December 2013
Briggs AH (2000) Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 17(5):479–500
Briggs A, Claxton K, Sculpher M (2006) Decision modelling for health economic evaluation. Handbooks in health economic evaluation. Oxford University Press Inc, New York
World Health Organisation (WHO) (2013) Choosing interventions that are Cost Effective (WHO-CHOICE): cost-effectiveness thresholds. http://www.who.int/choice/costs/CER_thresholds/en/index.html. Accessed 10 December 2013
International Monetary Fund (IMF) (2013) World Economic Outlook Database. http://www.imf.org/external/pubs/ft/weo/2012/02/weodata/index.aspx. Accessed 10 December 2013
NICE (2009) The management of rheumatoid arthritis in adults (Guidance CG 79). https://www.nice.org.uk/guidance/cg79. Αccessed 30 March 2014
Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ, British Society for Rheumatology Biologics R (2007) Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 56(1):13–20. doi:10.1002/art.22331
Karlsson JA, Kristensen LE, Kapetanovic MC, Gulfe A, Saxne T, Geborek P (2008) Treatment response to a second or third TNF-inhibitor in RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology (Oxford) 47(4):507–513. doi:10.1093/rheumatology/ken034
Furst DE, Gaylis N, Bray V, Olech E, Yocum D, Ritter J, Weisman M, Wallace DJ, Crues J, Khanna D, Eckel G, Yeilding N, Callegari P, Visvanathan S, Rojas J, Hegedus R, George L, Mamun K, Gilmer K, Troum O (2007) Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis 66(7):893–899. doi:10.1136/ard.2006.068304
van Vollenhoven R, Harju A, Brannemark S, Klareskog L (2003) Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann Rheum Dis 62(12):1195–1198
Acknowledgements
The authors acknowledge Susanne Wiegratz, MD, PhD (UCB Pharma, Germany), for publication coordination, and Simon Page, PhD (Costello Medical Consulting Ltd, Cambridge, UK), for editorial assistance in preparing this manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standard
This article does not contain any studies with human participants performed by any of the authors.
Study sponsor information
UCB Pharma funded this study and manuscript. UCB Pharma reviewed only for scientific and legal accuracy.
Rights and permissions
About this article
Cite this article
Tzanetakos, C., Tzioufas, A., Goules, A. et al. Cost-utility analysis of certolizumab pegol in combination with methotrexate in patients with moderate-to-severe active rheumatoid arthritis in Greece. Rheumatol Int 37, 1441–1452 (2017). https://doi.org/10.1007/s00296-017-3736-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3736-z