Abstract
Background
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often used to treat inflammation, pain, and fever, but no criterion standard exists for the management of postoperative pain following spinal surgery. In the present study, we compared the analgesic efficacy of loxoprofen sodium (loxoprofen) and celecoxib for the management of postoperative pain following spinal surgery.
Methods
One-hundred forty-one patients (mean age 62.2 years) were randomly assigned to two groups before spinal surgery: a loxoprofen group (n = 73, 180 mg/day) and a celecoxib group (n = 68, 200 mg/day). The drugs were administered from 1 day until 7 days after surgery. A numeric rating scale (NRS) was used to evaluate pain at nine predefined times every day and the findings were compared between the two groups. Laboratory data and adverse events were also recorded.
Results
There was no significant difference in the maximum and mean NRS scores on each day between loxoprofen and celecoxib, suggesting a comparable analgesic effect for these two NSAIDs. Greater improvement in the NRS score between preadministration (baseline) and 30 min or 2 h after administration was obtained for loxoprofen. This tendency was shown for both slight (NRS score <5 at baseline) and severe pain (NRS score ≥5 at baseline). Loxoprofen was discontinued in one patient on day 4 because of renal dysfunction. Celecoxib was discontinued in one patient on day 2 at the patient’s request.
Conclusions
Both loxoprofen sodium and celecoxib were well tolerated for the relief of acute postoperative pain after spinal surgery. A single administration of loxoprofen showed superior and rapid effectiveness compared with celecoxib for both slight and severe postoperative pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) have anti-inflammatory, antipyretic, and analgesic properties, and are among the most widely prescribed drugs worldwide [1]. NSAIDs are commonly used for the treatment for osteoarthritis, rheumatoid arthritis, and other inflammatory pain conditions, and act via the inhibition of cyclooxygenase enzymes (COX-1 and -2) [2]. Loxoprofen sodium (loxoprofen, Loxonin; Daiichi-Sankyo Co, Tokyo, Japan) is a prodrug in the propionic acid class, which is marketed in Asia, Mexico, and Brazil, but not in the United States [3].
In a recent survey of nonselective NSAID prescriptions in East Asia, loxoprofen was the physicians’ first-choice nonselective NSAID and the most frequently prescribed [4]. By contrast, celecoxib (Celecox; Pfizer Inc, Tokyo, Japan) was marketed in Japan in 2007, as the first NSAID of the coxib family and selective COX-2 inhibitor developed to target COX-2. Its inhibitory effect on COX-1 is minimal and it does not suppress the formation of COX-1-derived prostaglandin, and causes upper digestive tract injury less frequently and less severely than conventional NSAIDs [5–8]. NSAIDs have a significant role in postoperative pain control because they can reduce the use of opioids [9–11], which are associated with a variety of side effects, such as ventilatory depression, drowsiness and sedation, nausea and vomiting, pruritus, urinary retention, ileus, and constipation [12, 13].
COX-2 selective inhibitors are generally used worldwide, and the beneficial effects of their perioperative administration for postoperative analgesia have been reported for a number of surgical models [14–17]. In a recent systematic review, COX-2 selective inhibitors were found to be effective in providing analgesia in the extended postoperative period following orthopedic surgery with a minimal side-effect profile, while nonselective NSAIDs need to be used with greater caution [18]. Previous direct comparative studies of loxoprofen and celecoxib reported the superiority of celecoxib when considering the incidence of upper gastrointestinal or bowel injury induced after administration [19, 20]. Nevertheless, a meta-analysis indicated that nonselective NSAIDs produce better pain relief than COX-2 selective inhibitors in patients undergoing lumbar spine surgery [21], although there was no direct comparison of loxoprofen and celecoxib for postoperative pain after spinal surgery. The purpose of the present study was to analyze the efficacy and safety of loxoprofen and celecoxib for postoperative pain after spinal surgery.
Methods
After approval of the protocol for the present study by our institutional review board for clinical research and treatment, we conducted a randomized comparison of loxoprofen sodium and celecoxib in patients more than 20 years old who planned to undergo spinal surgery from May 2013 to April 2014 and satisfied all of the requirements for inclusion in the study, as follows. (1) Patients had not received NSAIDs during the 4 weeks before the surgery; and (2) had received an explanation in writing from the physician in charge of the study regarding its design and signed an accompanying informed consent form approved by the ethics committee of our university. This consent to participate was provided by each patient at their own discretion before the start of the study. Exclusion criteria were as follows: (1) Patients with diseases of the digestive tract; (2) patients with a history of hypersensitivity or allergy to any component of the test drug preparation; (3) patients with a history of myocardial infarction or angina; or (4) other patients judged by the attending physician to be inappropriate for this study.
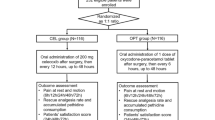
The study design and patient assignment are shown in Fig. 1. After obtaining written informed consent, 141 patients screened from 148 patients were randomly allocated to a loxoprofen group (180 mg/day) or to a celecoxib group (200 mg/day) using a random-number table. For each patient, age, sex, body weight, The American Society of Anesthesiologists (ASA) physical status [22], affected spinal level, and whether the surgery was conducted with or without fusion were recorded. Three types of analgesic were taken by patients included in this study, together with NSAIDs before surgery. The analgesics were pregabalin (Lyrica; Pfizer Inc., Tokyo, Japan), Tramcet (37.5 mg of Tramadol hydrochloride ± 325 mg of Acetaminophen/tablet; Janssen Pharmaceutics Inc., Tokyo, Japan), and Neurotropin (an extract from cutaneous tissue of rabbit inoculated with vaccinia virus, 4 Neurotropin Unit/tablet; Nippon Zoki Pharmaceutical Co., Osaka, Japan). After surgery, these analgesics were taken again from postoperative day 1. We determined the proportion of patients who were taking these analgesics and compared the dose in these patients between the two groups.
The surgery was performed under intravenous anaesthesia. After tracheal intubation, anesthesia was maintained with continuous intravenous infusion of propofol (5–10 mg/kg/h) and remifentanil (0.05–0.3 μg/kg/min). Intravenous fentanyl was also appropriately administered during surgery to relieve postoperative pain immediately after extubation. Intravenous flurbiprofen axetil, and pentazocine intravenously or intramuscularly were allowed on demand for 2 days after surgery. A rectal suppository of diclofenac sodium was also allowed on demand until 7 days postoperatively. Operative time and total bleeding, and use of medications on demand for postoperative pain were also recorded for each patient.
From the morning on postoperative day 1, administration of a tablet of loxoprofen (60 mg) or a tablet of celecoxib (100 mg) was started. Loxoprofen was administered after breakfast, lunch, and dinner (3 tablets/day), and celecoxib was administered after breakfast and dinner (2 tablets/day). Each meal was a hospital diet, which was furnished for each patient. The doses used are the approved doses for acute inflammatory pain used in Japan, are the most universally used dosages, and were given for seven consecutive days from day 1 after the surgery. Postoperative rehabilitation and orthosis were applied with the same indication in both groups. Briefly, on postoperative day 1–3, rehabilitation started at the bedside and was also conducted in a rehabilitation room, depending on the patients’ recovery. An orthosis, such as a cervical collar, canvas or hard corset, was applied depending on the diagnosis and surgical method, with the same indication in both groups.
The primary outcome measure was the analgesic effect of loxoprofen and celecoxib for postoperative pain. Postoperative pain was assessed using an 11-point numeric rating scale (NRS), with 0 being the absence of pain and 10 being the worst pain imaginable. In the loxoprofen group, NRS score was obtained at nine defined times, which were just before, as well as 30 min and 2 h after each administration. In the celecoxib group, NRS score was obtained nine times a day, as it was in the loxoprofen group. We obtained maximum and mean NRS scores for each day from postoperative days 1–7, and compared them between the two groups. To compare the effectiveness of a single administration, the mean NRS score before administration and changes (improvement or worsening) in NRS during 30 min and 2 h after each administration were also compared between the two groups. To further compare the effectiveness of loxoprofen and celecoxib in both slight and severe pain, the NRS score changes were also compared in patients with an NRS score <5 and in patients with an NRS score ≥5 before each administration. For all primary measurements, when intravenous flurbiprofen axetil, intravenous or intramuscular pentazocine, or rectal suppositories of diclofenac sodium were used on demand, NRS scores obtained within 24 h after their use were excluded from the comparisons. Of the 4536 scheduled measurements in the loxoprofen group and 4221 measurements in the cerecoxib group, 1449 measurements (31.9 %) and 1299 measurements (30.8 %) were excluded, and the remaining 3087 measurements in the loxoprofen group and 2922 measurements in the cerecoxib group were evaluated respectively. The frequency of use of additional analgesics administered on demand from day 0 to 7 was also measured.
To evaluate the safety of loxoprofen and celecoxib, any renal or hepatic dysfunction in patients was compared by evaluating laboratory data, including serum alanine aminotransferase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), creatinine, and estimated glomerular filtration rate (eGFR). Lab data for each of 138 patients at 1 day before surgery and 7 days after surgery were analyzed. Data for one patient in the loxoprofen group and two patients in the cerecoxib group were excluded because no postoperative data were obtained. Additionally, any adverse events treated (e.g., epigastric distress, abdominal pain or chest pain) were noted.
Statistical analyses were conducted using SPSS (version 13; SPSS, Chicago, IL). An unpaired t test was used to compare data from the two groups. Group differences were analyzed using a χ 2 test. p < 0.05 was considered significant.
Results
Of the 141 randomized patients included, 73 were in the loxoprofen group and 68 were in the celecoxib group. The mean age of the patients was 62.2 years. Table 1 shows demographic characteristics between the two groups at baseline and during the surgery. Age, sex, body weight, affected level in the spine, and ASA physical status before surgery were not different between the two groups.
Of other analgesics taken together preoperatively with NSAIDs, pregabalin was taken by 18 patients in the loxoprofen group (135 ± 78 mg/day) and 19 patients in the cerecoxib group (171 ± 77 mg/day), Tramcet was taken by two patients in the loxoprofen group (5.0 ± 4.2 tablets/day) and six patients in the cerecoxib group (3.3 ± 0.8 tablets/day). Neurotropin was taken by one patient in the loxoprofen group and five patients in the cerecoxib group (all patients were taking four tablets/day). No significant difference was noted in the percentage of patients who were taking these analgesics or the dose between the two groups.
The surgical method, which was with or without fusion surgery, was not significantly different between the two groups, but the total time of anesthesia and surgery was longer and there was more bleeding during surgery in patients from the loxoprofen group, indicating a difference in surgery between patients in the two groups. Nevertheless, the NRS score in the morning on postoperative day 1 was not significantly different between the groups (p = 0.15).
The frequency of additional intravenous flurbiprofen axetil, intravenous or intramuscular pentazocine, and rectal suppository of diclofenac sodium used postoperatively on demand in each group was 1.3 ± 2.2 vs 1.7 ± 2.3, 0.2 ± 0.7 vs 0.2 ± 0.7, and 0.5 ± 1.1 vs 0.4 ± 1.1, respectively. These frequencies are not statistically different between the two groups.
Maximum and mean NRS score on each day was not different between the two groups throughout the 7 days after the surgery (Fig. 2a, b). Yet, for each administration, improvement of NRS score was greater in the loxoprofen group. Mean NRS before administration was 3.03 ± 1.62 in the loxoprofen group and 3.01 ± 2.03 in the cerecoxib group, and there was no significant difference between them (P = 0.82), but significant differences in change of NRS score were noted both 30 min (0.3 ± 0.04 vs 0.2 ± 0.04) and 2 h (0.4 ± 0.05 vs 0.3 ± 0.05) after administration (Fig. 3). Improvement of NRS score was significantly greater for patients taking loxoprofen for subgroups with an NRS ≥5 and <5 both 30 min (1.0 ± 0.2 vs 0.6 ± 0.1 and 0.2 ± 0.03 vs 0.1 ± 0.02, respectively) and 2 h (1.2 ± 0.2 vs 0.8 ± 0.1 and 0.2 ± 0.04 vs 0.1 ± 0.04, respectively) after each administration (Fig. 4a, b). In both the loxoprofen and celecoxib groups, improvement in NRS was significantly greater in the subgroup with an NRS score ≥5 compared to the subgroup with an NRS score <5, suggesting that these two NSAIDs have a greater effect on severe postoperative pain than they do on slight pain after spinal surgery.
One patient discontinued loxoprofen 4 days after the surgery because of renal dysfunction. Renal function recovered within a week after suspension of loxoprofen without any treatment. There was no other adverse event related to treatment in any other patient. The levels of ALT, AST, BUN, creatinine, and eGFR were not significantly different between the two groups, either before or 7 days after administration (Table 2).
Discussion
To our knowledge, the present study is the first to directly compare the efficacy and safety of loxoprofen and celecoxib for postoperative pain after spinal surgery. Although little evidence exists that evaluates the effectiveness of loxoprofen for postoperative surgical pain, a Japanese study evaluating 16 postoperative cases found increased effectiveness within 30 min of medication dosing in eight cases (50 %), and some pain relief was obtained in 14 cases (87.5 %), without any severe side effect in any case [23]. Numerous papers report the effectiveness of celecoxib for postoperative pain. A systematic review reported that celecoxib significantly decreased postoperative pain compared with placebo [24], and a single administration of celecoxib (200 mg) is reported to provide analgesic efficacy inferior to that of ibuprofen (400 mg) in the early period (0–6 h), but superior from 12 h after administration in patients with dental extraction [25], suggesting the effectiveness of celecoxib is not rapid, but long-lasting compared with nonselective NSAIDs. In spinal surgery, celecoxib is reported to have an opioid-sparing effect for the first 8 h after spinal fusion surgery [26]. Conversely, perioperative administration of celecoxib was reported to have no significant opioid-sparing effect or benefits with pain levels around the wound after lumbar disc surgery [27].
Our results indicate comparative analgesic efficacy between loxoprofen and celecoxib for 7 days after spinal surgery, as evaluated by maximum and mean NRS score on each day (Fig. 2a, b). Yet, when evaluated after a single administration, loxoprofen demonstrated rapid and superior effectiveness, as shown in Fig. 3. This tendency was shown for both slight pain where NRS score was <5 and severe pain where NRS score was ≥5 (Fig. 4a, b). However, that we used celecoxib at a loading dose of 200 mg/day, which is less than the maximum dose of 400 mg/day that is permitted to be used for postoperative pain in Japan, should be considered.
From a clinical and pharmacological perspective, the major side effects of NSAIDs are lower gastrointestinal complications, renal disturbances, and cardiovascular events. In this study, loxoprofen was discontinued in one patient 4 days after the surgery because of renal dysfunction, but no adverse event appeared in any other patient. Laboratory data evaluating renal or hepatic dysfunction indicated the comparative safety of loxoprofen and celecoxib. Our study has several limitations. First, we did not include a control group of individuals who were not taking a NSAID. In this comparative study design, the influence of a natural course of recovery without NSAIDs cannot be completely excluded. Second, we did not compare the difference in NRS scores more than 2 h after drug administration. If there was a difference in NRS scores at longer times after drug administration, different characteristics between loxoprofen sodium and cerecoxib might be shown. Third, we only evaluated the effectiveness of postoperative NSAIDs with NRS scores, and without any other objective data. The clinical importance of small differences in NRS scores remains controversial. A multifaceted analysis for comparing effectiveness, including patients’ satisfaction, might be desirable in further study. Fourth, we did not examine the digestive tract using endoscopy or cardiac function using an electrocardiogram to assess safety, although no patient appeared to have discomfort of the upper and lower digestive tract or chest. In a case-controlled study in Japan, the risk of bleeding in the upper digestive tract was found to be 5.9 times higher in subjects taking loxoprofen than in subjects who did not take any NSAIDs [28]. Celecoxib strongly inhibits the COX-2 enzyme and has only a weak effect on COX-1, and is therefore considered not to interfere with homeostasis [7]. Further clinical studies, including the omitted tests, are required to compare any side effects between loxoprofen and celecoxib more thoroughly.
In conclusion, both loxoprofen sodium and celecoxib were well tolerated for the relief of acute postoperative pain on each of 7 days after spinal surgery. After a single dose of loxoprofen, the level of NRS score improvement was significantly higher than that for patients taking celecoxib. Further study in a larger number of patients is required to compare the different types of NSAIDs more thoroughly.
References
Scheiman JM, Hindley CE. Strategies to optimize treatment with NSAIDs in patients at risk for gastrointestinal and cardiovascular adverse events. Clin Ther. 2010;32(4):667–77.
Vane SJ. Differential inhibition of cyclooxygenase isoforms: an explanation of the action of NSAIDs. J Clin Rheumatol. 1998;4(5 Suppl):s3–10.
Tsumura H, Tamura I, Tanaka H, Chinzei R, Ishida T, Masuda A, Shiomi H, Morita Y, Yoshida M, Kutsumi H, Inokuchi H, Doita M, Kurosaka M, Azuma T. Prescription of nonsteroidal anti-inflammatory drugs and co-prescribed drugs for mucosal protection: analysis of the present status based on questionnaires obtained from orthopedists in Japan. Intern Med. 2007;46(13):927–31.
Arakawa T, Fujiwara Y, Sollano JD, Zhu Q, Kachintorn U, Rani AA, Hahm KB, Takahashi S, Joh T, Kinoshita Y, Matsumoto T, Naito Y, Takeuchi K, Yamagami H, Agustanti N, Xiong H, Chen X, Jang EJ, Furuta K. Terano A; IGICS study group. A questionnaire-based survey on the prescription of non-steroidal anti-inflammatory drugs by physicians in East Asian countries in 2007. Digestion. 2009;79(3):177–85.
Emery P, Zeidler H, Kvien TK, Guslandi M, Naudin R, Stead H, Verburg KM, Isakson PC, Hubbard RC, Geis GS. Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparison. Lancet. 1999;354:2106–11.
Singh G, Fort JG, Goldstein JL, Levy RA, Hanrahan PS, Bello AE, Andrade-Ortega L, Wallemark C, Agrawal NM, Eisen GM, Stenson WF. Triadafilopoulos G; SUCCESS-I Investigators. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I Study. Am J Med. 2006;119(3):255–66.
Simon LS, Weaver AL, Graham DY, Kivitz AJ, Lipsky PE, Hubbard RC, Isakson PC, Verburg KM, Yu SS, Zhao WW, Geis GS. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA. 1999;282(20):1921–8.
Sakamoto C, Soen S. Efficacy and safety of the selective cyclooxygenase-2 inhibitor celecoxib in the treatment of rheumatoid arthritis and osteoarthritis in Japan. Digestion. 2011;83(1–2):108–23.
Michaloliakou C, Chung F, Sharma S. Preoperative multimodal analgesia facilitates recovery after ambulatory laparoscopic cholecystectomy. Anesth Analg. 1996;82(1):44–51.
Ilan DI, Liporace FA, Rosen J, Cannavo D. Efficacy of rofecoxib for pain control after knee arthroscopy: a prospective, randomized, double-blinded clinical trial. Arthroscopy. 2004;20(8):813–8.
Ekman EF, Wahba M, Ancona F. Analgesic efficacy of perioperative celecoxib in ambulatory arthroscopic knee surgery: a double-blind, placebo-controlled study. Arthroscopy. 2006;22(6):635–42.
White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94(3):577–85.
Puura A, Puolakka P, Rorarius M, Salmelin R, Lindgren L. Etoricoxib pre-medication for post-operative pain after laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2006;50(6):688–93.
Buvanendran A, Kroin JS, Tuman KJ, Lubenow TR, Elmofty D, Moric M, Rosenberg AG. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. JAMA. 2003;290(18):2411–8.
Camu F, Beecher T, Recker DP, Verburg KM. Valdecoxib, a COX-2-specific inhibitor, is an efficacious, opioid-sparing analgesic in patients undergoing hip arthroplasty. Am J Ther. 2002;9(1):43–51.
Desjardins PJ, Shu VS, Recker DP, Verburg KM, Woolf CJ. A single preoperative oral dose of valdecoxib, a new cyclooxygenase-2 specific inhibitor, relieves post-oral surgery or bunionectomy pain. Anesthesiology. 2002;97(3):565–73.
Joshi GP, Viscusi ER, Gan TJ, Minkowitz H, Cippolle M, Schuller R, Cheung RY, Fort JG. Effective treatment of laparoscopic cholecystectomy pain with intravenous followed by oral COX-2 specific inhibitor. Anesth Analg. 2004;98(2):336–42.
Roberts M, Brodribb W, Mitchell G. Reducing the pain: a systematic review of postdischarge analgesia following elective orthopedic surgery. Pain Med. 2012;13(5):711–27.
Sakamoto C, Kawai T, Nakamura S, Sugioka T, Tabira J. Comparison of gastroduodenal ulcer incidence in healthy Japanese subjects taking celecoxib or loxoprofen evaluated by endoscopy: a placebo-controlled, double-blind 2 week study. Aliment Pharmacol Ther. 2013;37(3):346–54.
Mizukami K, Murakami K, Yamauchi M, Matsunari O, Ogawa R, Nakagawa Y, Okimoto T, Kodama M, Fujioka T. Evaluation of selective cyclooxygenase-2 inhibitor-induced small bowel injury: randomized cross-over study compared with loxoprofen in healthy subjects. Dig Endosc. 2013;25(3):288–94.
Jirarattanaphochai K, Jung S. Nonsteroidal antiinflammatory drugs for postoperative pain management after lumbar spine surgery: a meta-analysis of randomized controlled trials. J Neurosurg Spine. 2008;9(1):22–31.
Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2(3):281–4.
Hamamoto H, Takeda N, Neo M, Inagaki Y, Sawano H. Clinical experience of loxonine for postoperative and posttraumatic pain. Rinsyoiyaku. 1987;3(2):275–81 (in Japanese).
Barden J, Edwards JE, McQuay HJ, Moore RA. Single dose oral celecoxib for postoperative pain. Cochrane Database Syst Rev 2003 (2):CD004233.
Malmstrom K, Fricke JR, Kotey P, Kress B, Morrison B. A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: a single-center, randomized, double-blind, placebo- and active-comparator-controlled, parallel-group, single-dose study using the dental impaction pain model. Clin Ther. 2002;24(10):1549–60.
Reuben SS. Connelly NR Postoperative analgesic effects of celecoxib or rofecoxib after spinal fusion surgery. Anesth Analg. 2000;91(5):1221–5.
Karst M, Kegel T, Lukas A, Lüdemann W, Hussein S, Piepenbrock S. Effect of celecoxib and dexamethasone on postoperative pain after lumbar disc surgery. Neurosurgery. 2003;53(2):331–6.
Sakamoto C, Sugano K, Ota S, Sakaki N, Takahashi S, Yoshida Y, Tsukui T, Osawa H, Sakurai Y, Yoshino J, Mizokami Y, Mine T, Arakawa T, Kuwayama H, Saigenji K, Yakabi K, Chiba T, Shimosegawa T, Sheehan JE, Perez-Gutthann S, Yamaguchi T, Kaufman DW, Sato T, Kubota K, Terano A. Case-control study on the association of upper gastrointestinal bleeding and nonsteroidal anti-inflammatory drugs in Japan. Eur J Clin Pharmacol. 2006;62(9):765–72.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sekiguchi, H., Inoue, G., Nakazawa, T. et al. Loxoprofen sodium and celecoxib for postoperative pain in patients after spinal surgery: a randomized comparative study. J Orthop Sci 20, 617–623 (2015). https://doi.org/10.1007/s00776-015-0726-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-015-0726-4