Abstract
Background and Objectives
Several systemic treatments are available for metastatic hormone sensitive prostate cancer (mHSPC) including docetaxel (D), abiraterone and prednisone (A + P) and new anti-androgens (NA). In our study we performed a systematic review and meta-analysis assessing efficacy outcomes (survival and radiological-free survival), safety and survival on specific subgroups of patients.
Methods
Outcomes of interest were: (i) Risk of death, biochemical and radiological progression among all patients. (ii) Risk of death according to different pathological/clinical features. (iii) Evaluation of the relative risk (RR) and risk difference of serious toxicity defined as adverse events (AEs) with grade ≥ 3 specific AEs. Hazard ratios (HRs) and RR were measures adopted for endpoints 1–3.
Results
Overall, eight randomized trials were included in meta-analysis for a total of 9987 patients. Administration of D, A + P and NA resulted in improved overall survival (OS) and radiological progression-free survival (rPFS). Survival benefit was not confirmed in patients receiving NA and previously exposed to docetaxel (HR 0.948, 95% CI 0.671–1.338). Patients with visceral metastases and high lactate dehydrogenase (LDH) did not benefit from NA treatment, while it seems that patients with low Gleason score do not benefit from A + P. NA showed the more favorable safety profile.
Conclusion
NA may not provide survival benefit when adopted subsequently or in concomitant to D. Specific subgroups of patients may benefit more from A + P, D or NA. Safety profiles significantly differ among agents evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Several drugs have shown clinical efficacy in patients with mHSPC. |
Efficacy of these agents may be significantly different according to pathological and clinical variables. |
Safety profiles of each drug are significantly different. |
1 Introduction
Prostate cancer (PC) is the most common malignancy in men representing about 20% of oncological diagnosis [1]. Among this large group, only 3%–4% of patients present a metastatic hormone sensitive prostate cancer (mHSPC) as primary diagnosis [2].
Compared to patients developing metastasis after curative treatment, de-novo mHSPC is a disease correlated with worst prognosis and shorter time to develop a metastatic castration-resistant prostate cancer (mCRPC) [3].
Nonetheless, there are specific subgroups of patients with mHSPC who presented more favorable prognosis. Generally, these patients present low tumor burden, small metastatic disease and low Gleason [4].
Traditionally, systemic management of mHSPC has been carried out through an androgen deprivation therapy (ADT). However, in recent years, several trials evaluating the addition of systemic compounds to ADT showed that combination treatment results in improved clinical outcomes. Docetaxel was the first systemic agent tested in this setting [5,6,7,8,9], followed by abiraterone–prednisone [10, 11]. Very recently, data from randomized studies demonstrated that the administration of new anti-androgen compounds with ADT resulted in a survival and clinical advantage in patients with mHSPC [12,13,14].
To date, two different studies performed a comparison between these agents.
Marchioni et al performed a network meta-analysis showing that administration of new anti-androgen compounds with ADT does not reflect a survival advantage compared to docetaxel [15]. However, administration of hormonal compounds is associated with lower disease progression rates and better safety profile [16]. Similarly, Sathianathen et al performed a systematic review and network meta-analyses to characterize the comparative efficacy of combination treatments in patients with mHSPC [16]. Combination therapies between ADT and other compounds (both chemotherapy or hormonal agents) can improve patients’ prognosis and overall survival [16].
Here, we carried out a meta-analysis aimed to assess the toxicity profiles of these treatments, the survival and progression-free survival (PFS) benefit in all patients and the survival benefit in patients with specific clinical/pathological behaviors.
2 Methods
2.1 Evidences Acquisition
This meta-analysis has been carried out according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
2.2 Search Strategies
We searched all perspective, randomized Phase III clinical trials published between 01 January 2012 to 15 September 2019 evaluating a new hormonal agent or other compounds in addition to ADT in patients with mHSPC.
Keywords used for searching on Pubmed/Medline, Cochrane library, and Scopus, were: “metastatic hormone sensitive prostate cancer” OR “mHSPC” OR “metastatic prostate cancer” OR “Castration sensitive metastatic prostate cancer” AND “ apalutamide” OR “enzalutamide” OR “abiraterone” OR “docetaxel”; only papers published in peer-reviewed journals, and written in English language, were considered. Furthermore, proceedings of the main International Oncological and Urological meetings (American Society of Clinical Oncology, European Society of Medical Oncology, American Association for Cancer Research, European Association of Urology, and American Urological Association) were also evaluated for relevant abstracts. When more publications of the same study were available, we adopted the more recent publication with longer follow up. Four authors (VDN, FM, VM and MS) made a first study selection. Therefore, all authors reviewed all relevant studies selected before their inclusion in analyses.
2.3 Aims of the Meta-Analysis
Aims of the meta-analysis were:
-
(i)
To evaluate risk of death, biochemical and radiological progression-free survival (bPFS and rPFS) among patients with mHSPC randomized to ADT or ADT + experimental compound. For this aim, only studies reporting completed results of overall survival (OS), bPFS and rPFS analyses were evaluated. Our aim was to evaluate the different risks of death bPFS and rPFS in previously untreated and treated patients with mHSPC.
-
(ii)
To evaluate risk of death of specific patient subpopulations randomized to ADT or ADT + experimental compound. Variables of interest were: ECOG performance status (0 vs 1–2), age (< 70, ≥ 70), visceral metastases (yes/no), Gleason (< 8, ≥ 8), lactate dehydrogenase (LDH) levels (high/normal), high volume disease (yes/no). For this aim, only studies reporting completed survival outcomes in these subgroups as well as studies reporting bPFS analysis were considered.
-
(iii)
To evaluate the relative risk (RR) and risk difference of serious toxicity defined as adverse events (AEs) with grade ≥ 3 of specific AEs such as: fatigue, falls, seizure/dizziness, cardiovascular toxicity, death due to AE, hypertension, neutropenia, febrile neutropenia, anemia, thrombocytopenia, arthralgia and edema.
2.4 Data Extraction and Synthesis
The following data were extracted for each publication: (a) population on study (b) OS expressed as hazard ratio (HR) with 95% confidence interval (CI); (c) rPFS expressed as HR with 95% CI, (d) bPFS expressed as HR with 95% CI; (d) OS expressed as HR in patients with ECOG performance status 0, ECOG performance status 1, age < 70, age ≥ 70, absence of visceral metastases, presence of visceral metastases, high volume disease, Gleason score < 8 and Gleason score of 8 or more (g) serious AEs (fatigue, falls, seizure/dizziness, cardiovascular toxicity, death due to AE, hypertension, neutropenia, febrile neutropenia, anemia, thrombocytopenia, arthralgia and edema) with Grade 3 or more occurred among trials considered; (h) median follow up; (h) primary and secondary endpoints of trials exanimated.
Four separate authors (VDN, VM, MS and FM) independently conducted the search and identification at four different times. Results of the research were then shared among all authors before final inclusion in analysis. The same process was adopted for quantifying the risk of bias according to Cochrane tool for risk of bias assessment in randomized trials [17]. Evaluation of studies according to Cochrane tool for risk of bias was performed considering the presence of: (1) selection bias (presence/absence of bias due to inadequate generation of a randomized sequence or inadequate concealment of allocations before assignment); (2) performance bias (knowledge of the allocated interventions by participants and personnel during study); (3) detection bias (knowledge of the allocated interventions by outcome assessment); (4) attrition bias (presence of incomplete outcome data); (5) reporting bias (selective outcome reporting); (6) other bias.
2.5 Statistical Design
Endpoints of the meta-analysis: the evaluation of risk of death, risk of bPFS and rPFS all patients (endpoint 1), the different risk of death in specific subgroups (endpoint 2). Furthermore, we performed a safety analysis among clinical trials evaluating the pooled RR of each specific toxicity of interest (endpoint 3). Meta-analysis was performed using the MedCalc (ver 18.11.3); Excel was used for data collection.
Endpoint 1, 2: Summary measures were HRs with 95% CIs for rPFS, bPFS and OS. We applied the inverse variance technique for the meta-analysis of HRs. In OS/rPFS analyses, we adopted both a random and a fixed-effects model. Statistical heterogeneity between studies was examined using I2statistic [17,18,19,20,21,22].
Endpoint 3: The number of patients receiving experimental drugs, as well as the number of grade 3 or higher AEs in both treatment and control arms were extracted from all selected studies. Incidences, RR and 95% CIs were subsequently calculated as proposed by Altman et al [8]. Cochran’s Q statistic was employed to test heterogeneity between studies. The I2 statistic was chosen for quantification of inconsistency. Both the inverse variance fixed-effects model (weighted with inverse variance) and the random effect model was adopted. Studies with no AE in the treatment or control arms were corrected according to Yates. Risk difference was estimated as the difference between experimental and comparator arm, which was then expressed as percentage [17,18,19,20,21,22].
3 Results
3.1 Studies Selection
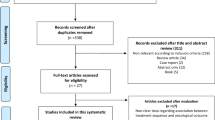
We selected ten publications [5,6,7,8,9,10,11,12,13,14] of the 2892 detected as potentially relevant studies. The main reasons for exclusion: other setting of intervention, review articles, not randomized clinical trials, letters, systematic review or meta-analysis (Fig. 1; Supplementary Material). At the end of the process, eight randomized clinical trials were selected.
In Table 1 we summarized the design of the study, patients enrolled, primary and secondary endpoints. Among the studies included, two were upgraded versions of previous published studies [6, 8]. One randomized trial was presented during an international meeting but lacked mature data on OS [14]. On the basis of the independent evaluation, seven studies were associated with low risk of bias. One study presented a moderate risk of bias mainly due to the immature follow up. Five of the eight studies selected were associated with high risk of bias in the blinding of outcomes and assessment section. This was mainly due to the lack of placebo/double-blind control. Additionally, one of these studies presented an uncertain risk of bias due to the absence of complete data about safety analyses [7, 8]. Overall, 9987 patients were included in this meta-analysis. Of these, 4994 patients received ADT monotherapy, while 4993 received ADT plus experimental compounds. In particular, among 4993 patients included in experimental arms, 1774 received docetaxel (593 also received zoledronic acid), 1557 received abiraterone, 1662 were treated with enzalutamide (n = 1137) and apalutamide (n = 525).
Of note, in the STAMPEDE trials, we considered only patients with metastatic disease for bPFS, rPFS, OS and sub-group analyses (Table 2).
3.2 Overall Survival (OS) and Radiological Progression-Free Survival (rPFS) Analysis
Seven of eight [5,6,7,8,9,10,11,12,13] studies were considered for OS analysis (1 study excluded as OS follow up was still immature [14]).
Overall, the administration of experimental compounds resulted in a survival advantage (pooled-random HR 0.714; CI 0.656–0.777; p value < 0.001; I2 = 15.66%, p = 0.31; Fig. 2a.1). The survival advantage was confirmed after the inclusion of previously untreated patients (pooled-random HR 0.697; CI 0.629–0.772; p value < 0.001; I2 = 37.78%, p = 0.13; Fig. 2a.2) and previous docetaxel or concomitant exposed patients (pooled-random HR 0.736; CI 0.662–0.819; p value < 0.001; I2 = 35.59%, p = 0.14; Fig. 2a.3).
Overall survival (OS) analysis among patients with metastatic hormone sensitive prostate cancer. a All patients included, including patients not previously exposed to docetaxel, patients previously exposed to docetaxel. b New anti-androgens overall, exposed and not previously exposed to docetaxel. c OS result among metastatic patients receiving docetaxel; OS result among metastatic patients receiving abiraterone
Survival benefit was demonstrated in patients treated with docetaxel (pooled-random HR 0.736; CI 0.662–0.819; p value < 0.001; I2 = 0.00%, p = 0.69; Fig. 2c.1), abiraterone (pooled-random HR 0.615, 95% CI 0.532–0.712; p value < 0.001; I2 = 0.00%, p = 0.91; Fig. 2c.2) and new anti-androgens (pooled-random for enzalutamide/apalutamide-treated patients: 0.690, 95% CI 0.568–0.838; p value < 0.001; I2 = 0.00%, p = 0.72; Fig. 2b.1).
Among patients treated with apalutamide or enzalutamide, the survival benefit was confirmed in previously untreated patients (pooled random HR 0.587, 95% CI, 0.467–0.736, p < 0.001, I2 = 0.00%, p = 0.46; Fig. 2b.2) but no survival benefit emerged in patients exposed (concomitant or subsequently) with docetaxel (pooled random HR 0.948, 95% CI 0.671–1.338, p = 0.760, I2 = 0%, p = 0.48; Fig. 2b.3).
Regarding rPFS analyses, we considered five of eight studies selected [5, 6, 10, 12,13,14] (three studies did not report data on rPFS [7,8,9, 11]). Overall, the administration of experimental compounds resulted in prolonged rPFS in overall cohort (pooled random HR: 0.475, 95% CI 0.390–0.579, p < 0.001). Heterogeneity was statistically significant with an I2 value of 74%, p = 0.004 (Fig. 3a). The radiological progression-free advantage was also achievable including patients previously untreated (Fig. 3b.1) and exposed (concomitant or subsequently) with docetaxel to docetaxel (in this case Heterogeneity was statistically significant. I2 value: 81.62%, p = 0.0002; Fig. 3b.2).
Radiological progression-free (rPFS) analysis. a All studies reporting rPFS. b All studies including patients who received docetaxel before experimental treatment, all studies including patients not exposed to docetaxel. c New anti-androgen treatment among patients who did not receive docetaxel and among patients previously exposed to docetaxel
When we consider only the three studies with a cohort of previously treated patients, the rPFS advantage was available in all patients, previously untreated patients (Fig. 3c.2) and previously treated patients (or patients who received concomitant docetaxel) (Fig. 3c.3). An extensive summary of the results achieved for this aim are available in the Supplementary Material.
In bPFS analyses, we collected data provided by four of eight studies [5, 6, 10, 13, 14]. In this analyses, administration of experimental compounds (docetaxel, enzalutamide or abiraterone) resulted in a significant improvement of bPFS, although heterogeneity was statistically significant (I2 = 93.99%, p < 0.0001). Similar results have been observed when analysis was restricted to patients who received hormonal experimental compounds (I2 = 85.9%, p = 0.0008) or enzalutamide (I2 = 92.38%, p = 0.0003) (Table 3).
3.3 Subgroup Analysis
Variable of interest in subgroups analyses were: Eastern Cooperative Oncology Group (ECOG) performance status 0 versus ECOG performance status 1–2, aged < 70 years versus aged ≥ 70 years, Gleason < 8 versus ≥ 8, presence or absence of visceral metastases, high volume versus low volume disease, normal level versus high LDH (Table 3).
In ECOG performance status analysis, all agents showed an improvement in survival regardless of initial performance status (Table 3). Similarly, patients aged < 70 years showed clear survival advantages with experimental treatments without particular differences among the classes of compounds adopted. However, patients aged ≥ 70 years did not show a clear survival benefit with experimental compounds and especially with the administration of docetaxel (a survival advantage emerged adopting fixed-effect model; however, due to the high level of heterogeneity observed, the pooled HR obtained with random-effect model should be considered).
Among patients with Gleason < 8, docetaxel and new anti-androgen compounds were associated with improved survival. Abiraterone did not prolong the OS of patients with low Gleason score (Table 3). In patients with higher Gleason score, the survival benefit was unclear with administration of docetaxel while both abiraterone and new anti-androgen were clearly associated with improved survival.
Data on efficacy in patients with visceral metastases were available in four of eight studies [7, 8, 10, 12, 13]. Of note, administration of apalutamide or enzalutamide did not reflect a statistically significant survival advantage in patients with visceral metastases (Table 3). Similarly, administration of docetaxel did not lead to an OS benefit in patients with low volume metastatic disease.
Patients with high serum level of LDH benefit from administration of abiraterone, while neither docetaxel nor apalutamide showed improved survival in this population. A detailed description of the results achieved in sub-group analyses is available in the Supplementary Material.
3.4 Safety Analysis
Overall, 9876 patients were included in safety analyses. Of these, 4865 received ADT while 5011 received experimental compounds (1807 docetaxel, 1545 abiraterone + prednisone, 1135 enzalutamide, 524 apalutamide).
In this analysis, administration of docetaxel was significantly associated with an increased RR to develop G3 or more neutropenia and neutropenia febrile (Table 4). There was no significant increased risk of high-grade anemia and thrombocytopenia observed with administration of docetaxel. Hormonal treatments were shown to prevent the onset of high-grade anemia. Docetaxel was associated with an increased risk of sensory toxicity.
In fatigue analyses, all agents were associated with increased risk of fatigue development. However, heterogeneity of studies evaluating docetaxel was statistically significant and no increased risk of high-grade fatigue emerged with abiraterone. Only administration of new hormonal anti-androgen was clearly associated with increased risk of this AE.
RR of seizure/dizziness, falls, arthralgia and edema resulting from enzalutamide/apalutamide administration were not statistically significant.
Increased risk of high-grade cardiovascular toxicity was clearly associated with abiraterone + prednisone treatment, while the risk of hypertension was not statistically relevant in patients receiving apalutamide or enzalutamide.
Risk of death due to AE was unclear in patients treated with docetaxel, while patients who received abiraterone or new-antiandrogen compounds did not show an increased risk of death (Table 4). An extensive description of the result obtained in safety analysis is available in the Supplementary Material.
4 Discussion
Here we report the result of a meta-analysis exploring the role of chemotherapy and new hormonal treatments in addition to ADT in patients with mHSPC.
Our results showed that all these agents are able to improve OS with the exception of the anti-androgen (apalutamide and enzalutamide) in patients previously exposed to docetaxel. Considering that all these agents showed a survival benefit in randomized clinical trials, our findings are not surprising. However, important data may be suggested by subgroup analyses.
Overall, all agents were also able to improve rPFS and bPFS, while the results of this last analysis were weighted by a large heterogeneity. Of interest, we isolated a specific subgroup of patients who may present worst survival after docetaxel (patients aged ≥ 70 years, high Gleason score at diagnosis, and higher LDH serum levels), abiraterone + prednisone (low Gleason score) and apalutamide/enzalutamide (visceral metastases, previously or concomitant docetaxel-exposed patients, high LDH serum levels) treatment.
There are several limitations, which may reduce the value of our analyses. First of all the included studies presented a heterogeneous population, which often consists of patients with both metastatic and non-metastatic disease (only patients with metastatic disease were included in OS and rPFS analyses) [9, 11], patients with different volume and risk [5,6,7,8,9,10,11] and patients previously exposed to other systemic treatment in addition to ADT [12,13,14]. Another limitation is the inclusion of data of sub-groups and exploratory analyses, which have the limitation of being previously unplanned analysis among trials explored. In subgroup analysis, some studies reported survival outcomes in patients aged < 65 years or > 65 years and thus, the same studies have not been included in this subgroup analysis. Moreover, age alone is not a useful variable as it does not take in account patients’ clinical conditions, comorbidities and performance status. Thus, age alone should be not be used as a parameter for planning therapeutic choices in clinical practice. Similarly, some studies [12, 14] randomized patients in ECOG performance status 0 or 1 (without enrollment of ECOG, 2 patients) and this may further underestimate the value of ECOG performance status subgroup analysis. Of note, we included two docetaxel arms of STAMPEDE trial in our analysis and this could translate in an over estimation of the overall effect. However, due to the missing impact of zoledronic acid on survival, it may be reasonable think that it did not influence the effect of docetaxel on OS.
Finally, our analysis stands alone as a quality of life comparison among experimental treatments and a cost-effectiveness evaluations.
Another important limitation emerged in the comparison of new anti-androgens after or during docetaxel treatment. Indeed, the settings in which docetaxel was administered was significantly different (previous docetaxel in TITAN study, concomitant docetaxel in ENZAMET trial) [12, 13]. This limited the value of our finding. However, in both subgroup analyses, patients who were previously exposed or patients with concomitant docetaxel did not seem to show a survival benefit from the addition of new anti-androgens. The limited number of patients considered, the high heterogeneity and the different modality of docetaxel administration (previous or concomitant) significantly limited the value of this analysis. Moreover, in the apalutamide arm, only 11 events occurred, and median overall survival is still not mature. Significantly higher percentage of patients who received docetaxel have been included in enzalutamide arm.
These limitations are mainly due to the lack of data on quality of life in patients receiving new anti-androgen.
Despite these issues, our meta-analysis is, to the best of our knowledge, the largest meta-analysis carried out on patients with mHSPC and offers a valuable insight into the management of the disease.
Overall survival benefit emerged with all agents considered (docetaxel, abiraterone and enzalutamide) and this is consistent with the single result reported by the trials considered. STAMPEDE, GETUG and ARCHES trials failed to report a significant improvement in terms of OS. This may be explained by an Inadequate selection of patients randomized to receive docetaxel (STAMPEDE and GETUG) or in a still immature OS follow up (ARCHES) [5, 6, 9, 14]. However, pooled-HR of studies adopting the same agents confirmed that each agent was associated with survival improvement raising the key value of a good selection of patients before treatment planning.
Of note, we identified that patients with high baseline LDH serum levels did not show a survival benefit with the addition of new anti-androgens. This result should be carefully evaluated as only three studies reported sub-group analysis in this subpopulation of patients [5, 6, 10, 12] and this might have influenced the final results. Indeed, only TITAN study [12] reported complete data about patients with low/high LDH, while no information is available from ARCHES and ENZAMET studies [13, 14]. Overall, enzalutamide was shown to be an effective treatment in patients with high volume disease [13] while neither enzalutamide nor apalutamide have shown any real advantage in patients with visceral metastases [12, 13].
Curiously, we detected a low level of heterogeneity in OS analysis, while higher heterogeneity emerged in rPFS and bPFS analyses. It is likely that the different modalities and timing of assessment influenced these results, increasing heterogeneity of rPFS and bPFS outcome. In particular, the high level of heterogeneity emerging from bPFS analysis may reflect the different definition and assessment that had been adopted by the clinical trials included. Thus, this analysis should be carefully interpreted as potentially affected by these biases. GETUG [5, 6] study provided significantly worse results in terms of rPFS and bPFS and was the only trial to adopt docetaxel in rPFS and bPFS. The results of this trial probably affected the high level of heterogeneity observed.
The selection of patients with mHSPC is a well-known issue, which emerged during primary studies evaluating docetaxel in this population. The evidence that patients with high-volume disease benefit from docetaxel, while in patients with low-volume disease, there was no clear evaluation of the results of GETUG-AFU-15, CHAARTED, and STAMPEDE trials [5,6,7,8,9]. Subsequently the combination ADT + abiraterone + prednisone was tested in patients with high-risk disease and in patients with lowest stage/grade of disease showing positive results on OS [10, 11]. A meta-analysis that aimed to compare OS in patients receiving docetaxel or abiraterone, failed to show a significant difference between these treatments [23, 24]. Subsequently, another analysis suggested that abiraterone + prednisone may be the most effective treatment; however, heterogeneity and variability of patients included in trials under investigation may have affected the final result [25].
Prevention of disease progression and better quality of life also emerged in a comparison analysis between docetaxel and abiraterone [26].
Toxicity may be another important issue to consider before treatment planning. In our study, we confirmed the classical hematological toxicity of docetaxel and also the sensorial neuropathy associated with this treatment. Abiraterone + prednisone were significantly associated with an increased risk of cardiological toxicity and hypertension, confirming the result of a previous analysis of this AE [26].
Classical AE of interest among new anti-androgen compounds are dizziness/seizures, falls and mental impairment. In our analysis, we showed that the risk of high-grade dizziness/seizures is low and infrequent during new anti-androgen treatment. Unfortunately, we were not able to perform an evaluation of mental impairment AE due to the lack of complete data and maybe the still immature follow-up. Curiously, an increased risk of neutropenia emerged with the administration of hormonal agents (no increased risk of febrile neutropenia). This outcome may reflect other complications related to prostate cancer (such as cancer medullary replacement and consequent neutropenia) more than a specific toxicity of hormonal treatment.
Of note, risk of death during the course of treatment was similar among the experimental and comparator arms. The inclusion of grade 3 or more AEs may limit the real impact of some toxicity. Indeed, low-grade fatigue (such as grade 2) may be an important toxicity experienced by patients, significantly reducing their quality of life.
Our analysis confirms the well-known toxicity profiles of new anti-androgens, docetaxel and abiraterone and does not add significant insight in the safety profile of these agents. However, summarizing evidences about safety profile of agents available for management of mHSPC may be an important issue, as this is a key element to plan our choices in clinical practice.
5 Conclusion
The addition of chemotherapy, abiraterone or new anti-androgens to ADT improves survival of patients with mHSPC. Our finding is not surprising considering results achieved by each drug in randomized studies.
The use of a new anti-androgen may not improve survival of patients receiving concomitant docetaxel or previous docetaxel. However, the large heterogeneity among studies evaluating this issue limits the value of this observation. According to our results, patients with visceral metastases did not seem to show a survival benefit with the administration of new anti-androgens. Initial Gleason score may be related to different outcomes among patients receiving docetaxel or abiraterone. Toxicity profiles of these drugs confirmed the known hematological toxicity of docetaxel and cardio-vascular toxicity associated with abiraterone. High-grade AEs typically associated with new anti-androgens rarely occur during or after treatment.
Results of our meta-analysis suggest that:
-
Patient selection is essential before treatment planning. Indeed, some patients do not benefit from a specific treatment (such as docetaxel for patients with low tumor volume or enzalutamide/apalutamide in patients previously exposed to chemotherapy)
-
Disease assessment may be an important issue to consider before treatment planning. Low Gleason score may be associated with lowest effect of abiraterone on survival. The presence of visceral metastases should discourage the adoption of apalutamide or enzalutamide.
-
Toxicity profile of agents should be carefully considered, and administration of enzalutamide/apalutamide may be a treatment of choice in frail patients. The cardiotoxicity of abiraterone should be considered in patients with high number of cardiovascular comorbidities, while patients with hematopoietic dysfunction or higher risk of infective disease should be discouraged from the adoption of docetaxel in this setting.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. https://doi.org/10.3322/caac.21551.
Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis. 2016;19:395–7.
C. Mosillo, R. Iacovelli, C. Ciccarese, Fantinel E, Bimbatti D, Brunelli M et al. De novo metastatic castration sensitive prostate cancer: state of art and future perspectives Cancer Treat Rev. 2018; 70:67–74. https://doi.org/10.1016/j.ctrv.2018.08.005.
Iacovelli R, Ciccarese C, Mosillo C, Bimbatti D, Fantinel E, Stefani L, et al. Comparison between prognostic classifications in de novo metastatic hormone sensitive prostate cancer. Target Oncol. 2018;13(5):649–55. https://doi.org/10.1007/s11523-018-0588-8.
Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149–58. https://doi.org/10.1016/S1470-2045(12)70560-0.
Gravis G, Boher JM, Joly F, Soulié M, Albiges L, Latorzeff I, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70(2):256–62. https://doi.org/10.1016/j.eururo.2015.11.005.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–46. https://doi.org/10.1056/NEJMoa1503747.
Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemo-hormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080–7. https://doi.org/10.1200/JCO.2017.75.3657.
James ND, Sydes MR, Clarke NW, Mason MD, Daernaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–77. https://doi.org/10.1016/S0140-6736(15)01037-5.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–60. https://doi.org/10.1056/NEJMoa1704174.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Daernaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–51. https://doi.org/10.1056/NEJMoa1702900.
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24. https://doi.org/10.1056/nejmoa1903307.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31. https://doi.org/10.1056/NEJMoa1903835.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;22:JCO1900799. https://doi.org/10.1200/jco.19.00799.
Marchioni M, Di Nicola M, Primiceri G, Novara G, Castellan P, Paul A et al. New anti-androgen compounds compared to docetaxel in metastatic hormone sensitive prostate cancer: results from a network meta-analysis. J Urol. 2019. https://doi.org/10.1097/ju.0000000000000636.
Sathianathen NJ, Koschel S, Thangasamy IA, Teh J, Alghazo O, Butcher G, et al. Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2019. https://doi.org/10.1016/j.eururo.2019.09.004.
Higgins JP, Altman DG, Gøtzsche PC, Gotzsche PC, Juni P, Moher D et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928.
Altman DG, Machin D, Bryant TN, and Gardner MJ. Statistics with confidence, second edition. British Medical Journal London; 2000, pp. 45–56.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Yates F. Contingency table involving small numbers and the x2 test. J R Stat Soc. 1934;1:217–35.
Wallis CJD, Klaassen Z, Bhindi B, Goldberg H, Chandrasekar T, Farrell AM, et al. Comparison of abiraterone acetate and docetaxel with androgen deprivation therapy in high-risk and metastatic hormone-naïve prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2018;73(6):834–44. https://doi.org/10.1016/j.eururo.2017.10.002.
Vale CL, Fisher DJ, White IR, Carpenter JR, Burdett S, Clarke NW, et al. What is the optimal systemic treatment of men with metastatic, hormone-naive prostate cancer? A STOPCAP systematic review and network meta-analysis. Ann Oncol. 2018;29(5):1249–57. https://doi.org/10.1093/annonc/mdy071.
Feyerabend S, Saad F, Li T, Ito T, Diels J, Van Sanden S, et al. Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: a network meta-analysis. Eur J Cancer. 2018;103:78–87. https://doi.org/10.1016/j.ejca.2018.08.010.
Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer 2018;16(3):e645–e653. https://doi.org/10.1016/j.clgc.2017.12.007(epub 2017 Dec 27).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No source of founding.
Conflict of interest
All authors declare no competitor interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Di Nunno, V., Santoni, M., Mollica, V. et al. Systemic Treatment for Metastatic Hormone Sensitive Prostate Cancer: A Comprehensive Meta-Analysis Evaluating Efficacy and Safety in Specific Sub-Groups of Patients. Clin Drug Investig 40, 211–226 (2020). https://doi.org/10.1007/s40261-020-00888-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00888-5