Abstract
Three prospective controlled clinical trials and numerous small series and case reports have confirmed that durable, drug-free remission in systemic sclerosis is possible via an autologous hematopoietic stem cell transplantation. Similar results have been seen in other autoimmune diseases. The exact mechanism by which this immune “reset” was achieved in some but not all cases remains elusive, but includes major reduction of autoreactive immune competent cells, re-establishment of T- and B cell regulatory networks and normalization of tissue niche function, particularly vascular. Some aspects regarding mobilization, conditioning and graft manipulation still remain open, but clearly a significant toxicity is associated with all effective regimens at present, and therefore patient selection remains a key issue. In the hematology/oncology arena, major efforts are being made to reduce genotoxic and other collateral toxicity induced by current mobilization and conditioning protocols, which may also translate to autoimmune disease. These include developments in rapid mobilization and antibody drug conjugate conditioning technology. If effective, such low-toxicity regimens might be applied to autoimmune disease at an earlier stage before chronicity of autoimmunity has been established, thus changing the therapeutic paradigm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the pre-biologic era of the mid-1990s, a growing frustration with the available therapeutic options for autoimmune diseases (ADs) led to a dialogue between hematopoietic stem cell transplantation (HSCT) experts and specialists treating ADs in several centers [1,2,3,4]. This evolved into an international collaboration [4, 5] (4), which continues to this day.
The concept arose from case reports describing patients receiving an HSCT for a conventional hematological/oncological indication and in whom a coincidental AD showed improvement [6, 7] and animal model AD data which indicated positive outcomes after both allogeneic HSCT and autologous HSCT (aHSCT) [8, 9]. Several AD patients were successfully treated [2, 10,11,12], case reports became small series, and eventually two decades later, over 3000 patients have received an HSCT as treatment of an AD. This includes four prospective controlled trials which have established a positive role of aHSCT in systemic sclerosis (SSc), also called scleroderma, [13,14,15] and multiple sclerosis (MS) [16]. This has been extensively and recently reviewed [17,18,19,20] and as the biologics and other targeted therapies appeared, many indications such as rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA) became less urgent (Fig. 1). However, no treatment of AD has induced durable drug-free remission as effectively as has aHSCT, and despite the toxicity (see Sect. 2), there may still be a place for aHSCT in such cases, given the cost of lifelong treatment with biologics, drug retention rates of 80–50% [21, 22] and relapse after stopping biologics (80% in RA) [23].

Adapted from Snowden et al. [18]
Transplants per year by disease. CD Crohn’s disease, MS multiple sclerosis, SLE systemic lupus erythematosus, SSc systemic sclerosis.
Recent data (August 2018) from the European Group for Blood and Marrow Transplantation (EBMT) shows that a total of 2549 patients have received an aHSCT for AD in Europe, including 1259 for MS, 546 for SSc and 192 for CD [24]. Furthermore, over 40% of these transplants occurred in the past 7 years, the majority of which being for MS, SSc and CD.
Thus far, SSc has defied the search for an effective disease-modifying therapeutic agent, and not surprisingly has been, along with MS, the main focus of attention and will be the subject of this review.
2 Clinical Results
Table 1 shows the results of the only five published clinical trials of aHSCT in AD, three of which are in SSc. The Autologous Stem cell Transplantation International Scleroderma (ASTIS) [13] and the Scleroderma: Cyclophosphamide Or Transplantation (SCOT) [14] trials employed very similar selection criteria and control arms, but differed in that ASTIS used cyclophosphamide (CYC) in the mobilization phase and a non-myeloablative conditioning regimen, i.e., CYC 200 mg/kg + anti-thymocyte globulin (ATG) versus CYC 120 mg/kg + ATG + total body irradiation (TBI) in SCOT. Both employed a CD-34 selected graft. The outcomes were positive in both trials regarding the primary outcomes, i.e., event-free survival (EFS) in ASTIS (events being death or permanent end organ failure) and, in SCOT, a Global Rank Composite Score at week 54.
The treatment-related mortality (TRM) differed: 10% at 12 months in ASTIS, and in the SCOT trial, 3% at 54 months and 6% at 72 months. There was no TRM in the SCOT trial in the first 12 months after transplant, in contrast to ASTIS and other studies employing CYC (2–4 g/m2 mobilization and 200 mg/kg conditioning). The reasons for this difference remain obscure given that early registry data have indicated that the increased toxicity of myeloablative regimens overall was not justified by a significant increased efficacy [25]. In the same study, low-intensity regimens such as thiotepa had a significantly lower efficacy. It could be that the higher doses of CYC are especially toxic to SSc-associated overt or covert cardiac disease, and indeed more intensive cardiac screening is now recommended (see Sect. 3). It could also be that the higher numbers of patients transplanted in ASTIS (twice those of SCOT) account for the difference, or that with time the increased incidence of secondary malignancy in SCOT may surpass that of ASTIS.
In addition, the same early registry data study from 2005 indicated that regimens which included CYC in the mobilization protocol had higher efficacy than those which did not [25]. SCOT used only granulocyte colony-stimulating factor (G-CSF) for mobilization, but perhaps this potentially negative factor was overshadowed by the more intensive myeloablative conditioning regimen.
It should be noted that registry data is often incomplete. In the EBMT registry of MS transplants, details of the conditioning regimens were absent in 9.5% of cases [26].
The ASSIST trial was smaller (19 patients) than both ASTIS and SCOT and used lower CYC doses for mobilization (2 g/m2 vs 4 g/m2), a non-myeloablative regimen (CYC/ATG) and no graft manipulation [15]. The primary outcome was improvement of the modified Rodnan skin score (mRSS) of > 25% and/or increased forced vital capacity of > 10% at 12 months and was positive, with benefit sustained out to at least 2 years. There was no TRM. A retrospective American and Brazilian study of 90 SSc patients treated with the intermediate intensity CYC/ATG regimen and an unselected graft showed a 6% TRM and 70% relapse-free survival up to 5 years. Eight of the 22 relapses were fatal [27].
3 Patient Selection
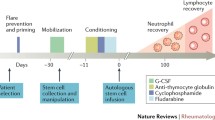
A major issue which emerged with time was patient selection. From the outset, it was advised that severely affected patients with irreversible end organ damage and precarious clinical state should not be transplanted, based on decades of experience in the hematology/oncology setting [28]. However, as the experience in AD grew, further refinements were added to patient selection in the various ADs, especially SSc. Patients with severely impaired cardio/pulmonary function were particularly at risk of TRM, especially during the phases of hyperhydration during CYC infusion and the cytokine storm induced by ATG and alemtuzumab. Protocols were adjusted accordingly with consensus selection criteria and organ screening recommendations have since been published (Table 2) [29, 30]. In essence, these recommendations are aimed at suggesting upper and lower limits to various functional parameters measuring pulmonary artery hypertension, left ventricular function, arrhythmias and pulmonary function.
Importantly, cardiac magnetic resonance imaging (MRI) has been recommended, since it has become clear that potentially fatal cardiac involvement in SSc may be more extensive than initially suspected [31].
In a study of causes of death in SSc from the EUSTAR database, 55% were directly attributable to the SSc, 35% pulmonary fibrosis, 26% pulmonary artery hypertension and 26% all cardiac [32].
It is hoped, but not guaranteed, that more careful cardiopulmonary screening will reduce the TRM, but clearly the experience of the treating center, including accreditation by the Joint Accreditation Committee of the International Society for Cellular Therapy (ISCT)-Europe and EBMT, known as JACIE, is of paramount importance [30]. Some cases of SSc have undergone successful aHSCT despite significant cardiac disease in such centers, including the use of defibrillating pacemakers (Matucci-Cerinic M., personal communication).
Clearly early rather than late cases are preferred since the most rapid deterioration of lung function occurs in the first 3–5 years of disease [33] and, although slowing or arrest of lung function deterioration due to fibrosis is to be expected, significant improvement after any treatment is unlikely [34]. Some case reports of markedly improved lung function and reduced High Resolution Computer Tomography (HRCT) opacification most likely reflect reduced inflammatory alveolitis rather than removal of fibrosis and re-establishment of alveolar/capillary architecture [35].
For reasons which are incompletely understood, significant improvement in symptoms related to hypomobility of the gastrointestinal tract, e.g., esophageal reflux, blind loop syndrome and megacolon rarely respond to any immune-modulating treatment, including aHSCT [36]. This may be due to established atrophy and fibrosis resulting from loss of myenteric plexus function early in disease [37].
In general, patients with the diffuse cutaneous form of SSc (dcSSc) have been the main target for aHSCT, but given the degree of overlap between dcSSc and the limited cutaneous form (lcSSc), a final decision to offer aHSCT to a patient with SSc should be a consensus between SSc experts and transplantation colleagues.
Currently, efforts are underway to determine which patients may be poor responders to conventional CYC or other immunomodulatory therapy at an early stage of the treatment. Based on clinical and laboratory data from the ASTIS and other studies, this may facilitate the optimal timing for an aHSCT (van Laar J., personal communication).
4 Relapse
Relapse after an apparently successful aHSCT has been observed in all AD, and the prediction of such an event is not currently possible. It has been observed that some patients respond well to immunodulatory agents which pre-transplant were ineffective. Others have undergone a successful second transplant.
5 Mechanisms
Most patients experience a marked improvement in the mRSS and other inflammatory features of SSc immediately after an aHSCT, most likely due to the known potent anti-inflammatory components of the mobilizing and conditioning regimens. In addition, the period of profound immunosuppression during the initial aplasia and later slow immune reconstitution adds to this effect.
However, in those patients who experience durable remissions years after the “once only” immunosuppressive impact of aHSCT has worn off, there must have been a re-setting of autoimmunity to account for the sustained improvement. In addition, improved tissue structure and function has been observed, so called “de-remodeling” or “reverse remodeling,” involving cells which are not directly targeted by the agents (CYC, ATG, alemtuzumab, etc.) employed in aHSCT. Examples include normalization of microcapillary structure in nail folds [38, 39] and skin [40], improved macrovascular changes in gastric antral vascular ectasia (GAVE) [41], and reversed collagen deposition in skin [42]. In other cases, secondary skin organs such as hair follicles and sweat glands have begun to function again after years of inactivity (Fig. 2).
Normalization of skin after aHSCT for SSc in a patient of Indian origin. 2006 Pre-transplant; thickened, shiny and hyperpigmented dry itchy skin; flexion contracture of the elbows. HSCT performed in 2007. 2010 Normal skin texture and pigmentation; no joint contractures; return of sweat gland and hair follicle function. ANA and Scl70 (Topo1) became and remained negative. Patient status in April 2019: Full drug-free remission with normal skin. aHSCT autologous HSCT, ANA antinuclear antibodies, HSCT hematopoietic stem cell transplantation, SSc systemic sclerosis
This implies some form of niche management must have taken place, presumably due to removal of autoaggressive putative cells, allowing normal homeostasis and repair to operate. Exactly which cells these are remains enigmatic, but presumably they must be susceptible to the agents used in aHSCT and therefore are most likely of hematopoietic origin. One candidate is the plasmacytoid dendritic cell which forms a bridge between the innate and adaptive immune system and mediates immune reactivity or tolerance [40, 43].
Attempts to target specific components of the immune system such as B cells, T cells and pan-lymphocyte monoclonal antibodies have not induced long-term drug-free remission.
Perhaps it requires the broad based, major reduction of a whole network of dysfunctional immune competent cells to allow re-establishment of a normal self-tolerant system. Some clues as to how this may take place are emerging.
6 Immune “Reset”
Early on it was appreciated that despite full reconstitution of the immune system post-aHSCT, some responding patients with systemic lupus erythematosus (SLE) and MS remained in remission. In seven SLE patients, both autoreactive and protective memory were lost post-aHSCT and replaced by normal B cell numbers and an expanded T-cell repertoire as evidenced by T-cell receptor Vβ gene usage at 3 years post-aHSCT. In addition, increased regulatory T cells (Treg) and recent thymic emigrants were evidence of increased thymic function post-aHSCT [44].
In MS, in CD4+ T cells, dominant T cell receptor (TCR) clones present before treatment were undetectable following reconstitution, and patients largely developed a new repertoire. However, dominant CD8+ clones were not effectively removed, and the reconstituted CD8+ T-cell repertoire was created by clonal expansion of cells present before treatment. Patients who failed to respond to treatment had less diversity in their T-cell repertoire early during the reconstitution process [45].
From the Utrecht group it was found that aHSCT induced functional renewal of regulatory T cells as well as a strong Treg TCR diversification in JIA and juvenile dermatomyositis. However, adding Treg to the graft did not lead to additional clinical improvement, but resulted in delayed donor T-cell reconstitution in a murine proteoglycan arthritis model [46]. This emphasizes the complexity of immune homeostasis following aHSCT including the normalisation of regulatory B cell networks (reviewed in [47]).
Fewer data are available for SSc after transplant. Comparing five “responders” to five “non-responders” at 6 years follow-up, “non-responders” had a more rapid T-cell immune reconstitution [48]. More extensive mechanistic data are expected soon from the SCOT study (Sullivan, personal communication).
Several groups have shown that a dysregulated dominant T helper 2 (Th2) cytokine profile exists in active SSc and that the Th1/Th2 ratio may normalize after aHSCT [49] [50].
The hope is that eventually a combination of clinical features and in vitro tests will provide a “responder” profile both for selecting cases suitable for an aHSCT and to determine which cases may require maintenance immunomodulation post-transplant. Some gene expression data in SSc suggests that predominant patterns such as “inflammatory” or “pro-fibrotic” patterns may be used to direct therapy [49,50,51,52].
7 The Future
7.1 Pre-Emptive AD Therapy
The past several years have seen an increasing interest and literature regarding the concept of pre-emptive treatment of AD, also referred to as preventative treatment [53]. The concept arose from immune regulatory data, especially autoantibody and cytokine levels, obtained from sera collected years before the first symptoms of AD became manifest [54]. One of the most extensive of such databases is the American Department of Defense Serum Repository (DODSR) in which 60 million sera from 10 million individuals are stored [55]. Fifty-five patients who later fulfilled the criteria for SLE manifested enhanced type II interferon (IFN) activity, followed by elevated INF-α and B-cell stimulator levels. Cases were distinguished by multivariate random forest models incorporating IFN-γ, macrophage chemoattractant protein (MCP)-3, anti-chromatin and anti-spliceosome antibodies (accuracy 93% > 4 years pre-classification; 97% within 2 years of SLE classification) [56].
Similar data were demonstrated in a study of 790 individuals randomly selected at health fairs and screened for double-stranded DNA (dsDNA), chromatin, SSA/Ro, SSB/La, Sm, Sm/RNP, RNP, ribosomal P, Scl-70, centromere B, and Jo-1. Fifty-seven (7%) were antinuclear antibody (ANA) positive, and elevated proinflammatory cytokines (IFN-γ, tumor necrosis factor [TNF], interleukin-17 [IL-17] and G-CSF) showed a stepwise increase from ANA-negative healthy, ANA-positive healthy and SLE patients. In contrast, only SLE patients showed elevated IFN-α, IFN-β, IL-12p40 and stem cell factor/c-kit. In addition, BlyS was elevated in SLE patients, but decreased in ANA-positive healthy individuals, and only in SLE patients was the protective factor IL-1 RA reduced [57].
Similar data have been observed in other ADs such as “pre-rheumatoid arthritis” [58].
In SSc, there are limited data so far, though this may change with increasing awareness of the Very Early Diagnosis Of Systemic Sclerosis (VEDOSS) project [59]. Patients with ANA positivity, Raynaud’s phenomenon and puffy fingers are so classified, and combined with gene expression “big data” mentioned above [52] may enable a pre-emptive therapeutic strategy for SSc.
7.2 Low-Toxicity Pre-emptive Treatment Regimens
A randomized placebo-controlled study in 83 “pre-RA” patients (anti-citrullinated peptide antibody (ACPA) or IgM rheumatoid factor positive arthralgia, but no objective arthritis) using dexamethasone 100 mg intramuscular injection at baseline showed no prevention of arthritis development after 6 weeks [60].
For SSc, a similar pre-emptive “Hit Hard and Early” study is being planned using a 12-week, randomized, double-blind, placebo-controlled trial analyzing the effects of high-dose intravenous methylprednisolone in very early SSc [61]. Thirty patients who fulfill the criteria for very early SSc will be randomly assigned in a 2:1 ratio to receive either a 1-g intravenous infusion of methylprednisolone or a placebo on 3 consecutive days over 3 consecutive months. In this study, the primary endpoint will be the change in capillary density between baseline and after 12 weeks of treatment.
It would be enticing to think that a relatively non-toxic, targeted, immunomodulatory regimen might turn off the autoaggressive immune response before an established and recalcitrant dysregulation is established. The recently published PRAIRI study has dampened some of these hopes [62]. Patients with arthralgia and positive ACPA, but no objective synovitis were randomized to receive one course of rituximab or placebo. Both groups developed clinically manifest RA with the same incidence, but with a 12-month delay in the treated group.
A superficial interpretation could be that the progress to RA was already inevitably programmed, and that B-cell reduction was insufficiently profound to prevent this. An alternate explanation could be that the process leading to RA is more complex than just B-cell dysregulation and that a more eclectic immune modulation such as aHSCT is still required to “reset” autoimmunity.
Unfortunately, currently there are no conditioning regimens available with such a potential and of a sufficiently low toxicity as to be acceptable to patients with minimal or even asymptomatic pre-clinical disease. This may change.
8 New Regimens
In the fields of hematology and oncology, efforts are underway to develop such mobilizing and conditioning strategies for, among other indications, non-malignant hematopoietic stem cell-based disorders such as sickle cell disease, thalassemia, metachromic leukodystrophy and various storage diseases. Currently, only an allogeneic HSCT or gene therapy is curative, but carries all the toxicity of conventional conditioning regimens and, in the case of allo HSCT, graft versus host disease (GvHD).
To circumvent this, antibody drug conjugate (ADC) technology is being refined to avoid the genotoxic and off-target toxicity of conventional regimens [63, 64]. Also referred to as a “Trojan horse” approach, ADC is based on a monoclonal antibody targeting a specific cell surface receptor and linked with a cytocidal toxin. The antibody transports the toxin into the cell, where it is then released to block a vital cell function, resulting in cell death. Various toxins targeting various pathways such as protein synthesis or microtubule formation have been used in oncology for some years and are being continually refined. Their application to AD is now being considered.
Autologous HSCT has now been recommended as treatment for selected cases of SSc by the European League Against Rheumatism (EULAR) [65], the American Society for Bone Marrow Transplantation (ASBMT) [66] and the Scleroderma Clinical Trials Consortium and Canadian Scleroderma Research group [67], and is reimbursed in several countries, including the UK, the Netherlands and Switzerland, but is limited by the inevitable toxicity of current regimens. If such toxicity could be reduced and combined with pre-emptive or very early treatment, a once only “reset” of an autoaggressive immune system could usher in a new era of therapy for AD, including SSc.
9 Conclusion
The use of aHSCT in treating severe therapy-resistant AD is being increasingly employed, and a combination of uncontrolled case series and controlled randomized trials has established that in many cases, a durable, drug-free “reset” of autoimmune processes is possible. In addition, clinically relevant reverse remodeling of tissues has been documented. Mostly MS and SSc are being so treated, with around 66% of cases showing an initial positive outcome.
The toxicity of all current regimens limits aHSCT to highly selected AD cases. Efforts are underway to develop less toxic but equally effective mobilizing and conditioning regimens in the hematology/oncology setting, and if successful, their translation to early onset/poor prognosis AD could usher in a paradigm shift.
References
Marmont A, Tyndall A, Gratwohl A, Vischer T. Hemopoietic stem cell precursor-cell transplants for autoimmune diseases. Lancet. 1995;345:978.
Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, et al. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of a pilot study. Bone Marrow Transplant. 1997;20:631–8.
Snowden JA, Biggs JC, Brooks PM. Autologous blood stem cell transplantation for autoimmune diseases. Lancet Lond Engl. 1996;348:1112–3.
Sullivan KM, Furst DE. The evolving role of blood and marrow transplantation for the treatment of autoimmune diseases. J Rheumatol Suppl. 1997;48:1–4.
Tyndall A, Gratwohl A. Blood and marrow stem cell transplants in auto-immune disease: a consensus report written on behalf of the European League against Rheumatism (EULAR) and the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 1997;19:643–5.
Lowenthal RM, Cohen ML, Atkinson K, Biggs JC. Apparent cure of rheumatoid arthritis by bone marrow transplantation. J Rheumatol. 1993;20:137–40.
McKendry RJ, Huebsch L, Leclair B. Progression of rheumatoid arthritis following bone marrow transplantation. A case report with a 13-year followup. Arthritis Rheum. 1996;39:1246–53.
Morton JI, Siegel BV. Transplantation of autoimmune potential. IV. Reversal of the NZB autoimmune syndrome by bone marrow transplantation. Transplantation. 1979;27:133–4.
Knaan-Shanzer S, Houben P, Kinwel-Bohré EP, van Bekkum DW. Remission induction of adjuvant arthritis in rats by total body irradiation and autologous bone marrow transplantation. Bone Marrow Transplant. 1991;8:333–8.
Tamm M, Gratwohl A, Tichelli A, Perruchoud AP, Tyndall A. Autologous haemopoietic stem cell transplantation in a patient with severe pulmonary hypertension complicating connective tissue disease. Ann Rheum Dis. 1996;55:779–80.
Marmont AM, van Lint MT, Gualandi F, Bacigalupo A. Autologous marrow stem cell transplantation for severe systemic lupus erythematosus of long duration. Lupus. 1997;6:545–8.
Cooley HM, Snowden JA, Grigg AP, Wicks IP. Outcome of rheumatoid arthritis and psoriasis following autologous stem cell transplantation for hematologic malignancy. Arthritis Rheum. 1997;40:1712–5.
van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311:2490–8.
Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med. 2018;378:35–47.
Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet Lond Engl. 2011;378:498–506.
Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321:165–74.
Tyndall A. Hematopoietic stem cell transplantation for autoimmune diseases: more than just prolonged immunosuppression. Curr Opin Hematol. 2018;25:433–40.
Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017;1:2742–55.
Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, Fassas A, et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 2017;74:459–69.
Del Papa N, Pignataro F, Zaccara E, Maglione W, Minniti A. Autologous hematopoietic stem cell transplantation for treatment of systemic sclerosis. Front Immunol. 2018;9:2390.
Alten R, Mariette X, Lorenz H-M, Galeazzi M, Cantagrel A, Nüßlein HG, et al. Real-world predictors of 12-month intravenous abatacept retention in patients with rheumatoid arthritis in the ACTION observational study. RMD Open. 2017;3:e000538.
Alten R, Mariette X, Lorenz H-M, Nüßlein H, Galeazzi M, Navarro F, et al. Predictors of abatacept retention over 2 years in patients with rheumatoid arthritis: results from the real-world ACTION study. Clin Rheumatol. 2019;38:1413–24.
Ebina K, Hashimoto M, Yamamoto W, Ohnishi A, Kabata D, Hirano T, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis—the ANSWER cohort study. PLoS One. 2018;13:e0194130.
Jessop H, Farge D, Saccardi R, Alexander T, Rovira M, Sharrack B, et al. General information for patients and carers considering haematopoietic stem cell transplantation (HSCT) for severe autoimmune diseases (ADs): a position statement from the EBMT Autoimmune Diseases Working Party (ADWP), the EBMT Nurses Group, the EBMT Patient, Family and Donor Committee and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Bone Marrow Transplant. 2019. https://doi.org/10.1038/s41409-019-0430-7.
Gratwohl A, Passweg J, Bocelli-Tyndall C, Fassas A, van Laar JM, Farge D, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant. 2005;35:869–79.
Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13:391–405.
Burt RK, Oliveira MC, Shah SJ, Moraes DA, Simoes B, Gheorghiade M, et al. Cardiac involvement and treatment-related mortality after non-myeloablative haemopoietic stem-cell transplantation with unselected autologous peripheral blood for patients with systemic sclerosis: a retrospective analysis. Lancet Lond Engl. 2013;381:1116–24.
Thomas ED. A history of haemopoietic cell transplantation. Br J Haematol. 1999;105:330–9.
Farge D, Burt RK, Oliveira M-C, Mousseaux E, Rovira M, Marjanovic Z, et al. Cardiopulmonary assessment of patients with systemic sclerosis for hematopoietic stem cell transplantation: recommendations from the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party and collaborating partners. Bone Marrow Transplant. 2017;52:1495–503.
Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47:770–90.
Bissell L-A, Dumitru RB, Erhayiem B, Abignano G, Fent G, Kidambi A, et al. Incidental significant arrhythmia in scleroderma associates with cardiac magnetic resonance measure of fibrosis and hs-TnI and NT-proBNP. Rheumatol Oxf Engl. 2019;58:1221–6.
Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR scleroderma trials and research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–15.
McNearney TA, Reveille JD, Fischbach M, Friedman AW, Lisse JR, Goel N, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemographic, and behavioral factors. Arthritis Rheum. 2007;57:318–26.
Roofeh D, Jaafar S, Vummidi D, Khanna D. Management of systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol. 2019;31:241–9.
Martini A, Maccario R, Ravelli A, Montagna D, De Benedetti F, Bonetti F, et al. Marked and sustained improvement 2 years after autologous stem cell transplantation in a girl with systemic sclerosis. Arthritis Rheum. 1999;42:807–11.
Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatol Oxf Engl. 2009;48(Suppl 3):36–9.
Roberts CGP, Hummers LK, Ravich WJ, Wigley FM, Hutchins GM. A case-control study of the pathology of oesophageal disease in systemic sclerosis (scleroderma). Gut. 2006;55:1697–703.
Aschwanden M, Daikeler T, Jaeger KA, Thalhammer C, Gratwohl A, Matucci-Cerinic M, et al. Rapid improvement of nailfold capillaroscopy after intense immunosuppression for systemic sclerosis and mixed connective tissue disease. Ann Rheum Dis. 2008;67:1057–9.
Miniati I, Guiducci S, Conforti ML, Rogai V, Fiori G, Cinelli M, et al. Autologous stem cell transplantation improves microcirculation in systemic sclerosis. Ann Rheum Dis. 2009;68:94–8.
Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK, et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One. 2008;3:e1452.
Bhattacharyya A, Sahhar J, Milliken S, Ma D, Englert H, Tymms K, et al. Autologous hematopoietic stem cell transplant for systemic sclerosis improves anemia from gastric antral vascular ectasia. J Rheumatol. 2015;42:554–5.
Nash RA, McSweeney PA, Crofford LJ, Abidi M, Chen C-S, Godwin JD, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood. 2007;110:1388–96.
van Bon L, Popa C, Huijbens R, Vonk M, York M, Simms R, et al. Distinct evolution of TLR-mediated dendritic cell cytokine secretion in patients with limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2010;69:1539–47.
Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2009;113:214–23.
Muraro PA, Robins H, Malhotra S, Howell M, Phippard D, Desmarais C, et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Invest. 2014;124:1168–72.
Delemarre EM, van den Broek T, Mijnheer G, Meerding J, Wehrens EJ, Olek S, et al. Autologous stem cell transplantation aids autoimmune patients by functional renewal and TCR diversification of regulatory T cells. Blood. 2016;127:91–101.
Malmegrim KCR, Lima-Júnior JR, Arruda LCM, de Azevedo JTC, de Oliveira GLV, Oliveira MC. Autologous hematopoietic stem cell transplantation for autoimmune diseases: from mechanistic insights to biomarkers. Front Immunol. 2018;9:2602.
Farge D, Arruda LCM, Brigant F, Clave E, Douay C, Marjanovic Z, et al. Long-term immune reconstitution and T cell repertoire analysis after autologous hematopoietic stem cell transplantation in systemic sclerosis patients. J Hematol OncolJ Hematol Oncol. 2017;10:21.
Michel L, Farge D, Baraut J, Marjanovic Z, Jean-Louis F, Porcher R, et al. Evolution of serum cytokine profile after hematopoietic stem cell transplantation in systemic sclerosis patients. Bone Marrow Transplant. 2016;51:1146–9.
Tsukamoto H, Nagafuji K, Horiuchi T, Mitoma H, Niiro H, Arinobu Y, et al. Analysis of immune reconstitution after autologous CD34+ stem/progenitor cell transplantation for systemic sclerosis: predominant reconstitution of Th1 CD4+ T cells. Rheumatol Oxf Engl. 2011;50:944–52.
Taroni JN, Mahoney JM, Whitfield ML. The mechanistic implications of gene expression studies in SSc: insights from systems biology. Curr Treat Opt Rheumatol. 2017;3:181–92.
Franks JM, Martyanov V, Cai G, Wang Y, Li Z, Wood TA, et al. A machine learning classifier for assigning individual patients with systemic sclerosis to intrinsic molecular subsets. Arthritis Rheumatol Hoboken NJ. 2019. https://doi.org/10.1002/art.40898.
Rose NR. Prediction and prevention of autoimmune disease in the 21st century: a review and preview. Am J Epidemiol. 2016;183:403–6.
Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33.
Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900–4.
Munroe ME, Lu R, Zhao YD, Fife DA, Robertson JM, Guthridge JM, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis. 2016;75:2014–21.
Slight-Webb S, Lu R, Ritterhouse LL, Munroe ME, Maecker HT, Fathman CG, et al. Autoantibody-positive healthy individuals display unique immune profiles that may regulate autoimmunity. Arthritis Rheumatol Hoboken NJ. 2016;68:2492–502.
Cope AP. Emerging therapies for pre-RA. Best Pract Res Clin Rheumatol. 2017;31:99–111.
Minier T, Guiducci S, Bellando-Randone S, Bruni C, Lepri G, Czirják L, et al. Preliminary analysis of the very early diagnosis of systemic sclerosis (VEDOSS) EUSTAR multicentre study: evidence for puffy fingers as a pivotal sign for suspicion of systemic sclerosis. Ann Rheum Dis. 2014;73:2087–93.
Bos WH, Dijkmans BC, Boers M, van de Stadt RJ, van Schaardenburg D. Effect of dexamethasone on autoantibody levels and arthritis development in patients with arthralgia: a randomised trial. Ann Rheum Dis. 2010;69:571–4.
van den Hombergh WMT, Kersten BE, Knaapen-Hans HKA, Thurlings RM, van der Kraan PM, van den Hoogen FHJ, et al. Hit hard and early: analysing the effects of high-dose methylprednisolone on nailfold capillary changes and biomarkers in very early systemic sclerosis: study protocol for a 12-week randomised controlled trial. Trials. 2018;19:449.
Gerlag DM, Safy M, Maijer KI, Tang MW, Tas SW, Starmans-Kool MJF, et al. Effects of B-cell directed therapy on the preclinical stage of rheumatoid arthritis: the PRAIRI study. Ann Rheum Dis. 2019;78:179–85.
Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9:33–46.
Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019;11:djz035.
Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76:1327–39.
Sullivan KM, Majhail NS, Bredeson C, Carpenter PA, Chatterjee S, Crofford LJ, et al. Systemic sclerosis as an indication for autologous hematopoietic cell transplantation: position statement from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2018;24:1961–4.
Fernández-Codina A, Walker KM, Pope JE, Scleroderma Algorithm Group. Treatment algorithms for systemic sclerosis according to experts. Arthritis Rheumatol Hoboken NJ. 2018;70:1820–8.
Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA. 2015;314(23):2524–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no funding associated with the preparation of this review.
Conflict of interest
AT is a co-founder and shareholder of Magenta Therapeutics.
Rights and permissions
About this article
Cite this article
Tyndall, A. Hematopoietic Stem Cell Transplantation for Systemic Sclerosis: Review of Current Status. BioDrugs 33, 401–409 (2019). https://doi.org/10.1007/s40259-019-00364-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-019-00364-3