Abstract
Background and Objective
More than 30% of patients with epilepsy have inadequate control of seizures with drug therapy. The goal of this study is to determine the budget impact (BI) of the introduction of brivaracetam to the portfolio of approved drugs in Spain as adjunctive therapy for the treatment of partial-onset epilepsy in patients over 16 years old with a 5-year time horizon in the Valencia Community, a Spanish region with a population of 5 million.
Methods
The BI model compares the pharmaceutical expenditure on antiepileptics in two scenarios: with and without brivaracetam. It assumes that the introduction and increased use of brivaracetam will lead to a proportional decrease in consumption of coexisting adjunctive antiepileptics and calculates the evolution of the consumption of brivaracetam over 5 years (2016–2020). The model was designed from the perspective of the Spanish National Health System. Data on the candidate population, consumption of antiepileptics, market share and pharmaceutical expenditure were obtained from real-world data. Finally, a sensitivity analysis was carried out on the set of variables involved in the evolution of costs using a Monte-Carlo simulation.
Results
The model estimates that the target population eligible for adjunctive antiepileptics will hold at around 2352 between 2016 and 2020. Annual expenditure on antiepileptics is approximately €3.6 million. The number of patients eligible for treatment with brivaracetam would increase from 42 to 179 and annual savings of 0.09–0.37% would be created, representing €41,873 over 5 years (0.23% of the total budget). The sensitivity analysis corroborates that the probability of achieving savings with brivaracetam is around 84%.
Conclusions
Brivaracetam is a therapeutic alternative that allows savings for the health system in patients with non-controlled epilepsy in monotherapy, having a fixed, predictable annual cost (independent of dose) from the first day of treatment as the lack of need for titration means the patient is within a range of therapeutic doses from the first dose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Brivaracetam is a new third-generation antiepileptic drug offering a therapeutic alternative for concomitant therapy in the treatment of partial-onset epileptic seizures, with or without secondary generalisation, in adults and adolescents above 16 years of age. |
The results from this budget impact analysis suggest that brivaracetam is a cost-saving therapeutic strategy for adjunctive therapy for epilepsy in Spain. |
1 Introduction
Epilepsy is one of the most common chronic neurological diseases in the world, with the World Health Organization estimating that it affects around 50 million people [1]. According a recent systematic review, the prevalence of active epilepsy was 6.38 per 1000 persons, while the lifetime prevalence was 7.60 per 1000 persons. The annual cumulative incidence of epilepsy was 67.77 per 100,000 persons, while the incidence rate was 61.44 per 100,000 person-years [2].
There are two types of epileptic seizure: generalised seizures, in which the entire surface of the brain is affected at the same time, and partial-onset or focal seizures, which begin by affecting one part of the brain [3, 4]. In Spain it is estimated that around 400,000 people are affected by epilepsy, with nearly 60% of patients having partial-onset or focal seizures [4].
Antiepileptic treatment centres on achieving the greatest reduction in the number of epileptic seizures while minimising adverse effects and long-term toxicity as far as possible. Clinical evidence shows that monotherapy with antiepileptic drugs (AEDs) is effective in 70% of patients [5]. The remaining 30% need adjunctive treatment to control the seizures [6] and, of these, approximately 25% have epilepsy that is difficult to control, refractory or resistant to AEDs. This implies difficulty for the neurologist in its management and the need to study other treatment strategies or optimise available pharmacological treatments. The importance of refractory epilepsy is in the significant decrease in quality of life with, moreover, the presence of associated morbidities (depression being the most frequent) and an increased probability of early death compared with patients with controlled epilepsy [7, 8].
The annual direct cost of epilepsy in Spain is estimated to be €2978/patient in the case of controlled epilepsy and between €4964 [9] and €6935 [4] per patient for non-controlled epilepsy, that is, the cost is between 1.7 and 2.3 times greater for non-controlled than controlled patients. This proportion reaches 2.7 times greater in infantile epilepsy [3]. Furthermore, non-controlled epilepsy is associated with a greater consumption of healthcare resources, lower quality of life and a greater incidence of severe depression. Therefore, it places a considerable burden on the National Health Service and society, as severe levels of anxiety and depression are associated with very high costs for the health system [10].
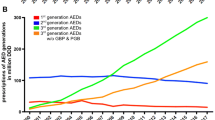
The neurologist has more than 20 AEDs available for the treatment of epilepsy, some of which have numerous adverse effects and interactions that can complicate patient treatment and management, especially for those with refractory epilepsy [5]. Since 1993, more than 12 new AEDs have been approved that have an effect on seizure control and a better tolerability profile, as well as a lower risk of drug interactions. To the four classic or first-generation AEDs (phenobarbital, phenytoin, carbamazepine and sodium valproate), eight second-generation (gabapentin, oxcarbazepine, topiramate, lamotrigine, vigabatrin, pregabalin, tiagabine and levetiracetam) and five third-generation (retigabine, eslicarbazepine, lacosamide, perampanel and zonisamide) AEDs have been added. Nevertheless, 30% of patients do not have entirely controlled epilepsy [6, 11].
The choice of the most suitable AED depends principally on the patient’s type of epilepsy, its effectiveness and the individual profile for tolerability and adverse effects. Generally, the new drugs are better tolerated, though not always more effective [12]. When comparing treatments, it is important to compare (1) drugs with the same indication [in this case, adjunctive drugs for partial-onset epileptic seizures (POS)]; (2) the need for titration and duration of it (speed in stabilising the patient); (3) available pharmaceutical forms for different clinical situations; (4) dosage (which will influence long-term compliance); (5) cost per treatment per day (affordable for the health service); (6) efficiency and effectiveness in real life; and (7) safety and interactions profile (associated with being a first-, second- or third-generation drug).
Brivaracetam is a new third-generation AED offering a therapeutic alternative for concomitant therapy in the treatment of POS, with or without secondary generalisation, in adults and adolescents above 16 years of age. This drug was approved by the European Medicines Agency in January 2016 [13]. Unlike other AEDs, it has a fixed cost independent of dosage, having no need for titration and ensuring the patient is within a therapeutic dosage range from the first day. It has a good tolerability profile and is commercialised in all pharmaceutical forms to deal with different patient profiles (out-patients and hospitalised patients) [14, 15].
When introducing a new medicine to the existing portfolio for a disease, the budget impact (BI) analysis (BIA) for the new medicine is an important tool in helping make decisions. A BIA is implemented to assess the sustainability of the use of a new technology, in this case a new drug. As such, the goal of this study was to determine the BI of the introduction of brivaracetam to the portfolio of approved drugs in Spain as adjunctive therapy for the treatment of POS in patients over 16 years of age with a 5-year time horizon in the Valencia Community (VC), a Spanish region with a population of 5 million.
2 Materials and Methods
2.1 Design
The BIA model was based on the latest methodological recommendations proposed by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) principles on good practice for BIA [16]. The model estimates the incremental BI of adopting brivaracetam as a treatment for POS, and is structured in six basic steps for estimating BI: (1) estimating the target population; (2) selecting a time horizon; (3) identifying the current and projected treatment mix; (4) estimating current and future drug costs; (5) estimating the change in disease-related costs; and (6) estimating and presenting changes in annual BI.
The starting point is the current market share of other AEDs in VC, obtained from real-word data from the regional electronic prescribing system. The model simulates brivaracetam entering the market and drawing a market share in pre-defined proportions from the other available therapies. Therefore, if in year 1 brivaracetam is assumed to reach 1.77% market share, the model simulates what proportion of this 1.77% is drawn from each of the other replacement therapies. This is due to the particular difficulty of establishing a market share in indications such as POS, given how many drugs are used in combination and the difficulty in obtaining market share data for the specific patient population (Fig. 1).
The assumptions and choices for the model are as follows: (1) all patients in year 1 are assumed to be a mix of incidental and prevalent patients; (2) the model does not take into account any treatment switches for any reason; (3) patients are assumed to be 100% compliant to each regimen they receive; (4) for all adjunctive lines it is envisaged that when brivaracetam is introduced, its market share may grow over time, and therefore the treatment mixes including brivaracetam can be adjusted from year 1 to year 5; and (5) the safety profile of AEDs is considered to be similar.
The growth rate was calculated assuming an annual population increase of 0.05%, in accordance with 2016 data from the National Statistics Institute (INE), and a mortality of 1.9% [4], taken from available data for 2013.
The third-generation drugs included in this comparison are those that, according to their summary of product characteristics (SmPC), have the same indications as adjunctive for POS, with or without secondary generalisation: lacosamide [17], eslicarbazepine [18], perampanel [19], retigabine [20] and zonisamide [21] (Table 1). Retigabine was withdrawn from the market in June 2017, but is nevertheless included as it was commercially available at the time of the study (January 2016).
The model was constructed using Microsoft Excel® (Microsoft Corp., Redmond, WA, USA) and is based on the international recommendations for evaluations of this kind [16].
2.2 Estimating the Target Population
The target population was patients over 16 years of age diagnosed with epilepsy and taking AEDs, both in monotherapy and as adjunctive treatment. This was extracted from the database of the Valencian Health Department (Generalitat Valenciana), which registers all holders of a health card for 2013. These data were anonymised and we selected the following variables per patient: age, sex, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes, drug dosage by Anatomical Therapeutic Chemical (ATC) code and pharmaceutical expenditure.
To avoid selecting any patient who was being treated with AEDs for diseases other than epilepsy, the diagnoses related to epilepsy selected were ICD-9-CM 345.90, 345.10 and 345.50, and these were cross-checked with the data for drug consumption corresponding to AEDs with ATC codes N03AA, N03AB, N03AD, N03AE, N03AF, N03AG and N03AX.
The number of patients with POS, with or without secondary generalisation, were estimated from existing epidemiological data in the literature, as were the data on incidence and prevalence [4].
2.3 Perspective and Time Horizon
The BI is determined from the perspective of the health service of the VC with a time horizon of 5 years, from 2016 to 2020.
2.4 Estimating Antiepileptic Drug Market Share and Treatment Mix
To obtain the market shares, the consumption data for AEDs was crossed-checked with the diagnoses of epilepsy in order to extract drug consumption for uses other than epilepsy.
Table 2 shows the total market share of each AED for treating epilepsy, including the total market share for each AED, the percentage of each AED used as monotherapy, the percentage of each AED used as adjunctive treatment and the total annual pharmaceutical expenditure.
The total pharmaceutical expenditure on AEDs was €15,342,650, with the AEDs included in the model accounting for 32.33% of the total (€4,960,118) with a market share of 9.78%. The percentage of patients receiving monotherapy was 3.25% (447 patients) and 14.10% (2395 patients) were treated with adjunctive therapy.
Market share change as each AED is introduced into the model as adjunctive therapy for the treatment of POS. Simulation of how market share varies on the introduction of brivaracetam is shown in Table 3.
To estimate the initial market share of brivaracetam, the patients considered eligible for treatment with brivaracetam were those who epilepsy was not controlled by the other therapies (Table 2). The model simulates the entry of brivaracetam onto the market with a predefined market share that is proportionally extracted from the other available therapies. This approach was adopted in order to reduce the work of compiling data on the present market share of all the relevant substitute therapies. Table 3 shows the number of patients that would be taking each therapy for each of the 5 years in the model.
2.5 Estimate of Costs
The base year for the costs considered in the model is 2016. To calculate the average daily costs for each drug, data were used from the Ministry of Health, Social Services and Equality (Ministerio de Sanidad Servicios Sociales e Igualdad) [22] and BOT-PLUS [23], using the ex-factory price.
All AEDs except brivaracetam have a titration phase on initiating the treatment, varying between several days and several weeks. During this phase treatment is not effective, as the dose is gradually increased daily until it reaches the effective dose. The costs associated with this titration period for each drug must be reflected in the model and were calculated from the dosage scale given in the approved SmPC for each over the time period established to reach the effective treatment dose [24]. These titration costs have been distributed over the 5 years of the study.
The average daily costs of the maintenance phase for each AED were calculated according to the average daily dosage; all drugs included in the model had the same indications as brivaracetam. The dose considered was that stated in the SmPC. In accordance with the ISPOR guidelines [16], costs were considered to have a discount rate of 0% for the base case. Table 4 shows the cost per treatment per day for each AED for the average dose considered and the additional cost of the initial titration phase.
The average daily cost of monotherapy treatment must also be added to the adjunctive treatment cost for each patient. This cost is calculated as an average of that for the most common therapies (carbamazepine, lamotrigine, oxcarbazepine, topiramate and valproate).
The dosage and frequency of administration is based on the SmPC for each product [13]. The pharmacological cost of the therapies studied is tied to the delivered dose. The number of days of treatment considered is 365 days per year.
Costs not related to the drugs, such as medical visits, hospital admissions and emergencies, have not been included in the BIA, which is limited only to the costs of the adjunctive AEDs.
2.6 Sensitivity Analysis
In order to analyse the robustness of the results, a sensitivity analysis was carried out with regard to those parameters of the model considered to have greater uncertainty associated with the values used in the base case [25].
A one-way sensitivity analysis of the BI was performed for the cost variation of the daily dosage of brivaracetam (alternative 1), and for increasing the brivaracetam market share by 10% (alternative 2), keeping the other variables constant.
Additionally, a probabilistic sensitivity analysis (PSA) was performed. In a Monte-Carlo simulation, 1000 interactions were carried out in which multiple variables introduced into the BIA varied simultaneously. The cost of the daily dose behaved as a random variable of normal distribution with an average price of €4 and a typical deviation of 5% of the average (€0.2), being able to adopt any value belonging to the distribution. Effectiveness randomly varied between 50 and 100%. Market share followed the random values of normal distribution with an average obtained from initial values and a typical deviation of 5% of the average. The discount rate varied randomly between 0 and 3%.
From the Monte-Carlo simulation we obtain the average BI and standard deviation and the cumulative probability distribution to establish the probability of a negative (savings) or positive (increased cost) BI.
3 Results
3.1 Market Size
In 2013 there were 4,714,840 people registered with a health card out of a VC population of 4,931,281, of whom 82.58% (3,893,421) were over 16 years old. A diagnosis of epilepsy had been given in 26,972 (50.8% men), with an average age of 51.32 years. Therefore, the percentage of patients with epilepsy among those over 16 years old in the VC for that year was 0.69%.
Given the prevalence of partial-onset epilepsy is 60% [3], the approximate number of patients with partial-onset epilepsy, calculated from the total number of patients diagnosed with epilepsy, will be 16,183, and of these a total of 15,015 will be prevalent and 1168 incidental.
Only 22,676 (84%) of the patients diagnosed with epilepsy in the database took AEDs for treatment and, as such, this study is centred on them. Of this 84% of patients, 61.9% are treated with monotherapy (14,035) and 38.1% with adjunctive treatments (8641).
The potential population for treatment with brivaracetam are those patients using adjunctive treatments. Of the 8641 patients being treated with adjunctive therapy, 14.10% (2395) take one of the AEDs considered in the BIA model.
For the first year studied (2016), the model is based on a population of 2352 patients, the result of extrapolation of the 2013 population to 2016, according to the population growth and mortality data considered.
3.2 Pharmaceutical Expenditure
The model presents results for the annual cost per patient, calculated from both the titration phase (only attributable to the first year) and maintenance (average dose for the following years).
Table 5 shows the evolution of the total daily costs of the medicines according to the evolution of the patients and the market share of each of the treatments. The total cost for each year is calculated according to the daily unit cost and the number of patients on each treatment (Table 3).
Brivaracetam has no titration costs as it can be initiated at an effective dosage, while the other AEDs have the additional costs of titration, as shown in Table 5.
Supposing for the base case that the share of brivaracetam increases from 1.77 to 7.59% in 5 years (Table 3), the drug with the greatest displacement would be lacosamide, which would lose a market share of 2.32% due to the way in which the calculations of drug displacement were made according to the initial market share of each drug.
3.3 Budget Impact
The population of the VC with POS and eligible to take brivaracetam was 2352 patients in 2016 and is expected to stay more or less constant until 2020, assuming that the market share will increase linearly with time. Table 6 shows the total medication cost in the reference scenario (without brivaracetam) and the new scenario (with brivaracetam).
In the reference scenario, the total cost of the medication is estimated to be €3.608 million in the first year, increasing to €3.615 million in the fifth year (up 0.20%), while in the new scenario the total cost would hardly vary over the 5 years (Fig. 2).
It can be seen in Table 6 that the BI, estimated as the difference between both scenarios, is negative, thus representing a saving, and the absolute value increases from €3085 to €13,257. Over the total of the 5 years of the study, the introduction of brivaracetam on the market entails savings of €41,873, that is, 0.23% of the total budget. Savings from lower acquisition costs represents 85.12% of the total and savings for reduced titration costs are 14.9%.
3.4 Sensitivity Analysis
Table 7 shows the result of a one-way sensitivity analysis. A 1% decrease in the daily dosage cost of brivaracetam implies an increase in budget savings of 19.7%, with the percentage of savings on the initial budget being 0.28% for a 5-year time horizon; that is, 0.05% greater than in the base case. An increase in cost of 1% would produce the opposite effect.
A variation of 10% greater than in the base case in the introductory market share of brivaracetam would result in 10% budget savings, with the percentage in savings on the initial budget being 0.26%; that is, 0.02% greater than in the base case.
In the PSA we obtained a pattern of normal distribution of BI, with an average of − €33,719 and a standard deviation of €33,844. The probability that the BI entails a saving for the National Health Service is 84%, which corroborates the robustness of the analysis with the probability obtained in these results (Fig. 3).
4 Discussion
The BIA compares the scenario with and without brivaracetam, taking into account the population eligible for treatment with brivaracetam, the market shares of other adjunctive treatments and their variation on linearly introducing brivaracetam.
The BI is conditioned by the displacement power of brivaracetam, which may be different to that considered and reflects an increasingly large budget saving from 0.09% in 2016 to 0.37% in 2020, an annual increasing average of 0.07%. Furthermore, the displacement of the other existing AEDs takes place as a function of their initial market share, as a result of which the most used drug will also be the most displaced in the model.
The data source for this model is a real-life database of AED consumption for epilepsy in the VC, including the correct figures for the adult population with health cards and the prevalence of epilepsy, as well as present consumption of different drugs on the market. The prevalence of epilepsy obtained was 0.69% of the adult population. The percentage of patients being treated with monotherapy is 61.89%, which is different to that stated in other international data of 70% [26].
The results obtained for the VC can be extrapolated for the national population, in which there were 47,155 adult patients with partial-onset epilepsy in 2016, to give savings of €824,431 over 5 years. This estimate of the target population for the whole of Spain was estimated based on 80% of the national population being over 16 years and epidemiological data from the literature, and not from real data on disease burden.
Therefore, this BIA shows that the gradual introduction of brivaracetam in the VC creates a saving in the health service budget, with the amount depending fundamentally on the estimates used concerning the brivaracetam market share, costs and market penetration throughout a 5-year time horizon.
In the base case, average global savings are estimated to be €41,873 over 5 years, which is 0.23% of the cost attributable in this period to antiepileptic therapies in patients with POS in adjunctive treatment.
The savings in titration costs become increasingly relevant in the period considered, as the titration costs of brivaracetam are zero, while the other AED therapies it would replace always have positive titration costs.
In the first year of the analysis, therapy using brivaracetam can create a positive BI, though these additional costs are compensated for by the savings in titration costs over the following years. Effectively, this lack of need for titration together with its fixed treatment cost per day (independent of dosage) are two of the reasons that would justify the potential savings associated with use of brivaracetam.
The budget savings obtained could be even greater, due to the treatment cost per day of brivaracetam being established at €4.00, independent of the dose used. Therefore, patients who need to increase their dose per day would cost the health system the same and it would help control very refractory patients. Any increase in dose of the other co-adjunctive AEDs considered would, by contrast, bring with it an increase in the treatment cost per day. This effect helps decision-making regarding health management, as the BI would not be affected by a change in brivaracetam dosage for a specific situation.
The majority of the limitations ascribable to the use of assumptions in this model have been dealt with by the sensitivity analysis carried out to test the robustness of the model and to determine the impact on the final result of changes in the most sensitive variables. Nevertheless, there are other kinds of limitations in the model where uncertainty could not be reduced and these must be taken into account.
First, it is a future projection model of the use of a drug based on multiple assumptions and on the attitude of clinicians to the introduction of brivaracetam to the AED market. If this attitude is different to that expected, the brivaracetam market share could be different to that analysed in this study. Nevertheless, the sensitivity analysis shows that even with significant variation in the expected market share, the savings for the health service remain important.
Second, only the costs of the medication were included, which implies that the analysis does not take into account other associated health costs, such as medical visits, etc. The results of the BIA presuppose, therefore, that these other costs are similar for any other scenario, and nor does it incorporate other supposed savings regarding costs of admissions or emergencies [27]. Nevertheless, these savings would be shared between all AEDs proportionally to their market share.
Third, the dosages considered in the base model could underestimate the average real dosages being used by the patients. In this case, the BIA obtained in the base case corresponds to a conservative scenario and the savings could be greater.
Fourth, the assumptions that the effectiveness of brivaracetam is 100%, the discontinuation rate is 0%, compliance is 100% and that all patients remain compliant to the end of the treatment imply a certain removal from clinical reality. Nevertheless, for the purpose of the BIA, this supposition is neutral, as it applies equally to all drugs considered.
Last, the assumption that there will be no dosage increase for any drug throughout the 5 years of the analysis is unrealistic in clinical practice, especially with certain drugs. This would, however, contribute to greater savings in the BIA.
We believe that these effects compensate each other and that, therefore, the figures we reach in our analysis show the real range of savings for the Spanish Health Service possible as a result of the introduction of brivaracetam. The analysis is sufficiently robust and shows savings for important variations of the parameters introduced in the analysis, given that the Monte-Carlo simulation shows the probability for savings is 84%, even when the parameters introduced in the analysis vary.
Having therapeutic alternatives available contributes to the sustainability of the health service, as well as increasing the treatment possibilities for patients and health service professionals. As such, brivaracetam is a therapeutic alternative that will provide savings to the health service for non-controlled epileptic patients receiving monotherapy [14, 15].
5 Conclusions
The BI shows that the introduction of brivaracetam on the Valencian market provides savings in costs, due in part to the lowering of acquisition costs, given that the price of brivaracetam is less than other drugs with a high market share presently, and also because of the decrease in titration costs in the scenario with brivaracetam.
Even with the limitations mentioned in Sect. 4, the analysis concludes that the use of brivaracetam in the Valencian market in patients who do not show a suitable response to conventional AEDs could produce net savings of €41,873 over 5 years.
Regional and national health services should promote the choice of rational and cost-effective therapeutic strategies, especially in chronic conditions such as epilepsy, which ensure long-term compliance with treatment and favour control of the pathologies.
Change history
23 July 2019
A Correction to this paper has been published: https://doi.org/10.1007/s40261-018-0630-8
References
World Health Organization (2017). Epilepsy fact sheet. http://www.who.int/mediacentre/factsheets/fs999/en/. Accessed 12 Jan 2016.
Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy. Neurology. 2017;88:296–303.
Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–85.
García-Ramos R, Pastor AG, Masjuan J, Sánchez C, Gil A. FEEN report on epilepsy in Spain [in Spanish]. Neurologia. 2011;26:548–55.
Brodie MJ. Practical use of newer antiepileptic drugs as adjunctive therapy in focal epilepsy. CNS Drugs. 2015;29:893–904.
Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9.
Sánchez-álvarez JC, Gil-Nagel A, Casas-Fernández C, Mauri-Llerda JA, Salas-Puig J, Sancho-Rieger J. Drug-resistant epilepsy: current recommendations for diagnosis and treatment in Spain. Neurologia. 2012;27:575–84.
Fazel S, Wolf A, Långström N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet. 2013;382(9905):1646–54. https://doi.org/10.1016/S0140-6736(13)60899-5.
Villanueva V, Girón JM, Martín J, Lahuerta J, Dolz M, Cuesta M. Quality of life and economic impact of refractory epilepsy in Spain: the ESPERA study. Neurologia. 2013;28:195–204.
Sancho J, Pena P, Rufo M, Palacios G, Masramon X, Rejas J, LINCE Study Collaborative Group. Health and non-health care resources use in the management of adult outpatients with drug-resistant epilepsy in Spain: a cost-of-illness study (LINCE study). Epilepsy Res. 2008;81:176–187.
Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59–70.
Duncan JS, Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–100.
European Medicines Agency (EMA). EPARs for authorised medicinal products for human use—Stelara. 2016. http://www.emea.europa.eu/humandocs/Humans/EPAR/stelara/stelara.htm. Accessed 29 Nov 2017.
Biton V, Berkovic SF, Abou-Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. 2014;55:57–66.
Ferlazzo E, Russo E, Mumoli L, Sueri C, Gasparini S, Palleria C, et al. Profile of brivaracetam and its potential in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2015;11:2967–73.
Sullivan SD, Mauskopf JA, Augustovski F, Caro JJ, Lee KM, Minchin M, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17:5–14.
European Medicines Agency. Vimpat. Summary of product characteristics. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000863/WC500050338.pdf. Accessed 27 Nov 2017.
European Medicines Agency. Eslicarbazepine. Summary of product characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000988/WC500047225.pdf. Accessed 27 Nov 2017.
European Medicines Agency. Perampanel. Summary of product characteristics. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002434/WC500130840.pdf. Accessed 29 Nov 2017.
European Medicines Agency. Retigabine. Summary of product characteristics. 2016;1–26. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001245/WC500104835.pdf. Accessed 29 Nov 2017.
European Medicines Agency Zonisamide. Summary of product characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004127/WC500204305.pdf. Accessed 29 Nov 2017.
BOE (2010) Real Decreto-Ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. Boe 20/5/2010:45070–45128. https://www.boe.es/diario_boe/txt.php?id=BOE-A-2010-8228. Accessed 29 Nov 2017.
Farmacéuticos CG de CO de BOTfarma. BOT Base de datos del medicamento. https://botplusweb.portalfarma.com/. Accessed 29 Nov 2017.
AEMPS. Informe de Posicionamiento Terapéutico de brivaracetam (Briviact®) en epilepsia. 2017;1–7. https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-brivaracetam-Briviact-epilepsia.pdf. Accessed 29 Nov 2017.
Belén Ferro-Rey M, Roca-Cusachs A, Sicras-Mainar A, Álvarez-Martín C, de Salas-Cansado M. Fixed drug combinations in hypertension: a budget impact analysis for the spanish health system on the marketing of a fixed combination of olmesartan/amlodipine [in Spanish]. Aten Primaria. 2011;43:345–55.
Simoens S. Pharmacoeconomics of anti-epileptic drugs as adjunctive therapy for refractory epilepsy. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):309–15.
Borghs S, Thieffry S, Noack-Rink M, Dedeken P, Hong LS, Byram L, et al. Health care cost associated with the use of enzyme-inducing and non-enzyme-active antiepileptic drugs in the UK: a long-term retrospective matched cohort study. BMC Neurol. 2017;17:59.
Acknowledgements
The opinions expressed in this paper are those of the authors and do not necessary reflect those of the afore-named. Any errors are the authors’ responsibility. We would also like to thank John Wright for the English editing.
Funding
This work was supported partially by the Instituto de Salud Carlos III-Ministerio de Economía y Competitividad and the European Union (FEDER [Fonds Européen de Développement Économique et Régional (European Fund for Economic and Regional Development)] funds)—FIS PI12/00037.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David Vivas Consuelo has received a grant from UCB Pharma. Isabel Barrachina Martínez and Anna Piera Balbastre have no conflicts of interest to declare.
Additional information
The text under the abstract, Conclusion was incorrectly published in the original publication. This is now updated in the original article.
A correction to this article is available online at https://doi.org/10.1007/s40261-018-0630-8.
Rights and permissions
About this article
Cite this article
Barrachina-Martínez, I., Vivas-Consuelo, D. & Piera-Balbastre, A. Budget Impact Analysis of Brivaracetam Adjunctive Therapy for Partial-Onset Epileptic Seizures in Valencia Community, Spain. Clin Drug Investig 38, 353–363 (2018). https://doi.org/10.1007/s40261-017-0615-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-017-0615-z