Abstract
Loxoprofen (Loxonin®, Loxonin® Pap, Loxonin® Tape) is a prodrug-type NSAID that is available in several formulations, including 60 mg tablets, 100 mg hydrogel patches and 50 or 100 mg tape. In active comparator-controlled trials, oral loxoprofen therapy (ranging from 2 days to 6 weeks’ duration depending on the pain type) provided analgesic efficacy that generally did not significantly differ from that of celecoxib for postoperative pain or frozen shoulder, ibuprofen for knee osteoarthritis or naproxen for lumbar pain. In double-blind, double-dummy, multicentre trials, loxoprofen hydrogel patches were noninferior to oral loxoprofen with regard to rates of final overall symptomatic improvement over 1–4 weeks in patients with knee osteoarthritis, myalgia or trauma-induced swelling and pain. Loxoprofen hydrogel patches were also noninferior to other commercially available patches (ketoprofen and indometacin) over 2 or 4 weeks in patients with knee osteoarthritis or myalgia in open-label studies. Oral and topical loxoprofen were generally well tolerated in clinical trials. Thus, loxoprofen is a useful analgesic option for patients with pain and inflammation, with topical loxoprofen potentially reducing the risk of gastrointestinal, cardiovascular and renal complications associated with oral NSAID use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Registered as 60 mg tablets for three-times-daily administration and 100 mg hydrogel patches and 50 and 100 mg tape for once-daily administration |
Oral therapy provides similar analgesic efficacy to other NSAIDs in patients with postoperative pain, knee osteoarthritis, lumbar pain or frozen shoulder |
Topical therapy provides high rates of clinical improvement in patients with knee osteoarthritis, myalgia and trauma-induced swelling and pain |
Generally well tolerated during short-term treatment |
1 Introduction
NSAIDs are widely used in the treatment of pain and inflammation associated with chronic conditions (e.g. osteoarthritis) [1–3], as well as short-term relief (e.g. ≤10 days) from relatively minor conditions, including headache, fever, toothache and back pain [4]. However, oral NSAIDs may increase the risk of cardiovascular (CV) and renal disorders [5, 6], and are known to interact with several medications, including antihypertensives, antithrombotics, antidepressants and corticosteroids [4]. The use of oral nonselective NSAIDs [i.e. inhibitors of both cyclooxygenase (COX)-1 and COX-2 enzymes] also carries an increased risk of upper gastrointestinal (GI) complications (e.g. erosive gastritis and bleeding) [6, 7].
Topical NSAIDs have similar analgesic and anti-inflammatory efficacy to oral NSAIDs, but offer a potentially improved safety profile due to their reduced systemic absorption [8]. As such, US and EU guidelines for osteoarthritis generally recommend topical rather than oral NSAIDs, particularly in patients aged ≥75 years [2, 9], and in those with comorbidities or an increased risk of GI, CV or renal adverse effects [9].
Loxoprofen (Loxonin®, Loxonin® Pap, Loxonin® Tape) is a prodrug-type, nonselective NSAID that was developed in Japan under the assumption that it would be associated with fewer NSAID-related adverse events (AEs) [3]. Oral formulations of loxoprofen have been available in Japan since 1986 [10], and the drug is widely used in clinical practice, being the most commonly prescribed NSAID in Japan and the second most common choice in China (after diclofenac) in 2007 [3]. Loxoprofen is also available in several other East Asian countries [3], as well as parts of Africa, the Middle East and Latin America. In China, loxoprofen 60 mg tablets are available for rheumatoid arthritis, osteoarthritis, pain and inflammation associated with surgery, trauma or tooth removal, and pain and fever caused by acute upper respiratory tract inflammation (URTI) [11]. Loxoprofen 100 mg hydrogel patches and 50 and 100 mg tape are also registered in China for the treatment of inflammation and pain in patients with osteoarthritis, myalgia or trauma-induced swelling and pain [12].
The purpose of this narrative review is to summarize the pharmacological properties and clinical data relevant to the use of oral and topical loxoprofen and provide discussion regarding its place in the management of pain and inflammation. Although 1 % loxoprofen topical gel is also registered in Japan [13], discussion of the use of this product is beyond the scope of this review.
2 Pharmacodynamic Properties of Loxoprofen
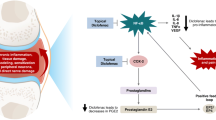
The pharmacodynamic profile of oral loxoprofen is well established and has been reviewed previously [14]. After oral or topical administration, the prodrug-type NSAID loxoprofen undergoes conversion to its active trans-alcohol (trans-OH) metabolite (Sect. 3), which potently suppresses prostaglandin biosynthesis via nonselective inhibition of COX enzymes [3, 10, 14]. In vitro, this trans-OH metabolite showed dose-dependent inhibition of human COX-1 and COX-2 enzymes, while loxoprofen did not appear to inhibit either COX enzyme [15].
In animal studies, oral [14] and topical (hydrogel patches) [16] loxoprofen were shown to have anti-inflammatory and analgesic effects that were similar to or greater than those of other commercially available NSAIDs, including indometacin, ketoprofen, flurbiprofen and felbinac. For instance, in rats with carrageenan-induced paw oedema, the anti-inflammatory effect of loxoprofen hydrogel patches was significantly (p < 0.01) greater than that of indometacin or felbinac patches, and not significantly different from that of ketoprofen or flurbiprofen patches [16]. In addition, the analgesic effect of loxoprofen hydrogel patches in rats with yeast-induced paw inflammation was significantly (p < 0.01) greater than that of indometacin, ketoprofen or felbinac patches, and not significantly different from that of flurbiprofen patches [16].
In patients with type 2 diabetes and overt nephropathy, short-term loxoprofen topical therapy for knee and/or low back pain did not significantly affect renal function or blood pressure [17]. In these patients, loxoprofen 100 mg tape once daily for five days significantly (p < 0.01) reduced visual analogue scale (VAS) pain scores without significantly affecting urinary prostaglandin E2 levels or estimated glomerular filtration rate (eGFR) [17].
2.1 Gastrointestinal Effects
In rats, oral loxoprofen was generally associated with less damaging effects on the GI mucosa than other oral NSAIDs [14]. In fasted rats, loxoprofen was associated with dose-dependent gastric mucosal erosion, with a median effective dose (ED50) of 16.1 mg/kg. By comparison, indometacin, ketoprofen and naproxen had ED50 values for gastric mucosal erosion of 6.4, 6.8 and 11.4 mg/kg, respectively. In well-fed rats, oral loxoprofen was also shown to have dose-dependent ulcerogenic effects on the intestinal mucosa, with an ED50 of 11.5 mg/kg (vs. 4.4, 8.1 and 19.4 mg/kg with indometacin, ketoprofen and naproxen, respectively) [14].
In healthy volunteers, oral loxoprofen was associated with less deleterious effects on the gastric mucosa than oral indometacin [18]. Following single-dose topical application of a loxoprofen 60 mg tablet (diluted in water and sprayed on to the gastric mucosa via endoscopy), there were no significant changes in gastric mucosal haemodynamics and no haemorrhagic erosions. By contrast, a single topical dose of indometacin 25 mg on the gastric mucosa was associated with significant (p < 0.05) decreases in mucosal haemoglobin content and haemoglobin oxygen saturation compared with both baseline and loxoprofen, indicating mucosal ischaemia; haemorrhagic erosions were also evident in the region where indometacin was applied [18].
In randomized studies in healthy volunteers, oral loxoprofen 180 mg/day for 2 weeks, either alone [19, 20] or coadministered with the proton pump inhibitor lansoprazole 15 mg/day [21], was associated with a significantly (p < 0.05) higher incidence of small bowel mucosal injury [19], gastroduodenal ulcers [20] or small intestine mucosal breaks [21] than oral celecoxib 200 mg/day (a COX-2-selective NSAID). When loxoprofen was coadministered with lansoprazole, loxoprofen recipients also had significantly (p = 0.0056) lower haemoglobin levels than celecoxib recipients [21].
3 Pharmacokinetic Properties of Loxoprofen
Loxoprofen was rapidly absorbed after a single oral dose of loxoprofen 60 mg in healthy adult volunteers, with peak plasma concentrations of loxoprofen and its active trans-OH metabolite being reached after ≈30 and ≈50 min [22]. After a single dermal application of four 1 % loxoprofen patches (400 mg) in healthy volunteers, peak plasma concentrations of loxoprofen and its metabolites were reached at 4 and 6 h [10]. In healthy volunteers, ≈10 % of a single topical dose of 1 % loxoprofen (100 mg) was estimated to have been transferred into the body over 12 h [10]. During multiple-dose administration of two 1 % loxoprofen patches (200 mg) once daily for 5 days, steady-state plasma concentrations of loxoprofen and its metabolites were reached on day 4–5 [10, 23].
In studies in healthy volunteers, loxoprofen 100 mg tape was bioequivalent to loxoprofen 100 mg hydrogel patches with regard to the total amount of loxoprofen in the stratum corneum after topical administration [10, 13, 24]. For instance, in one study, the geometric ratio of loxoprofen tape to hydrogel patch for the total amount of loxoprofen in the stratum corneum at 20 and 24 h post-dose had 90 % confidence intervals that were both within the predefined bioequivalence range of 0.8–1.25 [24]. In a subsequent modelled analysis of this study, which estimated the residual amount of loxoprofen in the uncollected stratum corneum, the 90 % confidence interval for the geometric ratio of loxoprofen tape to hydrogel patch for the total amount of loxoprofen in the whole stratum corneum at 20 h post-dose was also within this range, confirming the bioequivalence of these two topical formulations [25].
In healthy volunteers, the plasma protein binding of loxoprofen and its active trans-OH metabolite was 97 and 93 % at 1 h after oral administration of loxoprofen 60 mg [10]. In rats, a single radiolabelled dose of oral loxoprofen 2 mg/kg was predominantly distributed in the liver, kidney, skin, blood and extracellular spaces, with low levels of radioactivity found in the brain and skeletal muscle [14]. In rats, after systemic absorption from topical loxoprofen, distribution of loxoprofen and its metabolites in the blood and urine was generally similar to that observed with oral therapy [26]. However, at 48 h after application, the loxoprofen concentration directly under the application site was more than 9000- and 20-fold higher in skin and skeletal muscle, respectively, than under non-application sites [26].
Following absorption into the systemic circulation, loxoprofen is metabolized to its trans- and cis-OH metabolites predominantly by carbonyl reductase enzymes in the liver [10]. Both metabolites subsequently undergo glucuronidation in the liver to form glucuronide conjugates that are excreted in the urine [10]. In healthy volunteers, a single oral dose of loxoprofen 60 mg was rapidly excreted in the urine, predominantly as glucuronide conjugates of loxoprofen and its trans-OH metabolite (21 and 16 %, respectively), and had a half-life (t½) of ≈1.25 h [22]. Based on a study in rats, loxoprofen is thought to be metabolized to its trans-OH and cis-OH metabolites by carbonyl reductase in the skin and subcutaneous muscle tissue following topical administration, after which the parent drug and its metabolites enter the systemic circulation and are metabolized via a similar route to oral loxoprofen (i.e. excreted as glucuronide conjugates in the urine) [13].
After multiple-dose topical administration in healthy volunteers, daily urinary excretion of loxoprofen and its trans-OH metabolite remained generally consistent from the second 24-h period onwards [10, 13, 23]. From the first application to 48 h after the last patch was removed, the urinary excretion of free and conjugated compound accounted for ≈1 % of the administered dose for loxoprofen and its trans-OH metabolite and ≈0.5 % for its cis-OH metabolite (total cumulative urinary excretion of the free and conjugated compound of 2.67 %) [10, 13, 23]. During topical administration in this study, the mean t½ values of loxoprofen and its trans-OH metabolite were 7.8 and 8.2 h, respectively [23].
3.1 Potential Drug Interactions
Loxoprofen is a water-soluble drug that is not metabolized by cytochrome P450 (CYP) enzymes [22]. In vitro, loxoprofen did not affect the metabolism of drugs that are substrates of CYP1A1/2, CYP2A6, CYP2B6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E1 or CYP3A4 enzymes [22]. Nevertheless, similar to other NSAIDs [4], drug interactions may occur during concomitant use of oral loxoprofen and vitamin K antagonists (e.g. warfarin), sulfonylureas (e.g. tolbutamide), fluoroquinolones (e.g. enoxacin), methotrexate, lithium, thiazide diuretics or antihypertensive agents [22], although no clinically relevant drug interactions have been reported during topical loxoprofen therapy [10, 12]. Consult local prescribing information for further information regarding drug interactions with loxoprofen.
4 Therapeutic Efficacy of Loxoprofen
4.1 Oral Therapy
This section focuses on multiple-dose trials investigating the efficacy of oral loxoprofen in pain and inflammation. Five randomized, active comparator-controlled trials have evaluated the efficacy of oral loxoprofen 60 mg three times daily in patients with postoperative pain (Sect. 4.1.1) [27, 28], knee osteoarthritis (Sect. 4.1.2) [29], nonsurgical lumbar pain [30] (Sect. 4.1.3) or frozen shoulder [31] (Sect. 4.1.4). In addition, a large, noncomparative, multicentre trial investigated the use of oral loxoprofen 60 mg three times daily in elderly patients (aged ≥65 years) with lumbar pain (Sect. 4.1.3) [32]. Where specified, exclusion criteria were similar across trials, and generally included patients with GI disorders (i.e. gastric ulcers or intestinal bleeding) [27, 28, 30–32]. In this section, all drugs were administered orally [27–32].
Overall, loxoprofen was generally as effective as celecoxib [27, 28, 31], ibuprofen [29] and naproxen [30] in reducing pain and inflammation in patients with postoperative pain [27, 28], knee osteoarthritis [29] and lumbar [30] or frozen shoulder [31] pain, and was at least as effective as paracetamol (acetaminophen) in patients with postoperative pain [27].
4.1.1 Postoperative Pain
In adults (aged ≥18 years) who had undergone arthroscopic knee surgery, loxoprofen (n = 53), celecoxib 400 mg (first dose) then 200 mg twice daily (n = 53) or paracetamol 600 mg three times daily (n = 54) was administered from 3 h after surgery until postoperative day 2 [27]. In this trial, the primary outcomes were VAS scores (0–100 mm; higher scores indicate increasing severity) for knee pain at rest or on movement, which were assessed before and after surgery. At 48 h after surgery, loxoprofen and celecoxib both provided significantly (p < 0.05) lower mean VAS pain scores at rest than paracetamol (mean scores of 6.0, 6.0 and 9.8 mm for loxoprofen, celecoxib and paracetamol, respectively), although there was no significant difference between loxoprofen and celecoxib recipients. At 48 h after surgery, mean VAS pain scores on movement were also significantly (p = 0.03) lower with celecoxib than paracetamol (19.8 vs. 26.5 mm), but did not significantly differ between loxoprofen (22.5 mm) and either celecoxib or paracetamol. At 6 and 24 h after surgery, there were no significant between-group differences in either at rest or on movement mean VAS pain scores, other than between celecoxib and paracetamol at 24 h after surgery for pain at rest (10.2 vs. 15.4 mm; p = 0.02). Celecoxib was also associated with a significantly (p = 0.02) greater improvement in subjective global assessment of pain relief than paracetamol at 48 h after surgery, although loxoprofen recipients showed no significant differences compared with celecoxib or paracetamol recipients for this parameter [27].
In another trial, adults (aged >20 years) who had undergone spinal surgery received loxoprofen (n = 73) or celecoxib 100 mg twice daily (n = 68) for 1 week from the morning of postoperative day 1 [28]. The primary outcome was the analgesic effect for postoperative pain, as measured by the 11-point numeric rating scale (NRS). Loxoprofen had generally similar analgesic efficacy to celecoxib on each of the 7 days after spinal surgery, with no between-group differences in maximum and mean NRS pain scores observed (statistical significance not reported). However, after each single administration, loxoprofen was associated with significantly (p < 0.05) greater improvements from baseline (before administration) in mean NRS pain scores than celecoxib at 30 min (–0.3 vs. –0.2) and 2 h (–0.4 vs. –0.3) after administration. Similarly, there were also significantly (p < 0.05) greater improvements in NRS pain scores with loxoprofen than celecoxib at 30 min and 2 h after each administration in both patients with slight (NRS score <5) or severe (NRS score ≥5) pain at baseline. Within each treatment group, significantly greater improvements from baseline in NRS pain scores were observed among patients with a baseline NRS score ≥5 than those with a baseline NRS score <5, indicating that both loxoprofen and celecoxib may be more effective for severe than slight postoperative pain following spinal surgery [28].
4.1.2 Knee Osteoarthritis
In an open-label trial in adults (aged ≥40 years) with knee osteoarthritis (based on American College of Rheumatology criteria), loxoprofen (n = 20) or sustained-release ibuprofen 300 mg twice daily (n = 20) was administered for 4 weeks [29]. Overall efficacy, defined as the percentage of patients with ≥30 % clinical improvement after treatment, did not significantly differ between the loxoprofen and ibuprofen groups (80 vs. 75 %). Loxoprofen and ibuprofen were both associated with significant (p < 0.05) clinical improvements from baseline over 4 weeks in patients with knee osteoarthritis with regard to all assessed parameters, including active knee pain (based on 10 cm VAS scores), 15-metre walk time, joint tenderness, activities of daily life and patient self-assessment. However, the changes in these parameters did not significantly differ between the loxoprofen and ibuprofen groups [29].
4.1.3 Lumbar Pain
In patients aged ≥15 years with disc syndrome, spondylosis, mild spondylolisthesis, spinal stenosis and postural back pain or cumulative spine disorders, loxoprofen (n = 37) or naproxen 250 mg three times daily (n = 35) was administered for 6 weeks [30]. In this trial, both loxoprofen and naproxen recipients had significant (p = 0.0001) improvements from baseline after 6 weeks’ treatment in low back pain scores according to the Judging Criteria for Therapeutic Results on Lumbago (1984) of the Japanese Orthopaedic Academic Society, which includes subjective and objective evaluation of signs and symptoms and activities of daily life. Although pain scores at weeks 1 and 2 were similar to those at week 6 in the naproxen group, scores at week 6 were significantly (p = 0.0001) improved compared with those at weeks 1 and 2 in the loxoprofen group. There were no significant differences between the loxoprofen and naproxen groups with regard to these improvements [30].
In a noncomparative trial in elderly patients with lumbar pain associated with spondylarthritis, lumbar disc disease, spondylolysis and so-called low back pain (n = 4024), loxoprofen was initially administered for 4 weeks, with an extension to 8 weeks or more where possible [32]. After 4 weeks’ treatment with loxoprofen, there were subjective improvements from baseline in lumbar pain in 24 % of patients with severe pain (improved by 3 grades), 30 % of patients with moderate to severe pain (improved by 2 grades) and over 83 % of patients with mild to moderate pain (improved by ≥1 grade) [based on the Japanese Orthopaedic Association’s Standards for Drug Effect on Lumbar Pain] [32].
4.1.4 Frozen Shoulder
In patients with frozen shoulder (aged 26–83 years), loxoprofen (n = 33) or celecoxib 100 mg twice daily (n = 37) was administered for 1–2 weeks [31]. Loxoprofen and celecoxib both provided similar levels of analgesic activity after 1–2 weeks’ treatment, with mean VAS (0–5) pain scores that were significantly (p < 0.0001) improved from baseline in both treatment groups, but with no significant between-group difference. The proportion of patients with disappearance of nocturnal pain after treatment was significantly (p = 0.0281) higher in the celecoxib than the loxoprofen group (71 vs. 37 %), while the proportion of patients with disappearance of pain at rest (43 vs. 67 %) or pain during exercise (9 vs. 23 %) did not significantly differ between groups. There were significant (p < 0.001) improvements from baseline in overall shoulder range of motion in both groups, as determined by the range of motion for elevation/abduction and scores for internal/external rotation (with the arm down), although the between-group differences were not significant for any of these parameters [31].
4.2 Topical Therapy
Two randomized, double-blind, phase II trials in patients with knee osteoarthritis established the optimal dose [33] and administration frequency [34] of topical loxoprofen as one 100 mg hydrogel patch once daily. This section focuses on randomized, multicentre, noninferiority, phase III trials (three conducted in China [35–37] and five in Japan [38–42]) that compared the efficacy of once-daily loxoprofen 100 mg hydrogel patches with that of oral loxoprofen 60 mg three times daily [35–40] (Sect. 4.2.1) or active comparator patches [41, 42] (Sect. 4.2.2) in patients with knee osteoarthritis [35, 38, 41], myalgia [36, 39, 42] or trauma-induced swelling and pain [37, 40] (Table 1). Data from two of these trials [36, 37] are available only as abstracts. Two post-marketing surveillance studies investigating the use of topical loxoprofen have also been conducted in Japan [43, 44] (Sect. 4.2.3). No prospective clinical trials have directly investigated the efficacy of loxoprofen tape; however, as this formulation was shown to be bioequivalent to loxoprofen hydrogel patches (Sect. 3), it is likely to have comparable efficacy.
Where specified in phase III trials, the (co- [41, 42]) primary endpoints were the overall improvement rate at week 2 [35] and/or the final visit [35, 36, 38–42], and the consistency between the final overall improvement and patient pain assessment scores [41, 42]. Where specified [35, 36, 38–42], the overall improvement rate was defined as the proportion of patients with an overall symptomatic improvement of ≥50 % [i.e. ‘markedly improved’ (75–100 %) or ‘improved’ (50–74 %) in a seven-grade rating scale]. The clinical symptoms assessed were pain symptoms, inflammatory symptoms and disability in daily activities in patients with knee osteoarthritis [35, 38, 41]; pain at rest, pain on exertion, muscle tightness and restricted movement in patients with myalgia [36, 39, 42]; and pain symptoms, inflammatory symptoms and restricted movement in patients with trauma-induced swelling and pain [37, 40]. Where specified [35], patients with knee osteoarthritis were required to be aged ≥40 years and fulfil the American College of Rheumatology criteria for knee osteoarthritis. Where specified across trials, baseline patient characteristics were generally well balanced between treatment groups [35, 38–42].
4.2.1 Compared with Oral Loxoprofen
In six double-blind, double-dummy trials, patients with knee osteoarthritis (4 weeks’ treatment) [35, 38], myalgia (2 weeks’ treatment) [36, 39] or trauma-induced swelling and pain (1 week’s treatment) [37, 40] were assigned to receive loxoprofen hydrogel patch therapy or oral loxoprofen (Table 1).
Loxoprofen hydrogel patches were noninferior to oral loxoprofen with regard to the final overall improvement rate in patients with knee osteoarthritis [35, 38], myalgia [36, 39] or trauma-induced swelling and pain [37, 40] (Table 1). In the Chinese study in patients with knee osteoarthritis, the overall improvement rate did not significantly differ between loxoprofen patch and oral therapy at the co-primary timepoints of week 2 (27.2 vs. 27.7 %) or week 4 (Table 1) [35].
In two trials in patients with knee osteoarthritis, >50 % of patients in both the loxoprofen patch and oral therapy groups had improvements from baseline to week 4 in clinical pain symptoms (pain at rest, tenderness and pain on motion), inflammatory symptoms (swelling, effusion and burning) and disability in daily activities (squatting, standing and going up and down stairs) [35, 38]. In general, improvement in these symptoms showed no significant between-group differences [35, 38]; however, in the Chinese study, pain at rest showed significantly (p = 0.043) greater improvement with loxoprofen patch than oral therapy at week 4 [35].
Where specified in patients with myalgia [39], there were improvements from baseline to week 2 in pain at rest, tenderness, pain on exertion, muscle tightness and restricted movement in 71–85 % of patients in both the loxoprofen patch and oral therapy groups. In the Japanese study, symptomatic improvement in pain at rest, tenderness and restricted movement significantly (p < 0.05) favoured loxoprofen patch versus oral therapy, although improvements in pain on exertion and muscle tightness did not significantly differ between groups [39]. In the Chinese trial, improvements in rest pain, tenderness, pain on motion and disability in daily activities showed no significant between-group differences at week 1 or 2 [36].
In the Japanese trial in patients with trauma-induced swelling and pain, improvements from baseline to week 1 in pain symptoms (pain at rest, tenderness and pain on exertion), inflammatory symptoms (swelling and burning) and restricted movement were observed in most patients (≥92 %) in both the loxoprofen patch and oral therapy groups [40]. In the Chinese trial, symptomatic improvement in these parameters did not significantly differ between the loxoprofen patch and oral therapy groups [37].
Where reported, total drug exposure was lower with loxoprofen hydrogel patches than oral therapy [35, 37]. For instance, in the Chinese trial in patients with knee osteoarthritis, mean total loxoprofen exposure after 4 weeks’ treatment was 2750 mg in the hydrogel patch group and 4614 mg in the oral therapy group [35].
4.2.2 Compared with Active Comparator Patches
Two open-label, phase III trials compared the efficacy of once-daily loxoprofen 100 mg patches with that of twice-daily ketoprofen 30 mg patches in patients with knee osteoarthritis over 4 weeks [41] or twice-daily indometacin 70 mg patches in patients with myalgia over 2 weeks [42] (Table 1).
With regard to the final overall improvement rate (co-primary endpoint), loxoprofen hydrogel patches were noninferior to ketoprofen patches in patients with knee osteoarthritis over 4 weeks [41] and indometacin patches in patients with myalgia over 2 weeks [42] (Table 1). In both trials, the weighted κ consistency coefficient (co-primary endpoint) did not demonstrate clear consistency between the final overall improvement and patient pain assessment scores, although further analysis indicated a certain level of consistency in each trial [41, 42].
In patients with knee osteoarthritis, baseline to week 4 improvements in clinical symptoms (including those related to pain, inflammation and disability in daily activities) were observed in 57–87 % of patients in both the loxoprofen and ketoprofen groups, although there were no significant between-group differences in any of these symptomatic improvements [41]. Similarly, in patients with myalgia, there were baseline to week 2 improvements in pain at rest, tenderness, pain on exertion, muscle tightness and restricted movement in 78–87 % of patients in both the loxoprofen and indometacin groups, but these symptomatic improvements did not significantly differ between groups [42].
4.2.3 Post-Marketing Studies
Post-marketing surveillance studies based on case reports in Japan have confirmed the efficacy of loxoprofen topical therapy in the real-world setting [10, 43, 44]. In patients with osteoarthritis, myalgia or trauma-induced swelling and pain (n = 1374), once-daily loxoprofen 100 mg hydrogel patches for 12 weeks provided overall improvement rates of 96.5–98.5 % [10, 44]. In patients with osteoarthritis (n = 614), loxoprofen hydrogel patches were associated with an overall improvement rate of 95.5 % [10]. Similarly, loxoprofen 50 or 100 mg tape once daily for 12 weeks was associated with overall improvement rates of 95.0–98.9 % among patients with osteoarthritis, myalgia or trauma-induced swelling and pain (n = 955) [10, 43].
5 Tolerability of Loxoprofen
Loxoprofen, either as oral or topical therapy, was generally well tolerated in the clinical trials discussed in Sect. 4. According to labelling information, the most common AEs with oral loxoprofen include GI disorders (gastric discomfort, abdominal pain, nausea, vomiting or loss of appetite), hypersensitivity (oedema, rash, urticaria, pruritus and fever) and drowsiness [11, 22]. The Japanese interview form indicates that clinically relevant AEs may occur with oral loxoprofen, including shock or anaphylaxis, blood disorders (agranulocytosis, leukopenia, thrombocytopenia or haemolytic anaemia), toxic epidermal necrolysis or Stevens-Johnson syndrome, renal disorders (acute renal failure, nephrotic syndrome or interstitial nephritis), rhabdomyolysis, interstitial pneumonia, acute respiratory disorder, GI haemorrhage or perforation, hepatic impairment or jaundice, aseptic meningitis and congestive heart failure [22]. Oral loxoprofen has also been associated with palpitations and increased blood pressure [22].
In clinical trials, no AEs occurred in patients receiving oral therapy with loxoprofen or celecoxib for 2 days for postoperative pain following arthroscopic knee surgery [27]. In patients receiving loxoprofen or celecoxib for postoperative pain after spinal surgery for 1 week, one patient (1.4 %) stopped loxoprofen 4 days after surgery due to treatment-related renal dysfunction, which subsequently resolved without further treatment; there were no other treatment-related AEs in this trial [28]. The overall incidence of AEs was significantly (p < 0.01) lower with loxoprofen than ibuprofen (5 vs. 20 %) over 4 weeks in patients with knee osteoarthritis, with a mild GI reaction occurring in one loxoprofen recipient and upper abdominal pain and loss of appetite each occurring in two ibuprofen recipients [29]. Over 6 weeks’ treatment in patients aged ≥15 years with lumbar pain, mild GI irritation was reported in 4 of 37 loxoprofen and 3 of 35 naproxen recipients, and one patient in the naproxen group reported headache [30]. In this trial, significant GI irritation leading to treatment discontinuation occurred in two patients from each group [30]. In elderly patients with lumbar pain, 84 of 4024 loxoprofen recipients experienced ≥1 AE over 4 weeks, with GI disorders being the most common of these AEs [32]. Most AEs in this trial were mild, although moderate AEs requiring dosage reduction or treatment discontinuation occurred in 11 patients, and 1 patient experienced a severe AE (postduodenal bulb ulcer) [32]. Over 1–2 weeks’ treatment in patients with frozen shoulder, AEs occurred in 6 of 33 loxoprofen recipients (two cases of anorexia and one case each of gastric pain, constipation, diarrhoea and vomiting) and 2 of 37 celecoxib recipients (one case each of frequent bowel movements and constipation) [31].
The Japanese interview form for topical loxoprofen indicates that the most common AEs with loxoprofen hydrogel patches include skin symptoms (e.g. pruritus, erythema and contact dermatitis), GI symptoms (e.g. stomach discomfort) and abnormal laboratory findings (e.g. elevated aminotransferase levels) [10]. In patch and photo-patch testing studies in healthy adult volunteers, skin irritation or photosensitivity did not occur with loxoprofen 100, 200 or 400 mg hydrogel patches; therefore, loxoprofen hydrogel patches are considered to have favourable skin safety [10].
In general, the incidence of any AE with loxoprofen hydrogel patches was 1.4- to 1.9-fold lower than with oral loxoprofen over 1–4 weeks’ treatment in clinical trials, although these differences were not statistically significant [35–39]. For instance, over 2 weeks in patients with myalgia, AEs were reported in 14.3 and 22.0 % of patients in the loxoprofen patch and oral therapy groups in the Chinese trial [36] and 9.4 and 17.6 % of patients, respectively, in the Japanese trial [40]. However, in the Japanese trial in patients with trauma-induced swelling and pain, the incidence of AEs with loxoprofen was 7.8 and 6.9 % in the patch and oral therapy groups over 1 week [40]. In active comparator patch-controlled trials, the incidence of AEs with loxoprofen hydrogel patches did not significantly differ from that observed with ketoprofen patches over 4 weeks in patients with knee osteoarthritis (10.0 vs. 10.7 %) [41] or indometacin patches over 2 weeks in patients with myalgia (5.0 vs. 5.8 %) [42]. In general, serious AEs were not reported in any of the treatment groups in these trials [35–42], although a serious meniscus injury due to knee sprain was reported in one patient with knee osteoarthritis receiving oral loxoprofen [35].
Where specified in patients with knee osteoarthritis, the most common AEs with loxoprofen hydrogel patches or oral therapy (≥2.5 % incidence in either group) were GI disorders (8.4 vs. 12.9 %), skin or subcutaneous disorders (4.8 vs. 4.7 %) and infections (1.2 vs. 7.2 %) [35]. In this trial, routine laboratory tests showed mild anaemia and haematuria in two patients (2.4 %) in the loxoprofen patch group, and mild changes in haematological and urine tests in three patients (3.5 %) in the loxoprofen oral therapy group; these changes were considered possibly drug-related in one patient receiving oral loxoprofen [35].
Assessment of the global safety rating was included as a co-primary endpoint in the three Japanese trials evaluating loxoprofen hydrogel patch versus oral therapy [38–40]. In these trials, there were no significant differences between loxoprofen patches and oral therapy in global safety ratings in patients with knee osteoarthritis over 4 weeks (safety rate of 81.2 vs. 70.9 %; treatment difference 10.2 %, 95 % CI –2.4 to 22.9) [38], myalgia over 2 weeks (90.6 vs. 82.4 %; treatment difference 8.2 %, 95 % CI –0.9 to 17.2) [39] or trauma-induced swelling and pain over 1 week (92.2 vs. 93.1 %; treatment difference –1.0 %, 95 % CI –8.1 to 6.2) [40].
In post-marketing surveillance studies, the incidence of adverse drug reactions (ADRs) with loxoprofen hydrogel patch therapy over 12 weeks was 2.1 % in patients with osteoarthritis, myalgia or trauma-induced swelling and pain (n = 1427) [10, 44], and 3.7 % in patients with osteoarthritis (n = 624) [10]. Over 12 weeks’ treatment with loxoprofen tape, ADRs were reported in 3.4 % of patients with osteoarthritis, myalgia or trauma-induced swelling and pain (n = 987) [10, 43]. The most commonly reported ADRs with both formulations included contact dermatitis, pruritus and erythema at the application site [10, 43, 44]. There were no serious ADRs reported in these studies [43, 44].
6 Dosage and Administration of Loxoprofen
In China, oral loxoprofen is indicated for use in adults to reduce inflammation and pain induced by rheumatoid arthritis, osteoarthritis, lumbar pain, frozen shoulder, neck-shoulder-arm syndrome, surgery, wounds, tooth pain or tooth removal, and to reduce fever or pain induced by URTI [11]. In general, the recommended dosage is one tablet (60 mg) taken three times daily in patients with inflammation and pain (one to two tablets may be taken at a time with on-demand use), or one tablet at a time as needed (usually up to twice daily) in patients with URTI. Patients should not exceed three tablets (180 mg) daily, and should avoid taking oral loxoprofen on an empty stomach [11].
Loxoprofen 100 mg hydrogel patches and 50 and 100 mg tape are indicated in China as an analgesic and anti-inflammatory agent for patients with osteoarthritis, myalgia or trauma-induced swelling and pain [12]. The hydrogel patch or tape should be applied once daily to the skin over the affected area. Treatment is contraindicated in patients with a history of hypersensitivity to any of the product ingredients or aspirin-induced asthma [12]. Local prescribing information should be consulted for more information regarding durations of treatment, warnings and precautions and use in special patient populations.
7 Place of Loxoprofen in the Management of Pain and Inflammation
NSAIDs are very effective in the management of pain and inflammation; however, their use is associated with upper GI, CV and renal complications [1, 5–7]. The Chinese treatment guidelines for osteoarthritis recommend using NSAIDs when first-line treatment with paracetamol does not provide adequate pain relief in patients who do not have a high risk of GI, hepatic, renal or CV diseases [45]. Individualization of oral NSAID therapy according to patient risk factors and drug characteristics is recommended, with selective COX-2 inhibitors (e.g. celecoxib) or nonselective NSAIDs plus gastric mucosal protective agents (e.g. proton pump inhibitors) being considered potentially beneficial in patients with a high risk of GI disorders [45]. The EU guidelines for knee osteoarthritis recommend oral NSAIDs at the lowest effective dose for the shortest duration necessary for the advanced pharmacological management of persistently symptomatic patients [9], while the US osteoarthritis guidelines recommend not using oral NSAIDs in patients with chronic kidney disease stage IV or V (eGFR <30 mL/min) [2].
In general, the choice of NSAID is dependent on individual patient characteristics [7, 9]. According to the American Heart Association, nonselective NSAIDs may be considered when initial treatment with aspirin, paracetamol or narcotic analgesics (for acute pain) is not effective, tolerated or appropriate; however, these patients should have a low risk of CV thrombotic events and be prescribed the lowest effective dose to control their symptoms [7]. Based on clinical evidence, the EU treatment guidelines for knee osteoarthritis suggests that topical NSAIDs carry a lower risk of GI complications than oral NSAIDs, although they are often associated with an increased risk of mild skin reactions [9] (Sect. 5). Loxoprofen is a prodrug-type, nonselective NSAID that is registered for oral or topical (hydrogel patches or tape) use in the treatment of pain and inflammation in China (Sect. 6) [not licensed in the USA or EU]. The loxoprofen tape formulation has bioequivalent pharmacokinetic properties to the hydrogel patch (Sect. 3), but is less likely to fall off, due to its superior adhesive properties [10].
In active comparator-controlled trials, oral loxoprofen therapy (ranging from 2 days to 6 weeks’ duration depending on the pain type) provided analgesic efficacy that generally did not significantly differ from that of celecoxib for postoperative pain (Sect. 4.1.1) or frozen shoulder (Sect. 4.1.4), ibuprofen for knee osteoarthritis (Sect. 4.1.2) and naproxen for lumbar pain (Sect. 4.1.3), and provided significantly greater improvements in pain at rest than paracetamol in patients with postoperative pain following knee surgery (Sect. 4.1.1). Furthermore, there were significant improvements from baseline in lumbar pain in elderly patients receiving oral loxoprofen over 4 weeks in a single-arm trial (Sect. 4.1.3).
In double-blind trials, once-daily loxoprofen 100 mg hydrogel patches were noninferior to oral loxoprofen 60 mg three times daily with regard to the rate of final overall improvement in clinical symptoms over 1–4 weeks in patients with knee osteoarthritis, myalgia or trauma-induced swelling and pain (Sect. 4.2.1). Across these trials, the majority (60–98 %) of patients in both treatment groups had a ≥50 % overall improvement in clinical symptoms (Sect. 4.2.1). In open-label studies, loxoprofen hydrogel patches were also noninferior with regard to the final overall improvement rate to ketoprofen patches in patients with knee osteoarthritis over 4 weeks and indometacin patches in patients with myalgia over 2 weeks (Sect. 4.2.2). In post-marketing studies in Japan, loxoprofen hydrogel patches and tape were both associated with high rates of overall clinical improvement (≥95 %) among patients with osteoarthritis, myalgia or trauma-induced swelling and pain (Sect. 4.2.3).
NSAIDs as a class are mainly associated with GI, CV and renal complications [4], and the labelling information for oral loxoprofen includes warnings regarding the increased risks of congestive heart failure, renal disorders and GI haemorrhage or perforation (Sect. 5). In an analysis of Japanese clinical trials in patients with osteoarthritis or rheumatoid arthritis, the incidence of serious GI events was significantly higher with oral loxoprofen than oral celecoxib (p = 0.039), while the incidence of serious CV events did not significantly differ between treatments [46]. Although short-term topical loxoprofen had no effect on renal function or blood pressure in type 2 diabetic patients with nephropathy (Sect. 2), oral loxoprofen had ulcerogenic effects on the intestinal mucosa in well-fed rats (Sect. 2.1).
In clinical trials, loxoprofen was generally well tolerated, with the most common AEs being GI and hypersensitivity reactions with oral therapy, and skin and GI reactions and abnormal laboratory parameters with hydrogel patches (Sect. 5). In general, the overall incidence of AEs was numerically lower with loxoprofen hydrogel patches than oral therapy, although these differences were not significant (Sect. 5). Moreover, the overall AE incidence did not significantly differ between loxoprofen patches and ketoprofen or indometacin patches (Sect. 5). In post-marketing studies, contact dermatitis, pruritus and erythema at the application site were the most common ADRs over 12 weeks’ treatment with topical loxoprofen (Sect. 5).
In trials comparing loxoprofen hydrogel patches with oral therapy, where specified, total loxoprofen exposure was 1.7-fold lower with patches than oral therapy (Sect. 4.2.1). During long-term NSAID treatment, minimizing systemic exposure may help to reduce the risk of NSAID-related complications [35]. Although the overall incidence of AEs did not significantly differ between topical and oral therapy in these short-term studies (Sect. 5), it is possible that the risk-benefit ratio may favour topical over oral loxoprofen therapy with longer treatment durations in clinical practice [35]. Longer-term studies will be necessary to confirm this, and are awaited with interest.
It should be noted that clinical trials evaluating the use of loxoprofen for pain and inflammation have mostly been conducted in Japanese and Chinese patients, and extrapolation of these findings into other ethnic populations should be made with caution. Further studies investigating the use of loxoprofen in other patient populations would be of interest.
In conclusion, the water-soluble, prodrug-type NSAID loxoprofen is an effective and generally well-tolerated analgesic option for patients with pain and inflammation that is available in oral and topical formulations. In particular, loxoprofen hydrogel patches and tape may potentially benefit elderly patients and those with comorbidities or an increased risk of GI, CV or renal complications with oral NSAIDs.
Data selection sources:
Relevant medical literature (including published and unpublished data) on loxoprofen was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 11 July 2016], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Loxoprofen, Loxonin, CS-600, inflammat*, pain, osteoarthritis, myalgia, swelling.
Study selection: Studies in patients with musculoskeletal or orthopaedic pain and inflammation who received loxoprofen. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Burmester G, Lanas A, Biasucci L, et al. The appropriate use of non-steroidal anti-inflammatory drugs in rheumatic disease: opinions of a multidisciplinary European expert panel. Ann Rheum Dis. 2011;70(5):818–22.
Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465–74.
Arakawa T, Fujiwara Y, Sollano JD, et al. A questionnaire-based survey on the prescription of non-steroidal anti-inflammatory drugs by physicians in East Asian countries in 2007. Digestion. 2009;79(3):177–85.
Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061–75.
Scarpignato C, Lanas A, Blandizzi C, et al. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis—an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13:[article no. 55].
Scheiman JM. NSAID-induced gastrointestinal injury: a focused update for clinicians. J Clin Gastroenterol. 2016;50(1):5–10.
Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634–42.
Rannou F, Pelletier JP, Martel-Pelletier J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S18–21.
Bruyère O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 Suppl):S3–11.
Daiichi Sankyo Co Ltd. Loxonin® Pap 100 mg, Tape 50 mg and 100 mg (loxoprofen sodium hydrate patches): Japanese interview form on drugs. 2015. http://www.pmda.go.jp. Accessed 8 Jan 2016.
Daiichi Sankyo Co Ltd. Loxoprofen sodium tablets: Chinese prescribing information. 2009. http://www.daiichisankyo.com.cn. Accessed 2 Mar 2016.
Daiichi Sankyo Co Ltd. Loxoprofen patches: Chinese prescribing information. 2015. http://www.daiichisankyo.com.cn. Accessed 2 Mar 2016.
Daiichi Sankyo Co Ltd. Loxonin® Pap 100 mg, Tape 50 mg and 100 mg, Gel 1 %: Japanese summarized product information. 2014.
Tanaka K, Terada A, Iizuka Y, et al. Loxoprofen sodium (CS-600), a new non-steroidal anti-inflammatory drug. Ann Rep Sankyo Res Lab. 1984;36:1–43.
Noguchi M, Kimoto A, Gierse JK, et al. Enzymologic and pharmacologic profile of loxoprofen sodium and its metabolites. Biol Pharm Bull. 2005;28(11):2075–9.
Hamamoto T, Takeuchi S, Sasakura M, et al. Anti-inflammatory and analgesic effects of hydrogel patches containing loxoprofen sodium [Japanese with English abstract]. J Clin Ther Med. 2006;22(3):179–86.
Araki H, Kuwagata S, Soumura M, et al. Safety and efficacy of skin patches containing loxoprofen sodium in diabetic patients with overt nephropathy. Clin Exp Nephrol. 2014;18(3):487–91.
Kawano S, Tsuji S, Hayashi N, et al. Effects of loxoprofen sodium, a newly synthesized non-steroidal anti-inflammatory drug, and indomethacin on gastric mucosal haemodynamics in the human. J Gastroenterol Hepatol. 1995;10(1):81–5.
Mizukami K, Murakami K, Yamauchi M, et al. Evaluation of selective cyclooxygenase-2 inhibitor-induced small bowel injury: randomized cross-over study compared with loxoprofen in healthy subjects. Dig Endosc. 2013;25(3):288–94.
Sakamoto C, Kawai T, Nakamura S, et al. Comparison of gastroduodenal ulcer incidence in healthy Japanese subjects taking celecoxib or loxoprofen evaluated by endoscopy: a placebo-controlled, double-blind 2-week study. Aliment Pharmacol Ther. 2013;37(3):346–54.
Fujimori S, Hanada R, Hayashida M, et al. Celecoxib monotherapy maintained small intestinal mucosa better compared with loxoprofen plus lansoprazole treatment: a double-blind, randomized, controlled trial. J Clin Gastroenterol. 2016;50(3):218–26.
Daiichi Sankyo Co Ltd. Loxonin® (loxoprofen sodium) tablets 60 mg, fine granules 10 %: Japanese interview form on drugs. 2014. http://www.info.pmda.go.jp. Accessed 18 May 2016.
Sugawara S, Hasegawa S, Naganuma H, et al. Pharmacokinetic study of the hydrogel patch containing loxoprofen sodium (LX-A) following 5 days applications repeated once or twice a day [Japanese with English abstract]. J Clin Ther Med. 2006;22(4):279–92.
Chen X, Zhao Q, Hitsu E, et al. Dermatopharmacokinetic bioequivalence study of two types of topical patches containing loxoprofen sodium. Int J Clin Pharmacol Ther. 2014;52(10):927–32.
Chen X, Matsuzawa T, Hitsu E, et al. Model of the dermatopharmacokinetic profile of two loxoprofen patches for bioequivalence confirmation. Int J Clin Pharmacol Ther. 2015;53(5):412–3.
Matsuzawa T, Sairo H, Kurihara A, et al. Absorption, distribution, metabolism and excretion after dermal application of hydrogel patch containing loxoprofen sodium in rats [Japanese with English abstract]. J Clin Ther Med. 2006;22(3):187–203.
Onda A, Ogoshi A, Itoh M, et al. Comparison of the effects of treatment with celecoxib, loxoprofen, and acetaminophen on postoperative acute pain after arthroscopic knee surgery: a randomized, parallel-group trial. J Orthop Sci. 2016;21(2):172–7.
Sekiguchi H, Inoue G, Nakazawa T, et al. Loxoprofen sodium and celecoxib for postoperative pain in patients after spinal surgery: a randomized comparative study. J Orthop Sci. 2015;20(4):617–23.
Shi Y-Q, Han X-H. Clinical observation of loxoprofen sodium in treatment of patients with knee osteoarthritis [Chinese with English abstract]. Pharm Care Res. 2004;4(1):46–8.
Waikakul S, Soparat K. Effectiveness and safety of loxoprofen compared with naproxen in nonsurgical low back pain: a parallel study. Clin Drug Investig. 1995;10(1):59–63.
Ohta S, Komai O, Hanakawa H. Comparative study of the clinical efficacy of the selective cyclooxygenase-2 inhibitor celecoxib compared with loxoprofen in patients with frozen shoulder. Mod Rheumatol. 2014;24(1):144–9.
Aoki T. Effectiveness and safety of loxoprofen in elderly patients with lumbar pain. Drug Invest. 1992;4(6):477–83.
Sugawara S, Tateishi A, Tanaka M, et al. A dose finding study of a hydrogel patch containing loxoprofen sodium (LX-A) in patients with osteoarthritis of the knee (late phase II clinical study): a randomized, double-blind, placebo controlled comparative study [Japanese with English abstract]. J Clin Ther Med. 2006;22(4):293–310.
Sugawara S, Tateishi A, Tanaka M, et al. Evaluation of administration frequency of LX-A in patients with osteoarthritis of the knee: randomized, open label study compared between once-daily administration and twice-daily administration [Japanese with English abstract]. J Clin Ther Med. 2006;22(4):311–26.
Mu R, Bao CD, Chen ZW, et al. Efficacy and safety of loxoprofen hydrogel patch versus loxoprofen tablet in patients with knee osteoarthritis: a randomized controlled non-inferiority trial. Clin Rheumatol. 2016;35(1):165–73.
Zhao DB, Shi YQ, Li ZG, et al. Effectiveness and safety of hydrogel patches containing loxoprofen sodium in patients with myalgia: a randomized, controlled, double-blind, double-parallel, multicenter phase 3 trial [abstract no. APLAR-0134]. Int J Rheum Dis. 2013;16(Suppl 1):111.
Zhang H, Lin J, Sun T, et al. Double-blind, multicenter trial to evaluate safety and efficacy of hydrogel patch containing loxoprofen-sodium in treating swelling and pain caused by trauma [abstract no. APLAR-0153]. Int J Rheum Dis. 2013;16(Suppl 1):111.
Sugawara S, Kuroki Y, Tateishi A, et al. The effects of Loxonin® Pap 100 mg (hydrogel patch containing loxoprofen sodium) for the treatment of patients with osteoarthritis of the knee: randomized, double-blind clinical study with loxoprofen sodium tablets [Japanese with English abstract]. J Clin Ther Med. 2006;22(5):393–409.
Sugawara S, Kuroki Y, Tanaka M, et al. The effects of Loxonin® Pap 100 mg (hydrogel patch containing loxoprofen sodium) for the treatment of myalgia: randomized, double-blind study compared with loxoprofen sodium tablets [Japanese with English abstract]. J Clin Ther Med. 2006;22(5):411–26.
Sugawara S, Iwasaki Y, Aoki T. The effects of Loxonin® Pap 100 mg (hydrogel patch containing loxoprofen sodium) for the treatment of swelling or pain following injuries: randomized, double-blind study with loxoprofen sodium tablets [Japanese with English abstract]. J Clin Ther Med. 2006;22(5):427–42.
Sugawara S, Nobuoka F, Ogawa D, et al. Clinical efficacy of the hydrogel patch containing loxoprofen sodium (LX-A) on osteoarthritis of the knee: a randomized, open label clinical study with ketoprofen patch (phase III therapeutic confirmatory study) [Japanese with English abstract]. J Clin Ther Med. 2007;23(1):55–71.
Sugawara S, Nobuoka F, Ogawa D, et al. Clinical efficacy of the hydrogel patch containing loxoprofen sodium (LX-A) on myalgia: a randomized, open label clinical study compared with indometacin patch (phase III therapeutic confirmatory study) [Japanese with English abstract]. J Clin Ther Med. 2007;23(2):127–41.
Mizutani H, Miyano F, Uozu A, et al. Drug use result survey of Loxonin® Tape (patch containing loxoprofen sodium hydrate) [Japanese with English abstract]. J Clin Ther Med. 2010;26(10):727–41.
Mizutani H, Miyano F, Uozu A, et al. Drug use result survey of Loxonin® Pap (hydrogel patch containing loxoprofen sodium hydrate) [Japanese with English abstract]. J Clin Ther Med. 2010;26(3):227–40.
Chinese Orthopaedic Association. Diagnosis and treatment of osteoarthritis. Orthop Surg. 2010;2(1):1–6.
Sakamoto C, Soen S. Efficacy and safety of the selective cyclooxygenase-2 inhibitor celecoxib in the treatment of rheumatoid arthritis and osteoarthritis in Japan. Digestion. 2011;83(1–2):108–23.
Acknowledgments
During the peer review process, the manufacturer of loxoprofen was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Sarah Greig and Karly Garnock-Jones are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: X. Chen, Clinical Pharmacology Research Center, Peking Union Medical College Hospital, Beijing, China; H.-G. Xie, General Clinical Research Center, Nanjing First Hospital, Nanjing Medical University, Nanjing, China.
Rights and permissions
About this article
Cite this article
Greig, S.L., Garnock-Jones, K.P. Loxoprofen: A Review in Pain and Inflammation. Clin Drug Investig 36, 771–781 (2016). https://doi.org/10.1007/s40261-016-0440-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0440-9