Abstract
Background
Although studies assessing the cost effectiveness of genotype-guided warfarin dosing for the management of atrial fibrillation, deep vein thrombosis, and pulmonary embolism have been reported, no publications have addressed genotype-guided warfarin therapy in mechanical heart valve replacement (MHVR) patients or genotype-guided warfarin therapy under the fee-for-service (FFS) insurance system.
Objective
The aim of this study was to evaluate the cost effectiveness of genotype-guided warfarin dosing in patients with MHVR under the FFS system from the Korea healthcare sector perspective.
Methods
A decision-analytic Markov model was developed to evaluate the cost effectiveness of genotype-guided warfarin dosing compared with standard dosing. Estimates of clinical adverse event rates and health state utilities were derived from the published literature. The outcome measure was the incremental cost-effectiveness ratio (ICER) per quality-adjusted life-year (QALY). One-way and probabilistic sensitivity analyses were performed to explore the range of plausible results.
Results
In a base-case analysis, genotype-guided warfarin dosing was associated with marginally higher QALYs than standard warfarin dosing (6.088 vs. 6.083, respectively), at a slightly higher cost (US$6.8) (year 2016 values). The ICER was US$1356.2 per QALY gained. In probabilistic sensitivity analysis, there was an 82.7% probability that genotype-guided dosing was dominant compared with standard dosing, and a 99.8% probability that it was cost effective at a willingness-to-pay threshold of US$50,000 per QALY gained.
Conclusion
Compared with only standard warfarin therapy, genotype-guided warfarin dosing was cost effective in MHVR patients under the FFS insurance system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Genotype-guided warfarin dosing for optimal anticoagulation appears to represent a cost-effective strategy in mechanical heart valve replacement (MHVR) patients under the fee-for-service system. |
Our analysis indicates that genotype-guided warfarin dosing may be considered an integral part of healthcare prior to the initiation of warfarin therapy in MHVR patients under the fee-for-service system. |
1 Introduction
Warfarin is the most commonly prescribed anticoagulant used to prevent thromboembolic events [1, 2]. Because of its narrow therapeutic index and large inter-individual variation, an inappropriately high dose can cause serious adverse events such as intracranial hemorrhage (ICH), whereas inappropriately low doses can result in treatment failure including fatal ischemic stroke [3]. The polymorphisms in cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase complex subunit 1 (VKORC1) account for approximately 40% of inter-individual variation in warfarin maintenance dose requirements [4, 5], such that genotype-guided, individualized warfarin therapy is now recommended [3]. Despite reports indicating the cost effectiveness of genotype-guided warfarin dosing [6–9], the majority of insurance plans do not cover warfarin pharmacogenetic testing [3].
Previously published cost effectiveness of genotype-guided warfarin dosing studies focused on atrial fibrillation (AF) patients treated under the diagnosis-related group (DRG) system [6–12]. It was reported that genotype-guided warfarin dosing could be cost effective depending on the cost of CYP2C9 and VKORC1 genotyping in AF under the DRG system [6–9, 11, 12]. Mechanical heart valve replacement (MHVR) patients are at a markedly higher risk of thromboembolism than those with AF [13]. In addition, unlike other indications such as AF and deep vein thrombosis, MHVR patients can have a higher sensitivity to the warfarin caused by hemodynamic and hemostatic instability just after open heart surgery, with declining warfarin sensitivity over time [5, 14, 15]. Moreover, while MHVR patients should start anticoagulant therapy upon hospitalization for open heart surgery [1, 2], patients with AF or deep vein thrombosis can take anticoagulant medications as outpatients [2, 16]. Thus, a cost-effectiveness analysis in MHVR patients may have different results to those in other indications because they have a different risk of adverse events and utility value.
The fee-for-service (FFS) insurance system, the dominant payment method in Korea, is a model that separately bills treatment and hospitalization according to the type of clinical service, duration of hospitalization, and outpatient clinic visits [17]. While the cost of inpatient care is fixed under the DRG system, the cost can be reduced due to faster stabilization of patients and early discharge under the FFS system [18]. As yet, no reported studies have focused on genotype-guided warfarin dosing in MHVR patients or genotype-guided warfarin dosing under the FFS system. Therefore, we evaluated the cost effectiveness of genotype-guided versus standard warfarin dosing in MHVR patients under the FFS system.
2 Methods
2.1 Model Structure
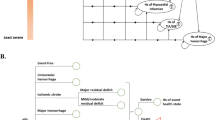
A decision-analytic Markov model was developed to simulate the outcomes of two treatments: genotype-guided warfarin therapy versus standard therapy (Fig. 1). The base-case analysis consisted of a hypothetical cohort of Asian MHVR patients commencing warfarin therapy [5]. Patients were categorized according to CYP2C9 and VKORC1 genotypes (CYP2C9 wild-type/VKORC1 –1639 AA, CYP2C9 wild-type/VKORC1 –1639 AG or GG, and CYP2C9 variant/VKORC1 AA). The CYP2C9 variant/VKORC1 AG or GG subgroups were not included due to a lack of available data on those patients [5, 7]. Genotype testing involved CYP2C9*3, CYP2C9*13, CYP2C9*14 and VKORC1 –1639 G>A based on East-Asian genotype profiles [5, 19].
Decision-analytic model. Mechanical heart valve replacement patients who start warfarin are classified according to treatment arms (genotype-guided warfarin therapy vs. standard therapy) and categorized according to CYP2C9 and VKORC1 genotype. The international normalized ratio control could be within, below, or above the target range, and patients might consequently experience no event, intracranial hemorrhage, extracranial hemorrhage, ischemic stroke, transient ischemic attack, or other death according to transition probabilities. CYP2C9 cytochrome P450 2C9, ECH extracranial hemorrhage, ICH intracranial hemorrhage, INR international normalized ratio, MHVR mechanical heart valve replacement, TE thromboembolism, TIA transient ischemic attack, VKROC1 vitamin K epoxide reductase complex subunit 1, VT variant, WT wild-type
Patients in the standard therapy group received initiation dosing following the 5 mg nomogram [20, 21]. After the initial dose, the warfarin dose was adjusted to find the stable warfarin dose based on INR monitoring through a trial-and-error process. In the genotype-guided warfarin therapy group, patients took an initial warfarin dose as calculated by a pharmacogenetic-based algorithm using demographic, clinical, and genetic data. The genotype-guided warfarin therapy group received CYP2C9 and VKORC1 genotyping before the initial warfarin dose. Based on our previous data, we assumed that patients in both groups received warfarin therapy during 2 weeks of initial hospitalization and subsequent outpatient clinic visits [5]. International normalized ratio (INR) control could be within, below, or above the target INR range, and time spent in INR ranges were used to determine the probability of adverse events.
In MHVR patients commencing warfarin therapy, we assumed schedule of INR measurements as follows: once per day when the patient was not within the INR target range during hospitalization, once per week when the patient remained in the INR target range, once per week after hospital discharge (until the patient reached a stable warfarin dose), and once per month after the patient reached a stable warfarin dose. The patients were followed for a lifetime. Four tiers of outcomes were simulated for each study arm: no event, bleeding, thromboembolism, and death. Total direct medical cost and quality-adjusted life-years (QALYs) gained were calculated on a weekly cycle.
Major bleeding events were classified as ICH or extracranial hemorrhage (ECH), including gastrointestinal bleeding [22]. Major thromboembolic events were defined as ischemic stroke and transient ischemic attack (TIA) [9]. The model was constructed using the TreeAge Pro software package (TreeAge Software Inc., Williamstown, MA, USA).
2.2 Data Sources
In this study, we used data from our previous study which included 265 MHVR patients [5]. Briefly, the previous study was an observational cohort study over 1 year of follow-up. It suggested that the CYP2C9 and VKORC1 genotypes influenced warfarin dosing in an early phase as well as steady state of warfarin therapy in Korean patients with MHVR. We also developed the genotype-based predictive algorithm for the warfarin maintenance dose.
2.3 Clinical Input
We retrieved the clinical inputs for the model from the literature. INR values were used as a surrogate marker for warfarin efficacy and safety. The percentage of time spent in, below, and above target INR ranges were used to determine the probability of an adverse event or therapeutic failure for each genotype subgroup. The mean percentage of time spent below, within, and above target INR ranges (<1.7; 1.7–2.8; >2.8), were determined for each genotype subgroup using data from our previous study [5], and was assumed to reflect standard warfarin therapy in Asian MHVR patients [5, 23, 24]. The percentage of time spent in the various INR ranges was calculated using the method described by Rosendaal et al. [25]. Figure 2 depicts the percentage of time spent in the different INR ranges according to CYP2C9 and VKORC1 genotypes during months 1, 6, and 12 of warfarin therapy. In the genotype-guided warfarin therapy group, we estimated that the patients with genotype-guided warfarin therapy would spend 12.6% more time within the target INR range, 9% less time below the target INR range, and 3.6% less time above the target INR range during the first month on the basis of the findings of Verhoef et al [9].
Percentage of time spent in, below, and above target international normalized ratio ranges in patients with mechanical heart valve replacement at a 1 month, b 6 months, and c 12 months, estimated based on our previous data [5]. The bars represent the percentage of time spent in, below, and above target INR ranges, stratified by CYP2C9 and VKORC1 genotype. CYP2C9 cytochrome P450 2C9, INR international normalized ratio, VKROC1 vitamin K epoxide reductase complex subunit 1, VT variant, WT wild-type
The risk of thromboembolism and major bleeding events was derived from published clinical literatures [26, 27]. The relative risks of thromboembolism and major bleeding events were increased in patients below or above the target INR range, respectively [23, 27, 28]. We assumed that the risk of adverse events in the target INR range of 2–3 was similar to the risk associated with the target range employed in Korea of 1.7–2.8 [5, 23, 24]. A total of 29% of the major bleeding events were ICH [29]; we assumed that all of the other major bleeding events were ECH [6, 7, 22, 27]. We also assumed that ECH patients would recover within 1 month [6, 9]. ICH patients had a 48.6% probability of dying and a 34% probability of recovery with sequelae [22]; the remaining patients (17.4%) recovered from ICH. A total of 60% of the thromboembolic events were classified as ischemic stroke; the remaining events (40%) were classified as TIA [9]. We assumed that 11.7% of stroke patients died [30], 43.6% recovered with sequelae [30], and the remaining patients (44.7%) recovered from stroke. It was assumed that all of the TIA patients were fully recovered within the subsequent month [9]. The annual mortality rate for MHVR patients was estimated at 7% per year [31]. The mortality rate during sequelae was estimated at 0.73% per month [32]. The mortality rates were obtained by the slope of linear regression of the log-transformed survival data [33]. The model input parameters are listed in Table 1.
2.4 Utility and Cost Input
Our hypothetical cohort was composed of Korean MHVR patients, and a utility value of 0.73 for MHVR patients was obtained from The Netherlands population-based MHVR patients’ EQ-5D scores [34]. The utility estimates associated with adverse events were derived from US population-based EQ-5D scores [35, 36]. A disutility value of 0.194, for initial warfarin therapy during hospitalization for open heart surgery, was applied [37], with a disutility value of 0.013 used for long-term anticoagulant care [35, 36]. Costs were determined from a healthcare sector perspective [33]. All relevant direct medical costs covered by the national insurance were considered. The costs and effectiveness were discounted at 3% per year in accordance with the World Health Organization (WHO) guideline [38]. All costs were updated to 2016 values using data obtained from the Korean National Health Insurance Service (NHIS). The Korean won to US dollar (US$) yearly average exchange rate was approximately 0.001 in 2016 [39]; i.e., 1000 Korean won was calculated to be US$1; all costs were expressed in US dollars. We estimated the cost of CYP2C9 and VKORC1 genotyping assay to be US$140, based on current pricing in Busan Paik Hospital, although this cost varies widely in the available data [40]. The cost of anticoagulant care during hospital admission days and clinical visits for INR monitoring were estimated using the micro-costing method using public data obtained from the Health Insurance Review & Assessment service [40]. Hospital admission costs comprised the general room, food service, prescription dispensing, INR monitoring, and doctors’ fees. The total cost of the clinical visit comprised the cost of INR monitoring and the doctor’s fee. The cost of warfarin (US$0.07 in Korea) was not included in this model [40]. The cost of valve replacement, ischemic stroke, TIA, ICH, ECH, and sequelae after stroke or ICH were estimated via the gross-costing method using Korean NHIS data from July 2015 to June 2016 [41].
2.5 Analyses
Base-case estimates of the costs and QALY values associated with the genotype-guided and standard warfarin dosing strategies were first determined, followed by one-way sensitivity analysis to evaluate the impact of different input parameters on the results. Parameters were varied according to their 95% confidence intervals, and varied by 20% if a confidence interval was not available. The risk of thromboembolism or major bleeding events was varied by 50%. The effect of genotype-guided warfarin dosing on increasing time spent within the target INR range (12.6% in base-case analysis) was varied by 50% (6.3–18.9%). Genotyping costs varied between US$70 and US$210 and the costs of anticoagulant treatment were varied by 50%. The costs of valve replacement, ischemic stroke, TIA, ICH, ECH, and sequelae after stroke or ICH were varied by 100% (because the Korean NHIS claims data does not include out-of-pocket insurance costs).
A probabilistic sensitivity analysis, using the Monte-Carlo simulation method, was performed to examine the combined impact of uncertainties concerning the values of multiple input parameters on the estimated cost-effectiveness of genotype-guided warfarin dosing. This also enabled us to calculate the probability that genotype-guided warfarin dosing would be cost effective at a certain threshold or at the willingness-to-pay value. During the probabilistic sensitivity analysis, the simulation was conducted by randomly sampling distributions of all variables, which were defined with 95% confidence intervals or plausible ranges. Gamma distribution was used for cost data [33]. Dirichlet distribution was used for the probabilities with more than two possible results (e.g., genotype and outcomes of adverse events). Beta distribution was used for utilities [33]. Log-normal distribution was used for the relative risk of adverse events and mortality [33]. Normal distribution was used for the percentage of time spent in, below, and above target INR ranges [42]. Finally, results of the Monte-Carlo simulation were ordered by using a cost-effectiveness acceptability curve to test for the probability of the estimated incremental cost-effectiveness ratios (ICERs) being under an assumed willingness-to-pay threshold of US$50,000 per QALY gained [43].
2.6 Estimation of Time Taken to Reach Stable Anticoagulation
Stable anticoagulation was defined as two consecutive INRs in the therapeutic range, with no dose adjustment during the follow-up interval (weekly) [44]. We estimated the time taken to reach stable warfarin dosing in our MHVR patients in both the genotype-guided and standard warfarin dosing arms, using simulation data obtained from our Markov model.
2.7 Model Validation
To assess the predictive validity of the model, we compared the cumulative probabilities of ischemic stroke and major hemorrhage associated with standard warfarin dosing to the clinical trial results of a randomized evaluation of long-term anticoagulation therapy over a 2-year period [29]. Model outputs for the 2-year cumulative probability of ischemic stroke and ICH were 2.11%/year and 1.13%/year, respectively, compared with 2.02%/year and 1.10%/year, respectively, for Asian patients.
3 Results
3.1 Base-Case Analysis
Table 2 shows the results of the cost-effectiveness analysis. Compared to standard warfarin therapy, the genotype-guided approach increased QALYs marginally (0.005; i.e., 2 days of perfect health) and increased costs by US$6.8. The ICER was US$1356.2 per QALY gained, which was less than the generally acceptable threshold for willingness to pay of US$50,000 per QALY gained.
3.2 Sensitivity Analysis
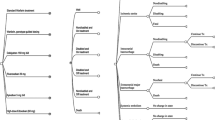
Figure 3 depicts a tornado diagram summarizing the results of the one-way sensitivity analysis of the clinical and economic parameters on the cost effectiveness. The ICER varied between –US$19,850 per QALY gained (less costly and more effective) and US$27,643 per QALY gained when the genotype-guided warfarin dosing increased the time within the target INR range by between 6.3% and 18.9%. This effect of genotype-guided warfarin dosing had the greatest influence on the cost effectiveness. The ICER varied between –US$12,662 per QALY gained (less costly and more effective) and US$15,375 per QALY gained when the genotyping costs varied between US$70 and US$210. The ICER varied between –US$4763 per QALY gained (less costly and more effective) and US$10,620 per QALY gained when the absolute risk of thromboembolism varied between 1%/year and 3%/year. The ICER varied between –US$8670 per QALY gained (less costly and more effective) and US$12,426 per QALY gained when the relative risk of thromboembolism below the target INR range varied between 2.92 and 8.8. We performed extensive sensitivity analysis on the costs of ischemic stroke, TIA, ICH, ECH, and long-term sequelae (the Korean NHIS claim data do not include out-of-pocket insurance costs). Although the costs of adverse event increased two-fold, they did not affect the robustness of the base-case results, with the exception of the cost of long-term sequelae treatment. However, The ICER was <US$0 per QALY gained (dominant cost effectiveness) when the cost of sequelae was >US$412. In the base-case analysis, we assumed that genotype-guided warfarin dosing did not affect hospital admission days for MHVR open heart surgery. If genotype-guided warfarin dosing reduces hospital stay by 1 day, the ICER (–US$16,223 per QALY gained) exhibits dominant cost effectiveness.
Tornado diagram of one-way sensitivity analysis. The horizontal bars represent the range of the incremental cost-effectiveness ratio for one-way sensitivity analysis over the range of the parameter in parentheses. The dotted vertical line indicates the incremental cost-effectiveness ratio of genotype-guided warfarin dosing at base-case analysis. ECH extracranial hemorrhage, ICH intracranial hemorrhage, INR international normalized ratio, QALY quality-adjusted life-year, TE thromboembolism, TIA transient ischemic attack, % TTR percentage of time within therapeutic INR range
In the probabilistic sensitivity analysis, genotype-guided warfarin dosing was more effective and more costly in 17.3% of simulations (Fig. 4) and was the dominant strategy (less costly, more effective) in 82.7% of simulations. There was also a 99.8% probability that genotype-guided warfarin dosing was cost effective at a willingness-to-pay threshold of US$50,000 per QALY gained. Figure 4 also indicates the probability that genotyping would be cost effective over a range of likely thresholds.
3.3 Reduced Time to Reach Stable Anticoagulation in Genotype-Guided Warfarin Dosing
The analysis of time taken to reach stable anticoagulation indicated that patients treated with genotype-guided warfarin dosing achieved a stable dose faster than those who were treated using standard warfarin dosing (Fig. 5). The median time taken to reach a stable warfarin dose in the genotype-guided versus standard dosing group was 12 and 20 days, respectively; 90% of patients receiving genotype-guided versus standard dosing reached a stable warfarin dose after 26 and 42 days, respectively. The mean value of hospitalization days in MHVR patients was 17 based on our previous data [5], and the proportion of patients achieving stable anticoagulation under genotype-guided and standard warfarin dosing was 67.4 and 42.4%, respectively.
Time taken to reach stable anticoagulation. The time taken to reach stable anticoagulation in a proportion of patients is shown on the x-axis. The dotted horizontal lines represent the proportion of patients reaching stable anticoagulation (50 and 90%). The dotted vertical line represents the average hospital stay (17 days) calculated from our previous study [5]. The two curves indicate the rates of patients reaching stable anticoagulation for each treatment arm: genotype-guided warfarin therapy (solid curve) vs. standard therapy (dotted curve). The proportion of patients in each treatment arm over time was generated from simulation of a Markov model
4 Discussion
This is the first study to assess the cost effectiveness of genotype-guided warfarin dosing in MHVR patients and genotype-guided warfarin dosing under the FFS system. In a base-case analysis, we demonstrated that MHVR genotype-guided warfarin dosing represents a cost-effective strategy of reducing the risk of bleeding and thromboembolism. In this study, half of MHVR patients carrying the CYP2C9 wild-type/VKORC1 AG or GG genotype were below the target INR range during early-phase warfarin therapy compared with 27% of MHVR patients carrying the CYP2C9 wild-type/VKORC1 AA genotype. It has been reported that the risk of thromboembolic events is significantly increased in patients below the target INR range [27, 28]. MHVR patients are also at significantly greater risk of thromboembolism than AF patients during early-phase treatment [2, 15], such that genotype-guided dosing represents a safer warfarin dosing strategy [45]. In total, 24% of the CYP2C9 variant/VKORC1 AA genotype carriers were above the target INR range compared with 11% of CYP2C9 wild-type/VKORC1 AA genotype carriers. The risk of hemorrhagic complications is significantly increased when the INR exceeds the target INR [27, 28, 46].
This is the first cost-effectiveness analysis of genotype-guided warfarin dosing considering Asian patients who have lower minor allele frequencies of CYP2C9 and VKORC1 than other ethnicities (Asian 0% vs. Caucasian 12% for CYP2C9*2; Asian 2% vs. Caucasian 8% for CYP2C9*3; Asian 7.5% vs. Caucasian 61.5% for VKORC1 G allele) [47, 48]. In addition, Asian patients receiving standard warfarin therapy have been reported to face a higher risk of stroke and adverse events than non-Asian patients [29, 49]. Thus, genetic effects on warfarin response are different among different ethnic populations [47, 50]. However, previously published reports about the cost effectiveness of genotype-guided warfarin dosing have not focused on Asian patients [6–12]. In general, genetic testing was cost effective in instances of higher minor allele frequency [51, 52]. Our results showed that genotype-guided warfarin dosing was a cost-effective strategy in Asian patients, despite low minor allele frequencies of CYP2C9 and VKORC1, which indicates that genotype-guided warfarin dosing may also be cost effective in other ethnicities with higher minor allele frequencies of CYP2C9 and VKORC1.
The cost effectiveness of new interventions needs to be evaluated in specific diseases under a specific healthcare system. The previously reported cost-effectiveness analyses included costs only under the DRG system [6–12], which is a medical care payment system used to eliminate variance in hospital costs based on categories of patient illness [53]. Costs of inpatient anticoagulation therapy under the DRG system do not increase markedly when patients undertake additional therapy; in contrast, under the FFS system, additional anticoagulant monitoring and hospitalization days are associated with increased costs. In our study, we assumed that the number of hospital days in the genotype-guided dosing group was identical to that of the standard dosing group, because hospital discharge was not contingent on warfarin dose stability. However, if genotype-guided warfarin dosing reduces hospitalization duration by 1 day, it represents the dominant strategy. In this study, the time taken to reach stable anticoagulation was faster using genotype-guided warfarin dosing than standard warfarin dosing. At the time of discharge (average hospitalization duration calculated from our previous report = 17 days) [5], 67.4% of MHVR patients managed using a genotype-guided warfarin regimen had achieved a stable dose compared with 42.4% of patients under a standard warfarin dosing regimen. Due to greater control of anticoagulation following discharge, less-frequent dose adjustment and INR monitoring are required; under the FFS system, the clinical costs of anticoagulant treatment are reduced with genotype-guided warfarin dosing regimens. This may also indicate that genotype-guided dosing confers greater safety and efficacy. However, the reduction of hospital resource use considering a patient or societal perspective cannot be reflected under the DRG system because of a fixed payment including out-of-pocket and indirect costs. Thus, we cannot directly compare societal perspectives between the two systems.
Previous studies indicate marked variability in the effects as well as cost effectiveness of genotype-guided warfarin dosing [6–9]. We therefore conducted extensive sensitivity analyses, in which genotype-guided dosing was a consistently cost-effective strategy under the assumptions used in this study. Genotyping costs are decreasing over time, increasing the probability for cost effectiveness. In their analyses, Eckman et al. [6] used a figure of US$400 to represent the genotyping cost and reported that genotype-guided warfarin dosing was not cost effective for their model, except in AF patients at high risk of bleeding [6]. They reported that in other circumstances, genotyping was cost effective when reduced to US$200. You et al. [8] used a figure of US$72 to represent genotyping cost, and demonstrated that genotype-guided warfarin dosing is cost effective. More recently, Verhoef et al. [9] used a figure of €40 (US$62 at the time of publication) to represent the genotyping cost and similarly demonstrated the cost effectiveness of genotype-guided warfarin dosing [9]. Provided that the cost of genotyping continues to decline, genotype-guided warfarin dosing can be cost effective as demonstrated by these studies [6, 8, 9]. Our results showed that the cost effectiveness of genotype-guided warfarin dosing remained robust, despite the potential variability of clinical and economic parameters included in our analyses.
Currently, new oral anticoagulants (NOACs) are used as alternatives to warfarin for the prevention and treatment of some thromboembolic diseases such as non-valvular AF, deep vein thrombosis, or pulmonary embolism [54]. It was already known that these drugs are highly cost effective compared with warfarin standard therapy. However, NOACs are not approved for the treatment of MHVR patients yet [54]. The use of warfarin with optimal dosing strategies such as genotype-guided dosing should be considered for anticoagulation of MHVR patients [55].
Healthcare in Korea is provided via a compulsory national health insurance and in many other countries mainly via a public health insurance system. Each insurance system decides whether to include a new intervention in the reimbursement list and how to determine the price of its national health insurance [56]. Whether an intervention is covered by health insurance has a significant influence on the prescribing behavior of physicians. Therefore, our study results can provide health policy makers, health insurance providers, and healthcare providers with important information on the cost effectiveness of genotype-guided warfarin dosing in MHVR patients.
A limitation of this study was that this it did not include indirect costs, such as patient time and caregiver time. The effect of genotype-guided warfarin dosing on increasing time spent within the target INR range could result in reducing the time to taken to reach stable anticoagulation and reducing the risk of adverse events. In addition, the genotype-guided dosing strategy could generate different indirect costs, which may affect the cost-effectiveness results when considering a societal perspective.
5 Conclusion
Compared with standard dosing, genotype-guided warfarin dosing resulted in a faster time to reach stable anticoagulation, a lower risk of adverse outcomes, and appears to represent a cost-effective treatment strategy for MHVR patients under the FFS system.
References
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):2438–88.
Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33(19):2451–96.
Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90(4):625–9.
Jonas DE, McLeod HL. Genetic and clinical factors relating to warfarin dosing. Trends Pharmacol Sci. 2009;30(7):375–86.
Kim HS, Lee SS, Oh M, Jang YJ, Kim EY, Han IY, et al. Effect of CYP2C9 and VKORC1 genotypes on early-phase and steady-state warfarin dosing in Korean patients with mechanical heart valve replacement. Pharmacogenet Genomics. 2009;19(2):103–12.
Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med. 2009;150(2):73–83.
Meckley LM, Gudgeon JM, Anderson JL, Williams MS, Veenstra DL. A policy model to evaluate the benefits, risks and costs of warfarin pharmacogenomic testing. Pharmacoeconomics. 2010;28(1):61–74.
You JH, Tsui KK, Wong RS, Cheng G. Cost-effectiveness of dabigatran versus genotype-guided management of warfarin therapy for stroke prevention in patients with atrial fibrillation. PLoS One. 2012;7(6):e39640.
Verhoef TI, Redekop WK, Veenstra DL, Thariani R, Beltman PA, van Schie RM, et al. Cost-effectiveness of pharmacogenetic-guided dosing of phenprocoumon in atrial fibrillation. Pharmacogenomics. 2013;14(8):869–83.
Patrick AR, Avorn J, Choudhry NK. Cost-effectiveness of genotype-guided warfarin dosing for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2009;2(5):429–36.
You JH. Pharmacogenetic-guided selection of warfarin versus novel oral anticoagulants for stroke prevention in patients with atrial fibrillation: a cost-effectiveness analysis. Pharmacogenet Genomics. 2014;24(1):6–14.
Jh Y. Universal versus genotype-guided use of direct oral anticoagulants in atrial fibrillation patients: a decision analysis. Pharmacogenomics. 2015;16(10):1089–100.
DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: a pathophysiologic approach. 8th ed. New York: McGraw-Hill Education, LLC.; 2011. p. 311–52.
Rose JP, Rihn TL, Long SF. Warfarin sensitivity after mechanical heart valve replacement. Pharmacotherapy. 1998;18(4):856–9.
Ageno W, Turpie AG. Exaggerated initial response to warfarin following heart valve replacement. Am J Cardiol. 1999;84(8):905–8.
Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S–47S.
Jeong H, Lee H, Lee JH, Lee T. Payment reform for the improvement of primary care in Korea. J Korean Med Assoc. 2013;56(10):881–90.
Schuetz P, Albrich WC, Suter I, Hug BL, Christ-Crain M, Holler T, et al. Quality of care delivered by fee-for-service and DRG hospitals in Switzerland in patients with community-acquired pneumonia. Swiss Med Wkly. 2011;141(18):w13228.
Lee SJ, Jang YJ, Cha EY, Kim HS, Lee SS, Shin JG. A haplotype of CYP2C9 associated with warfarin sensitivity in mechanical heart valve replacement patients. Br J Clin Pharmacol. 2010;70(2):213–21.
Casner PR. Warfarin: less may be better. Ann Intern Med. 1997;127(4):332–3.
Lee BK, Lee JY, Jeong YM, Lee MK, Ahn H. Determination of practical dosing of warfarin in Korean outpatients with mechanical heart valves. Korean J Thorac Cardiovasc Surg. 2005;38(11):761–72.
Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120(8):700–5.
You J, Chan F, Wong R, Cheng G. Is INR between 2.0 and 3.0 the optimal level for Chinese patients on warfarin therapy for moderate-intensity anticoagulation? Br J Clin Pharmacol. 2005;59(5):582–7.
Rhie S, Choi JY, Jang IS, Kim JW, Lee CE, Park HO. Relationship between the occurrence of thromboembolism and INR measurement interval in low intensity anticoagulation after aortic mechanical valve replacement. Korean J Thorac Cardiovasc Surg. 2011;44(3):220–4.
Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–9.
Labaf A, Grzymala-Lubanski B, Stagmo M, Lövdahl S, Wieloch M, Själander A, et al. Thromboembolism, major bleeding and mortality in patients with mechanical heart valves-a population-based cohort study. Thromb Res. 2014;134(2):354–9.
Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333(1):11–7.
Reynolds MW, Fahrbach K, Hauch O, Wygant G, Estok R, Cella C, et al. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis. Chest. 2004;126(6):1938–45.
Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44(7):1891–6.
Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349(11):1019–26.
Ruel M, Chan V, Bédard P, Kulik A, Ressler L, Lam BK, et al. Very long-term survival implications of heart valve replacement with tissue versus mechanical prostheses in adults <60 years of age. Circulation. 2007;116(11 suppl):I294–300.
Lai SM, Alter M, Friday G, Sobel E. Prognosis for survival after an initial stroke. Stroke. 1995;26(11):2011–5.
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: OUP Oxford; 2006. p. 77–120.
Maliwa MA, van der Heijden GJ, Bots ML, van Hout BA, Casselman FP, van Swieten H, et al. Quality of life and NYHA class 30 years after mechanical aortic valve replacement. Cardiovasc Surg. 2003;11(5):381–7.
Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Mak. 2006;26(4):410–20.
Oregon Health and Science University. EQ-5D index score calculators for the UK. http://www.ohsu.edu/epc/mdm/UKcalculators.cfm. Accessed 10 Jan 2017.
Halpin LS, Barnett SD, Martin LM, Hunt SL, Henry L, Ad N. Survival and quality of life following elective open-heart surgery. J Nurs Care Qual. 2008;23(4):369–74.
Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, et al., editors. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: WHO; 2003. http://www.who.int/choice/cost-effectiveness/generalized/en/. Accessed 12 Sept 2016.
OANDA. Average exchange rates. https://www.oanda.com/currency/average. Accessed 10 Jan 2017.
Health Insurance Review & Assessment Service. http://www.hira.or.kr/. Accessed 10 Jan 2017.
National Health Insurance Service. http://nhis.or.kr/. Accessed 10 Jan 2017.
Janzic A, Kos M. Cost effectiveness of novel oral anticoagulants for stroke prevention in atrial fibrillation depending on the quality of warfarin anticoagulation control. Pharmacoeconomics. 2015;33(4):395–408.
Weinstein MC, Jiegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–8.
Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360(8):753–64.
Epstein RS, Moyer TP, Aubert RE, O’Kane DJ, Xia F, Verbrugge RR, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study). J Am Coll Cardiol. 2010;55(25):2804–12.
Yasaka M, Minematsu K, Yamaguchi T. Optimal intensity of international normalized ratio in warfarin therapy for secondary prevention of stroke in patients with non-valvular atrial fibrillation. Intern Med. 2001;40(12):1183–8.
Moyer TP, O’Kane DJ, Baudhuin LM, Wiley CL, Fortini A, Fisher PK, et al. Warfarin sensitivity genotyping: a review of the literature and summary of patient experience. Mayo Clinic Proc. 2009;84(12):1079–94.
Lee SS, Kim KM, Thi-Le H, Yea SS, Cha IJ, Shin JG. Genetic polymorphism of CYP2C9 in a Vietnamese Kinh population. Ther Drug Monit. 2005;27(2):208–10.
Sabir I, Khavandi K, Brownrigg J, Camm AJ. Oral anticoagulants for Asian patients with atrial fibrillation. Nat Rev Cardiol. 2014;11(5):290–303.
Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369(24):2283–93.
Veenstra DL, Higashi MK, Phillips KA. Assessing the cost-effectiveness of pharmacogenomics. AAPS PharmSci. 2000;2(3):E29.
Hagaman JT, Kinder BW, Eckman MH. Thiopurine S-methyltranferase testing in idiopathic pulmonary fibrosis: a pharmacogenetic cost-effectiveness analysis. Lung. 2010;188(2):125–32.
Kahn KL, Rubenstein LV, Draper D, Kosecoff J, Rogers WH, Keeler EB, et al. The effects of the DRG-based prospective payment system on quality of care for hospitalized Medicare patients. An introduction to the series. JAMA. 1990;264(15):1953–5.
Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467–507.
Finkelman BS, Gage BF, Johnson JA, Brensinger CM, Kimmel SE. Genetic warfarin dosing: tables versus algorithms. J Am Coll Cardiol. 2011;57(5):612–8.
Yang BM, Bae EY, Kim J. Economic evaluation and pharmaceutical reimbursement reform in South Korea’s National Health Insurance. Health Aff (Millwood). 2008;27(1):179–87.
Author contributions
Ho-Sook Kim and Jae-Gook Shin conceived and designed the study. Dong-Jin Kim and Ho-Sook Kim collected and assembled the data. Dong-Jin Kim, Minkyung Oh, Eun-Young Kim, and Ho-Sook Kim contributed to the data analysis and interpretation of data. Dong-Jin Kim and Ho-Sook Kim drafted the manuscript. All of the authors had roles in conception and design of the study; collection and assembly of data; data analysis and interpretation; and preparation, review, and approval of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI15C1537).
Conflict of interest
Dong-Jin Kim, Ho-Sook Kim, Minkyung Oh, Eun-Young Kim, and Jae-Gook Shin have no conflicts of interest.
Ethical approval
Formal consent is not required for this type of study.
Rights and permissions
About this article
Cite this article
Kim, DJ., Kim, HS., Oh, M. et al. Cost Effectiveness of Genotype-Guided Warfarin Dosing in Patients with Mechanical Heart Valve Replacement Under the Fee-for-Service System. Appl Health Econ Health Policy 15, 657–667 (2017). https://doi.org/10.1007/s40258-017-0317-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-017-0317-y