Abstract
Ceramides are a class of sphingolipid that is the backbone structure for all sphingolipids, such as glycosphingolipids and phosphosphingolipids. While being a minor constituent of cellular membranes, ceramides are the major lipid component (along with cholesterol, free fatty acid, and other minor components) of the intercellular spaces of stratum corneum that forms the epidermal permeability barrier. These stratum corneum ceramides consist of unique heterogenous molecular species that have only been identified in terrestrial mammals. Alterations of ceramide molecular profiles are characterized in skin diseases associated with compromised permeability barrier functions, such as atopic dermatitis, psoriasis and xerosis. In addition, hereditary abnormalities of some ichthyoses are associated with an epidermal unique ceramide species, omega-O-acylceramide. Ceramides also serve as lipid modulators to regulate cellular functions, including cell cycle arrest, differentiation, and apoptosis, and it has been demonstrated that changes in ceramide metabolism also cause certain diseases. In addition, ceramide metabolites, sphingoid bases, sphingoid base-1-phosphate and ceramide-1-phosphate are also lipid mediators that regulate cellular functions. In this review article, we describe diverse physiological and pathological roles of ceramides and their metabolites in epidermal permeability barrier function, epidermal cell proliferation and differentiation, immunity, and cutaneous diseases. Finally, we summarize the utilization of ceramides as therapy to treat cutaneous disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ceramides are a class of sphingolipid that are key constituents in the formation of a competent epidermal permeability barrier in the skin, and they play both physiological and pathological roles in skin cells. |
We discuss diverse functions of ceramides and their metabolites in barrier function, epidermal cell proliferation and differentiation, immunity, and cutaneous diseases, and summarize the utilization of ceramides to treat cutaneous disease. |
We also discuss the limitations of current knowledge and the available experimental data on ceramides to help guide future studies. |
1 Introduction
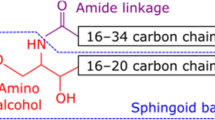
In the 1880s, Johann Ludwig Wilhelm Thudichum, a German physician who conducted biochemical research in London, discovered a previously unidentified lipid species in brain tissues. His isolated lipids had a previously unseen amphipathic feature, with unknown biological roles and structures. Faced with these mysterious lipids, and because the legendary Sphinx is said to have guarded the entrance to the Greek city of Thebes, asking a riddle to travelers to allow them passage, Thudichum named these lipids ‘sphingolipids’ [1]. Sphingolipids are a class of lipid that contain a long-chain (C16-20) amino alcohol (so-called sphingol or sphingoid base). The spingoid base’s amino residue is acylated to become ceramides, which are the backbone structure of all sphingolipids (i.e., glycosphingolipids and phosphosphingolipids). Ceramides are also a minor constituent of both plasma and cellular organellar membranes, while changes in cellular ceramide levels on the plasma membrane generate a signal to modulate cellular function; that is, to suppress cellular proliferation, or promote differentiation and apoptosis (see review articles [2,3,4]). Ceramides also create pores on mitochondrial membranes, causing cell death through increased membrane permeability [5]. Ceramide metabolites, such as the sphingoid bases, sphingosine-1-phosphate and ceramide-1-phosphate, also serve as modulator lipids to regulate cellular function [2, 6, 7]. Additionally, ceramides have a unique role in terrestrial animals’ stratum corneum [8,9,10,11]. A heterogenous ceramide molecular species is a key lipid component that combines with cholesterol and free fatty acid in the extracellular domain of the stratum corneum to form optimal barrier permeability [8,9,10,11]. Moreover, some ceramide species, that is, ω-O-acylceramide (acylceramide) and 6-hydroxysphingoid base-containing ceramide species, and cornified envelope-bound ω-hydroxy ceramide, are only found in terrestrial mammals [8]. Heterogenous ceramide species are abbreviated as previously reported [12, 13] with modification (Fig. 1).
Ceramide structures and heterogeneous ceramide species. Heterogenous ceramide species are abbreviated as previously reported [12, 13]. ADS N-2-OH dihydrosphingosine, AH N-2-OH-acyl-6-OH sphingosine, AP N-2-OH acyl-4-OH dihydrosphingosine, AS N-2-OH acylsphingosine, EODS ω-O-N-acyldihydrosphingosine, EOH ω-O-N-6-OH sphingosine, EOP ω-O-N-phytosphingosine, EOS ω-O-N-acylsphingosine, NDS N-acyldihydrosphingosine, NH N-acyl-6-OH sphingosine, NP N-acyl-4-OH dihydrosphingosine, NS N-acylsphingosine
Although there are several recently published review articles about skin ceramide, most of them are related to permeability barrier and skin diseases associated with a barrier abnormality (190 review articles appeared in a PubMed search with ‘ceramide’ and ‘skin’ as key words on March 13, 2021; article subjects were barrier [70%], lipid modulator [5%], sphingolipidosis [5%], and other [melanoma, lipidomics, general skin health; 20%]). Ceramides have multiple functions in cells, and here we discuss diverse physiological and pathological roles of ceramides and their metabolites in epidermal permeability barrier function, epidermal cell proliferation and differentiation, immunity, and cutaneous diseases. Finally, we summarize the utilization of ceramides as medicine to treat cutaneous disease. Further details regarding the specific roles of ceramides and their metabolites can be learned from prior publications cited in each section of this review.
2 Role of Ceramide in Skin Barrier
The skin of terrestrial mammals deploys multiple protective barriers: (1) a permeability barrier that suppresses excess evaporation of water and loss of small molecules from skin, while also quelling penetration of exogenous molecules, allergens and microorganisms into skin [14]; (2) an oxidative stress barrier, made up of antioxidant molecules such as glutathione and ascorbic acid [15]; (3) an ultraviolet irradiation (UV) barrier (urocanic acid is endogenous and UV-absorbent) [16]; (4) a mechanical stress barrier [17]; (5) an antimicrobial barrier [18]; and (6) a thermal barrier [19, 20]. The permeability barrier also serves as an antimicrobial barrier (preventing invasion of microbes into the skin and their colonization) and a thermal barrier (excess water evaporation decreases body temperature) in the stratum corneum [19, 20]. Both tight junctions in nucleated layers of epidermis and extracellular lipid lamellar structures in the stratum corneum are responsible for permeability barrier functions [21], while the permeability barrier in the stratum corneum serves as its ‘first line of defense’ and its ‘last line for preserving life’.
Unique heterogeneous ceramides are the key constituent of the epidermal permeability barrier in the stratum corneum (note: cholesterol and certain chain lengths of fatty acid, as well as ceramides, are also required to form a secure lamellar membrane structure in the stratum corneum [22, 23]). These ceramides are synthesized in late stages of differentiated keratinocytes. The majority of ceramide species are N-non-hydroxy acylsphingosine (NS) (carbon chain lengths of acyl residue are 16–24) in proliferated and early stages of differentiated keratinocytes [24]. As a major ceramide species, NS is universally present in cells of mammalian tissues. Heterogenous ceramides with appropriate ratios and amide-linked fatty acids (carbon chain length and saturation) are required for generation of a competent, vital barrier (the synthetic pathway of these ceramides is summarized in Figs. 2, 3, 4 and 5; see also reviews [25,26,27,28,29,30,31,32]). Decreases in acylceramide and N-acyl 4-hydroxydihydrosphingosine (N-non-hydroxy acylphytosphingosine [NP]), and changes in a ceramide’s fatty acid chain length affect the lamellar structure periodicity and lipid packing in the stratum corneum, conditions that are seen in the common skin disease, atopic dermatitis (Sect. 5.1). Amongst ceramide species, acylceramides are essential for a vital epidermal permeability barrier [9, 33,34,35], and certain amounts and molecular species of acylceramide are important to form stable lamellar membrane structures [36, 37]. Research using X-ray and Fourier-transform infrared spectroscopy (FTIR) analyses of lipid lamellar structures has successfully characterized lamellar structures and also proposed several models of lamellar membrane structures in the stratum corneum (see review articles [8, 11, 38, 39]). Moreover, neutron scattering analysis has also characterized the contribution of lipids in forming lamellar nanostructures in the stratum corneum [40, 41]. Yet, the structure and organization of lamellar membranes in in vivo skin still need to be elucidated (see Sect. 2.1).
2.1 What We Do Not Know
The following areas need more clarification: (1) the acceptable ranges of changes in ceramide composition to maintain a vital permeability barrier function; (2) why deficiency of specific ceramide species and changes in fatty acid composition occur in certain skin diseases; (3) how changes in pH, enzyme activities (proteases, lipases, ceramidases), temperature, and humidity are involved in dynamic (from moment to moment) alteration of lamellar membrane structure and function in the limited part of the stratum corneum in in vivo skin; (4) why, despite humans and other mammals (albeit characterization of the ceramide profile is limited to a small number of animals, such as mouse, guinea pig, dog, and pig) having different ceramide species profiles, these mammals when healthy have competent permeability barrier function [42,43,44,45]; (5) identification of the enzyme that synthesizes 6-hydroxy sphingoid base; (6) why although the corneocyte lipid envelope is thought to be a scaffold of lamellae in the stratum corneum [46, 47], there is no experimental evidence about its function; and (7) the regulatory mechanism that forms vital lamellar membrane structures in the stratum corneum.
3 Roles of Ceramides in the Modulation of Epidermal Proliferation and Differentiation
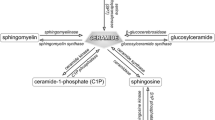
Ceramides and their metabolites (sphingoid base, sphingoid base-1-phosphate and ceramide-1-phosphate) are lipid mediators that modulate cellular function. Ceramide and ceramide metabolite-mediated modulations of cell function are very apparent in cells. NS, present in all mammalian tissue cells as a major ceramide species, has been shown to be a modulator ceramide [2, 48, 49]. Phytosphingosine and phytosphingosine-1-phosphate have been investigated as pharmacological modulators [50], while recent studies demonstrated that phytosphingosine-1-phosphate does not have the ability to modulate cellular function [51, 52]. Interestingly, prior studies have demonstrated that acylglucosylceramide promotes keratinocyte differentiation through increasing cellular Ca2+ concentration and protein kinase C activation [53, 54], but it has not been proven that other ceramide species identified in the epidermis (in vivo) have the ability to modulate cellular function.
Ceramides and sphingosine can add anti-mitotic and pro-cell death features to cells, including in keratinocytes. Sphingomyelin (N-acyl-sphingonyl-1-phosphocholine) located on plasma membranes produces ceramide by activation of acidic or neutral sphingomyelinase in response to extracellular stimuli such as inflammatory cytokines [55, 56]. Increased ceramide creates a platform that can mediate a death-inducing signaling complex [57]. Ceramide that is generated on plasma membranes can be changed back to sphingomyelin by plasma membrane residential sphingomyelin synthase (SMS) 2, an isoform of SMS [48, 58, 59]. SMS2 is partly involved in the maintenance or regulation of cell proliferation, differentiation and cell death [48, 58, 59].
An increase in intracellular ceramide induces apoptosis and mitosis, and it modulates insulin signaling through binding to certain proteins (ceramide-binding proteins) [3, 49], such as ceramide-activated protein phosphatases (protein phosphatase [PP]1 and PP2A) [60], protein kinase C zeta [61], and cathepsin D [62]. Ceramides also affect membrane fluidity that alters mitochondrial function, including electron transport via suppression of the respiratory chain [63] and mitophasy via interaction with LC3BII (microtubule-associated proteins 1A/1B light chain 3B) [64].
In keratinocytes, inhibition of ceramide hydrolysis by a specific inhibitor of alkaline ceramidase, 4-(dimethylamino)-pyridine (DMAP), and by conversion of ceramide to glucosylceramide by a specific glucosylceramide synthase inhibitor, d-threo-1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol (d-PPMP) increases caspase-14 expression at the transcriptional levels [65]. Caspase-14 hydrolyzes filaggrin to generate natural moisturizing factor (NMF) [66]. Sphingoid bases also modulate cellular functions. Prior studies demonstrate that (1) phytosphingosine increases transactivation of peroxisome proliferator-activated receptors (PPARs) [50]; (2) topical phytosphingosine suppresses phorbol ester-mediated inflammation (other sphingoid bases were not examined) [50]; and (3) lipidomic and transcriptomic analyses show that among sphingoid bases, dihydrosphingosine (sphinganine) is potent in promoting differentiation and ceramide production in keratinocytes [67].
Sphingosine-1-phosphate (S1P), which is synthesized from sphingosine by sphingosine kinase (SPHK) 1 [68] and SPHK2 [69], modulates cellular function by S1P receptor-dependent and -independent pathways. Five S1P receptors (all class G protein coupled receptors) have been identified, and keratinocytes express all of them [70]. S1P is secreted from cells via an ATP-binding cassette (ABC) transporter, spinster homolog 2 (SPNS2), that is a member of the large family of non-ATP-dependent organic ion transporters and of the major facilitator superfamily domain containing 2A (Mfsd2a) [71]. S1P modulates cellular function in an autocrine and a paracrine fashion in S1P receptor-dependent pathways. S1P is a pro-mitogenic lipid mediator in cells and suppresses proliferation of certain cells, including KC (i.e., S1P stimulates KC differentiation) [72]. S1P also modulates cellular function via a receptor-independent pathway. S1P generated by SPHK2 in the nucleus inhibits histone deacetylase (HDAC) 1 and 2 [73]. In addition, S1P synthesized by sphingosine kinase 1 (but not SPHK2) activates cathelicidin antimicrobial peptides (CAMP/LL-37) in cells, including human keratinocytes (KC) [52, 74] (see Sect. 4) (Fig. 6). S1P also binds to two heat shock proteins, (HSP) 90 and HSP90α, and endoplasmic reticulum residential GRP94, leading to formation of a signaling complex with TRAF2, TRADD, and RIP1, followed by activation of NF-κB and then C/EBPα [74]. Yet, the S1P-independent pathway is not completely characterized as compared with the S1P receptor-dependent mechanism.
A modulator role of ceramide metabolites, ceramide-1-phosphate and sphingosine-1-phosphate, in the regulation of innate immune response through antimicrobial peptide production in response to external stressors [51, 52, 74, 82, 83]. 1) cPLA2 cytosolic phospholipase A2; 2) 15d-PGJ2 15-Deoxy-Delta-12,14-prostaglandin J2; 3) PPARs peroxisome proliferator-activated receptors; 4) STAT signal transducer and activator of transcription; 5) hBD human ß-defensin; 6) NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells; 7) c/EBP CCAAT-enhancer-binding protein α; 8) CAMP cathelicidin antimicrobial peptide
3.1 What We Do Not Know
These studies suggest that pharmacological modulation of ceramide and sphingoid base amounts in skin should be useful for treatment of skin diseases associated with abnormal epidermal proliferation and differentiation, and inflammation. However, the physiological roles of ceramide, ceramide-1-phosphate, sphingoid base, and sphingoid base-1-phosphate mediated regulation in epidermal proliferation and differentiation in vivo still need to be determined.
4 Roles of Ceramides and Their Metabolites in Modulation of Skin Immunity
Sphingomyelin (whose backbone ceramide structure is NS) is a major lipid species in plasma membranes. Sphingomyelinase hydrolyzes sphingomyelin to generate ceramides. Five isoforms of sphingomyelinase (one acidic, three neutral and one alkaline sphingomyelinase) are identified in mammals [75, 76]. Both the acidic and neutral sphingomyelinase 2 are involved in immunity. Sphingomyelinase in T cells is activated to generate ceramide in response to stimuli, such as cytokines and clustering of receptors, including CD95, CD40 and tumor necrosis factor (TNF) receptor 1. Generated ceramide promotes T-cell migration through cytoskeletal polarization and interacts with endothelial cells, contributing to homing CD4+ T cells [77]. CD3/CD28 stimulation-mediated CD4+ T-cell activation is required for acidic sphingomyelinase [78]. However, exogenous ceramide does not rescue regulation of CD3/CD28 stimulation-mediated CD4+ T-cell activation [78]. These studies elucidated that acidic sphingomyelinase interacts with CD3/CD28, blocks the downstream signaling pathway of CD3/CD28, and results in suppressing differentiation of CD4+ T cells to Th17 cells [78].
Neutral sphingomyelinase 2 is localized at the cytosolic leaflet of the plasma membrane, and its activity helps maintain mitochondrial function and energy production in stimulated CD4+ T cells [79]. Lack of neutral sphingomyelinase 2 activity causes hyper-responsiveness to CD3/CD28 co-stimulation by excessive elevation of mitochondrial function (i.e., accumulation of ATP and increases in glycolytic activity) [79]. These studies suggest that ceramide produced from sphingomyelin regulates CD4+ T-cell functions through governing mitochondrial energy metabolism.
S1P levels are high in blood and lymph in the sub-micromolar range and low in intracellular and interstitial fluids, resulting in the creation of an S1P gradient. Because S1P has a chemotactic feature, this gradient mediates the egress of lymphocytes between lymphoid tissues and general circulation. Topically, S1P and FTY720 suppress Langerhans cell migration from skin to the draining auricular lymph node in hapten-treated skin via S1P1 receptor activation (keratinocytes expressed all five receptors, while immune cells express S1P1). In addition, migration of dendritic cells is reduced by S1P and FTY720 [80]. S1P also attenuates the antigen uptake of epidermal dendritic cells [81], so pharmacological modulation of S1P metabolism can be translated into disease treatment.
S1P and ceramide-1-phosphate stimulate innate immunity through antimicrobial peptides, namely, the cathelicidin antimicrobial peptide (CAMP) and human ß-defensin 2 and 3, respectively, in response to external stress, such as ultraviolet irradiation and other type of oxidative stress, and epidermal permeability barrier perturbation [74, 82, 83]. S1P and ceramide-1-phosphate increase the production of these antimicrobial peptides via external stress-mediated endoplasmic reticulum (ER) stress independent from microbial infection [74, 82, 83].
4.1 What We Do Not Know
Still to be determined: (1) whether ceramide modulates an immune response in residential T cell function of skin; (2) although topical administered S1P suppresses Langerhans cell migration and antigen presentation, whether increases in S1P during inflammation serve as an endogenous regulator to suppress Langerhans cell migration and antigen presentation; and (3) whether increases in S1P occur during inflammation, leading to directly suppressing inflammation, similar to pro-resolving lipid mediators, such as lipoxins, resolvins, protectins and maresins [84].
5 Skin Disease and Abnormalities in Ceramides and Their Metabolites
Characterization of the unique ceramide profile in the stratum corneum in the late 1970s and early 1980s led to scientific exploration of ceramide in skin. Moreover, finding changes in ceramide profiles in the following cutaneous diseases facilitated utilization of ceramide as part of topical agents that treat barrier-compromised skin.
5.1 Atopic Dermatitis (AD)
In 1986, Melnik et al. reported bulk ceramide content in non-inflamed plantar skin collected by biopsy [85], and later (1991), two Japanese groups, Yamamoto et al. [86] and Imokawa et al. [87] independently reported changes in ceramide profiles in atopic dermatitis skin. Both Japanese labs showed decreases in ω-O-N-acylsphingosine (EOS) in AD lesional skin [86, 87]. However, Yamamoto’s group found no changes in bulk ceramide levels, while Imokawa’s group found decreases in total ceramide levels in both lesional and non-lesional stratum corneum [87]. The number of subjects used for studies were different between the two groups, and samples were obtained by ethanol extraction directly from skin surface in Yamamoto’s study and by extraction from cyanoacrylate-stripped stratum corneum by Imokawa et al. The methodological differences used may have generated these varying results. Decreases in (1) acylceramide and α-hydroxy acylsphingosine (AS); and (2) the ratio of bulk ceramide to cholesterol content in AD stratum corneum lipid (samples are collected by cyanoacrylate stripping); and a correlation between decreases in AS levels and increases in TEWL were demonstrated [88]. However, another study showed that NP, but not AS, are decreased in AD stratum corneum [89]. Since these earlier studies (including [88], but not [89]) did not separately quantitate AS and NP by thin-layer chromatography, such analytical limitations likely caused these differing results. Moreover, HPLC-mass spectrometry analysis characterizes an alteration of the amide-linked fatty acid profile in ceramides in addition to confirming previous findings of decreases in acylceramide, NP, and N-non-hydroxy acyl 6-hydroxy sphingosine (NH) in atopic dermatitis stratum corneum [89]. Conversely, NS and AS are increased in the stratum corneum of atopic dermatitis subjects [89].
In skin lesions, the content of saturated free fatty acids with very long carbon chains (≥C24) is significantly reduced, whereas short-chain free fatty acid (C16:0 and C18:0) levels are increased [11, 90]. In parallel to changes in fatty acid composition of ceramides in AD stratum corneum, free fatty acid composition also changes (i.e., increases in shorter and longer fatty acids and also increases in monounsaturated fatty acid species) [22]. Although loss-of-function mutations in the filaggrin gene (FLG) are a risk factor for atopic dermatitis and ichthyosis vulgaris [91, 92], there is no correlation between filaggrin mutations and a change in a ceramide’s fatty acid chain length [22].
Ultrastructural analysis shows extrusion of lamellar bodies that is required for formation of lamellar membranes in the extracellular spaces of stratum corneum [93]. X-ray analysis and FTIR verified alterations in the formation of well compacted lamellar membrane structures in atopic dermatitis (i.e., increases in less compacted hexagonal lateral packing and decreases in orthorhombic lateral packing [compacted]) [90, 94, 95].
How is the ceramide profile changed in atopic dermatitis skin? (See also review article [96]). Inflammatory cytokines, IL-4, interferon (IFN)-γ and TNF-α decreased the level of acidic sphingomyelinase and ß-glucocerebrosidases at mRNA levels and conversely increased acid-ceramidase in cultured human keratinocytes [97]. Another study shows that IFN-γ decreased mRNA expression of elongase in long-chain fatty acids (ELOVLs) and in ceramide synthases in cultured human keratinocytes and epidermal sheets [98]. Ceramide production is also decreased by Th2 cytokines, IL-4 and IL-6, accompanied by decreases in mRNA expression of serine-palmitoyl transferase-2, acid sphingomyelinase, and ß-glucocerebrosidase in reconstructed human epidermal equivalents [99]. Moreover, sphingomyelin deacylase activity in lesional as well as nonlesional skin was reduced [100].
However, as described below, changes in inflammatory cytokine levels occur in not only atopic dermatitis, but also other inflammatory skin diseases. Inflammatory cytokines contribute to changing the ceramide profile in atopic dermatitis, but this abnormality is not specific to atopic dermatitis.
Colonization of Staphylococcus aureus is often found in atopic dermatitis skin, while Pseudomonas aeruginosa (P. aeruginosa) is also found in AD patient skin. Neutral ceramidase is secreted from P. aeruginosa, increasing S1P production, which then increases inflammatory cytokines IL-8 and TNF-α through NF-κB [101].
5.2 Netherton Syndrome
Netherton syndrome is caused by mutations in the serine protease inhibitor Kazal-type 5 gene that encodes the lympho-epithelial Kazal-type–related inhibitor, and patients with this disease have a severely compromised epidermal permeability barrier. Lipid analysis of the stratum corneum in Netherton syndrome patients reveals that total amounts of both free fatty acids and ceramides are decreased in the stratum corneum of patients, while longer chain free fatty acids and monounsaturated free fatty acids are decreased and increased, respectively [102]. This study further showed that levels of short-chain ceramides and longer-chain ceramides are increased and decreased, respectively; and the ratio of acylceramide to acylglucosylceramide is increased [102]. As expected, lamellar membrane organization is changed [102]. Moreover, a recent study characterized decreases in ß-glucocerebrosidase, which converts glucosylceramide to ceramide, and increases in acidic sphingomyelinase, which converts sphingomyelin to ceramide [103]. This study suggests that insufficient conversion from glucosylceramide to ceramide increases hydrophilic glucosylceramide, which affects the formation of lamellar membrane structures in the stratum corneum in Netherton syndrome patients [103].
5.3 Psoriasis
Levels of ceramide-containing phytosphingosine (NP and N-2-OH acyl-4-OH dihydrosphingosine [AP]) and EOS are lower in the scale of lesional areas of psoriatic skin (male subjects) than in normal human stratum, while NS and AS are higher in psoriatic skin compared with normal (derived from abdomen skin samples of six normal subjects of both sexes) [12]. Another recent study demonstrated that (1) the ratios of NP/NS, NH/NS, NP/AS, NH/NS, NDS/AS, ceramide AH/AS and ceramide EOP/AS are lower in psoriatic skin; and (2) these ratios are lower in lesional skin compared with non-lesional skin. However, (3) ceramide content of these species are not decreased in psoriatic skin compared with normal skin [104]. Since both studies reported no information about any prior drug treatments (topical/systemic) when skin samples were obtained, it is difficult to assess the influence of drugs on ceramide metabolism. Bulk ceramide synthesis was assessed in psoriatic epidermis using radio-labeled serine incorporation. In this study, psoriasis patients were not treated (either systemically or topically) for at least 1 month prior to collecting their epidermal samples. Reduction of ceramide synthesis in the lesional epidermis compared with non-lesional epidermis (epidermis was obtained by biopsies) was correlated with psoriasis area and severity index (PASI) score [105]. Another study examined ceramide species (except acylceramide) in the stratum corneum obtained from tape stripping and found that (1) the ratio of ceramides carrying long-chain fatty acids to total ceramide is lower in psoriasis patients than in healthy controls; (2) an in vitro study using a reconstructed skin model further showed that IFN-γ decreases the mRNA expression of ELOVL 1, 4 and 7, and of ceramide synthase (CerS) 3, 4 and 6 enzymes; and (3) ELOVL4 and CerS 3 expression was regulated by a transcriptional factor STAT1 [98]. The last study [98] partially characterized the mechanism responsible for changes in ceramide profile in psoriatic skin.
What we do not know Increases in NS occur in AD, Netherton syndrome, and psoriatic skin. Because NS is the dominant ceramide species in both undifferentiated and differentiated keratinocytes, and because heterogeneous species of ceramide and ceramide containing ultra-long-chain fatty acids are synthesized in a late stage of differentiated keratinocytes; and because bulk ceramide production is increased in late stages of differentiated keratinocytes, abnormal proliferation and differentiation change ceramide profiles in atopic dermatitis, psoriatic, and other inflammatory epidermis. Hence, it is unknown whether the changing ceramide profiles are unique features of atopic dermatitis and psoriasis. Nevertheless, normalization of ceramide levels in epidermis should be used as a therapeutic strategy to improve barrier integrity.
5.4 Autosomal Recessive Congenital Ichthyoses (ARCI)
Enzymes coded by CYP4F22, SLC27A4, CERS3, PNPLA1, and NIPAL4 are responsible for steps required for an essential ceramide species (acylceramide) to form a competent epidermal permeability barrier (Fig. 4). Mutation of these genes causing acylceramide deficiency is a pathogenesis of some autosomal recessive congenital ichthyoses (ARCI). ALOX12B and ALOXE3 are involved in the generation of the vital cornified envelope-bound ω-hydroxy ceramide. In addition, mutation of the triacylglyceride lipase activator coded by ABHD5 causes an ARCI, Chanarin-Dorfman syndrome (Note: the triacylglyceride lipase that provides linoleic acid from triacylglyceride in epidermis is unidentified) [31]. Fatty acids up to carbon chain length (16) are elongated by ELOVLs. Ultra-long-chain fatty acids (C-26-C34) synthesized by ELOVL4 are used for acylceramide synthesis [30]. The pathogenesis of the autosomal dominant disease Stargardt Macular Dystrophy involves an ELOVL4 mutation. In rare cases, patients with mutations on both alleles of ELOVL4 demonstrate ichthyosis symptoms [106].
5.5 Cutaneous Cancer
Alterations in sphingolipid metabolism are reported in cancers, including melanoma and squamous cell carcinoma. Increases in acidic ceramidase and sphingosine kinase 1 are shown in head and neck squamous cell carcinoma skin [107], and changes in sphingolipid levels contribute to tumorigenesis [108].
5.6 Other Skin Diseases
Reduction in ceramide levels has been reported in the skin of patients (who carry mutations in 3-ketodihydrosphingosine reductase [KDSR], which is required for sphingoid base synthesis) with inherited recessive forms of progressive symmetric erythrokeratoderma [109, 110]. Although alkaline ceramidase-null mice show hair loss through reduction of hair follicle stem cells and stemness, the importance of this ceramidase isoform in human hair follicles has not been demonstrated [111].
6 Topical Application of Ceramides in the Treatment of Skin Disease
Several molecular species of ceramide and structural mimetic pseudoceramides (pseudoceramides have different physical and physiological properties compared with natural ceramides) have been formulated in topical skin care products and also in medicine to treat xerosis, psoriasis, and atopic dermatitis [112, 113]. Topically applied ceramides could improve barrier function in three different ways: (1) ceramides can form liquid crystalline structures, lamellar liquid crystalline, and gel structures with other chemicals formulated in the agent, forming a permeability barrier on the skin surface [114]; (2) ceramides can be incorporated into lamellar bilayer structures to enhance barrier integrity in the stratum corneum [115], and (3) topically applied ceramides can penetrate into nucleated layers of epidermis [116, 117]. The absorption rate is dependent upon a barrier’s integrity and formulation. Absorbed ceramides are hydrolyzed to a sphingoid base and fatty acid, which are utilized in endogenous ceramide synthesis. In (1) above (stable liquid crystalline structures, lamellar liquid crystalline, and gel structures), any ceramide species and structural mimetic pseudoceramides can be used for products, as long as the formulation makes stable liquid crystal structures on the skin surface. However, in (2) above (contribution to lamellar bilayer structure formation in the stratum corneum), because changes in ceramide species cause skin diseases, ceramide species used for topical agents must be considered carefully (i.e., NS and short chain length of ceramide are increased in atopic dermatitis skin). Acylceramide is a ceramide species essential in forming a competent barrier. But as demonstrated in recent studies, certain amounts of acylceramide can make more stable bilayer structures [36]. In addition, racemates of N-nonhydroxyacyldihydrosphingosine (NDS), which are mixtures of endogenous (2S,3R [D-erythro]dihydrosphingosine) and non-endogenous [(2S,3S and 2R,3R, and 2R3S [L-threo] dihydrosphingosine] ceramide, are sold by cosmetic companies. Lamellar membrane structure packing is known to be attenuated in the lipid membranes containing L-threo ceramide, for example [118]. Thus, adequate selection of ceramide molecular species is required for preparation of an effective topical formula.
Oral glycosylceramide and sphingomyelin from plants and milk are used (as nutraceuticals) to improve epidermal permeability barrier (see review articles [119,120,121]). In particular, these nutraceuticals have become popular in Japan. They are categorized into two types: (1) specified health use and (2) foods with function claim in Japan. These claims are required for approval by Japan’s Consumer Affairs Agency (https://www.caa.go.jp/en/). Some oral sphingolipids are hydrolyzed by digestive enzymes and enzymes derived from intestinal microbes, absorbed through the intestinal membrane, and are transferred to the liver where they are further metabolized and circulated to peripheral tissues, including skin. During circulation, some sphingolipids are incorporated into lipoproteins, and sphingolipids may affect gut immunity. However, exactly how oral sphingolipids improve barrier function is still not completely understood [119].
7 Conclusion
Ceramides serve as structural and modulator lipids that maintain cellular functions (Fig. 7). In addition, unique to terrestrial mammals, ceramides are essential constituents in the formation of an epidermal permeability barrier in the stratum corneum, the outermost layer of skin, which serves as the ‘first line of defense’ and the ‘last line for preserving life’. Not only bulk, but also heterogenous species of ceramide are required for vital barrier generation. Alterations of ceramide molecular species occur in skin diseases that are associated with barrier abnormalities. Moreover, ceramide and its metabolites can modulate keratinocyte proliferation and differentiation, as well as modulate immunity in skin. Therefore, pharmacological manipulation of ceramide metabolism at an inter- and intra-cellular level can be a strategy to prevent and to treat skin diseases.
References
Thudicum JLW. A treatise on the chemical constitution of brain. London: Bailliere, Tindall and Cox; 1884.
Uchida Y. Ceramide signaling in mammalian epidermis. Biochim Biophys Acta. 2014;1841:453–62.
Summers SA, Chaurasia B, Holland WL. Metabolic messengers: ceramides. Nat Metab. 2019;1:1051–8.
Albeituni S, Stiban J. Roles of ceramides and other sphingolipids in immune cell function and inflammation. Adv Exp Med Biol. 2019;1161:169–91.
Colombini M. Ceramide channels. Adv Exp Med Biol. 2019;1159:33–48.
Presa N, Gomez-Larrauri A, Dominguez-Herrera A, Trueba M, Gomez-Munoz A. Novel signaling aspects of ceramide 1-phosphate. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158630.
Berwick ML, Dudley BA, Maus K, Chalfant CE. The Role of ceramide 1-phosphate in inflammation, cellular proliferation, and wound healing. Adv Exp Med Biol. 2019;1159:65–77.
Schmitt T, Neubert RHH. State of the art in stratum corneum research: the biophysical properties of ceramides. Chem Phys Lipids. 2018;216:91–103.
Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008;51:77–87.
Uchida Y, Hamanaka S. Stratum corneum ceramides: function, origins, and therapeutic applications. In: Elias PM, Feingold KR, editors. Skin barrier. New York: Taylor & Francis; 2006. p. 43–65.
van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta. 2014;1841:295–313.
Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R. Ceramide composition of the psoriatic scale. Biochim Biophys Acta. 1993;1182:147–51.
Masukawa Y, Narita H, Shimizu E, Kondo N, Sugai Y, Oba T, et al. Characterization of overall ceramide species in human stratum corneum. J Lipid Res. 2008;49:1466–76.
Elias PM. Skin barrier function. Curr Allergy Asthma Rep. 2008;8:299–305.
Thiele JJ. Oxidative targets in the stratum corneum. A new basis for antioxidative strategies. Skin Pharmacol Appl Skin Physiol. 2001;14(Suppl 1):87–91.
Mildner M, Jin J, Eckhart L, Kezic S, Gruber F, Barresi C, et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol. 2010;130:2286–94.
Bow JR, Sonoki Y, Uchiyama M, Shimizu E, Tanaka K, Dauskardt RH. Lipid loss increases stratum corneum stress and drying rates. Skin Pharmacol Physiol. 2020;33:180–8.
Afshar M, Gallo RL. Innate immune defense system of the skin. Vet Dermatol. 2013;24:32-8.e8-9.
Uchida Y, Park K. Stratum corneum. In: Kabashima K, editor. Immunology of the skin. Tokyo: Springer; 2016. p. 15–30.
Uchida Y, Park K. Anti-microbial peptides in skin barrier functions. J Skin Barrier Res. 2013;15:1–8.
Yokouchi M, Kubo A. Maintenance of tight junction barrier integrity in cell turnover and skin diseases. Exp Dermatol. 2018;27:876–83.
van Smeden J, Janssens M, Kaye EC, Caspers PJ, Lavrijsen AP, Vreeken RJ, et al. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol. 2014;23:45–52.
Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841:280–94.
Hamanaka S, Nakazawa S, Yamanaka M, Uchida Y, Otsuka F. Glucosylceramide accumulates preferentially in lamellar bodies in differentiated keratinocytes. Br J Dermatol. 2005;152:426–34.
Yamamoto H, Hattori M, Chamulitrat W, Ohno Y, Kihara A. Skin permeability barrier formation by the ichthyosis-causative gene FATP4 through formation of the barrier lipid omega-O-acylceramide. Proc Natl Acad Sci USA. 2020;117:2914–22.
Ohno Y, Kamiyama N, Nakamichi S, Kihara A. PNPLA1 is a transacylase essential for the generation of the skin barrier lipid omega-O-acylceramide. Nat Commun. 2017;8:14610.
Ohno Y, Nakamichi S, Ohkuni A, Kamiyama N, Naoe A, Tsujimura H, et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc Natl Acad Sci USA. 2015;112:7707–12.
Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci USA. 2010;107:18439–44.
Uchida Y, Hama H, Alderson NL, Douangpanya S, Wang Y, Crumrine DA, et al. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J Biol Chem. 2007;282:13211–9.
Vasireddy V, Uchida Y, Salem N Jr, Kim SY, Mandal MN, Reddy GB, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (>=C28) and the unique {omega}-O-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16:471–82.
Uchida Y, Cho Y, Moradian S, Kim J, Nakajima K, Crumrine D, et al. Neutral lipid storage leads to acylceramide deficiency, likely contributing to the pathogenesis of dorfman-chanarin syndrome. J Invest Dermatol. 2010;130:2497–9.
Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet. 2012;21:586–608.
Hansen HS, Jensen B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and alpha-linolenate. Biochim Biophys Acta. 1985;834:357–63.
McIntosh TJ, Stewart ME, Downing DT. X-ray diffraction analysis of isolated skin lipids: reconstitution of intercellular lipid domains. Biochemistry. 1996;35:3649–53.
Bouwstra JA, Gooris GS, Dubbelaar FE, Weerheim AM, Ijzerman AP, Ponec M. Role of ceramide 1 in the molecular organization of the stratum corneum lipids. J Lipid Res. 1998;39:186–96.
Opalka L, Kovacik A, Pullmannova P, Maixner J, Vavrova K. Effects of omega-O-acylceramide structures and concentrations in healthy and diseased skin barrier lipid membrane models. J Lipid Res. 2020;61:219–28.
de Jager M, Gooris G, Ponec M, Bouwstra J. Acylceramide head group architecture affects lipid organization in synthetic ceramide mixtures. J Invest Dermatol. 2004;123:911–6.
Schmitt T, Neubert RHH. State of the art in stratum corneum research. Part II: hypothetical stratum corneum lipid matrix models. Skin Pharmacol Physiol. 2020;33:213–30.
Nakazawa H, Imai T, Hatta I, Sakai S, Inoue S, Kato S. Low-flux electron diffraction study for the intercellular lipid organization on a human corneocyte. Biochim Biophys Acta. 2013;1828:1424–31.
Schmitt T, Gupta R, Lange S, Sonnenberger S, Dobner B, Hauss T, et al. Impact of the ceramide subspecies on the nanostructure of stratum corneum lipids using neutron scattering and molecular dynamics simulations. Part I: impact of CER[NS]. Chem Phys Lipids. 2018;214:58–68.
Schmitt T, Lange S, Sonnenberger S, Dobner B, Deme B, Langner A, et al. The long periodicity phase (LPP) controversy part I: The influence of a natural-like ratio of the CER[EOS] analogue [EOS]-br in a CER[NP]/[AP] based stratum corneum modelling system: a neutron diffraction study. Biochim Biophys Acta Biomembr. 2019;1861:306–15.
Kawana M, Miyamoto M, Ohno Y, Kihara A. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS. J Lipid Res. 2020;61:884–95.
Uchida Y, Iwamori M, Nagai Y. Distinct differences in lipid composition between epidermis and dermis from footpad and dorsal skin of guinea pigs. Jpn J Exp Med. 1988;58:153–61.
Wertz PW, Downing DT. Ceramides of pig epidermis: structure determination. J Lipid Res. 1983;24:759–65.
Angelbeck-Schulze M, Stahl J, Brodesser S, Rohn K, Naim H, Hewicker-Trautwein M, et al. Comparison of three different sampling methods for canine skin lipids. Vet Dermatol. 2013;24:233-e51.
Akiyama M. Corneocyte lipid envelope (CLE), the key structure for skin barrier function and ichthyosis pathogenesis. J Dermatol Sci. 2017;88:3–9.
Elias PM, Gruber R, Crumrine D, Menon G, Williams ML, Wakefield JS, et al. Formation and functions of the corneocyte lipid envelope (CLE). Biochim Biophys Acta. 2014;1841:314–8.
Taniguchi M, Okazaki T. Ceramide/sphingomyelin rheostat regulated by sphingomyelin synthases and chronic diseases in murine models. J Lipid Atheroscler. 2020;9:380–405.
Summers SA. Ceramides: nutrient signals that drive hepatosteatosis. J Lipid Atheroscler. 2020;9:50–65.
Kim S, Hong I, Hwang JS, Choi JK, Rho HS, Kim DH, et al. Phytosphingosine stimulates the differentiation of human keratinocytes and inhibits TPA-induced inflammatory epidermal hyperplasia in hairless mouse skin. Mol Med. 2006;12:17–24.
Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, et al. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol. 2013;33:752–62.
Shin KO, Kim KP, Cho Y, Kang MK, Kang YH, Lee YM, et al. Both sphingosine kinase 1 and 2 coordinately regulate cathelicidin antimicrobial peptide production during keratinocyte differentiation. J Invest Dermatol. 2019;139:492–4.
Uchida Y, Iwamori M, Nagai Y. Activation of keratinization of keratinocytes from fetal rat skin with N-(O-linoleoyl) omega-hydroxy fatty acyl sphingosyl glucose (lipokeratinogenoside) as a marker of epidermis. Biochem Biophys Res Commun. 1990;170:162–8.
Uchida Y, Ogawa T, Iwamori M, Nagai Y. Enhancement of keratin synthesis induced by lipokeratinogenoside, N-(O-linoleoyl)-omega-hydroxy fatty acyl sphingosyl glucose, in association with alteration of the intracellular Ca(2+)-content and protein kinase in cultured keratinocytes (FRSK). J Biochem. 1991;109:462–5.
Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–56.
Clarke CJ, Hannun YA. Neutral sphingomyelinases and nSMase2: bridging the gaps. Biochim Biophys Acta. 2006;1758:1893–901.
Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7.
Taniguchi M, Okazaki T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim Biophys Acta. 2014;1841:692–703.
D’Angelo G, Moorthi S, Luberto C. Role and function of sphingomyelin biosynthesis in the development of cancer. Adv Cancer Res. 2018;140:61–96.
Dobrowsky RT, Hannun YA. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992;267:5048–51.
Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–23.
Heinrich M, Wickel M, Winoto-Morbach S, Schneider-Brachert W, Weber T, Brunner J, et al. Ceramide as an activator lipid of cathepsin D. Adv Exp Med Biol. 2000;477:305–15.
Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, et al. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–95.
Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–8.
Jiang YJ, Kim P, Uchida Y, Elias PM, Bikle DD, Grunfeld C, et al. Ceramides stimulate caspase-14 expression in human keratinocytes. Exp Dermatol. 2013;22:113–8.
Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233–41.
Sigruener A, Tarabin V, Paragh G, Liebisch G, Koehler T, Farwick M, et al. Effects of sphingoid bases on the sphingolipidome in early keratinocyte differentiation. Exp Dermatol. 2013;22:677–9.
Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–8.
Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–20.
Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55:1596–608.
Wang Z, Zheng Y, Wang F, Zhong J, Zhao T, Xie Q, et al. Mfsd2a and Spns2 are essential for sphingosine-1-phosphate transport in the formation and maintenance of the blood-brain barrier. Sci Adv. 2020;6:8627.
Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schafer-Korting M, et al. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol. 2001;117:1241–9.
Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–7.
Park K, Ikushiro H, Seo HS, Shin KO, Kim YI, Kim JY, et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc Natl Acad Sci USA. 2016;113:E1334-42.
Clarke CJ, Wu BX, Hannun YA. The neutral sphingomyelinase family: identifying biochemical connections. Adv Enzyme Regul. 2011;51:51–8.
Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531:38–46.
Collenburg L, Beyersdorf N, Wiese T, Arenz C, Saied EM, Becker-Flegler KA, et al. The activity of the neutral sphingomyelinase is important in T cell recruitment and directional migration. Front Immunol. 2017;8:1007.
Bai A, Kokkotou E, Zheng Y, Robson SC. Role of acid sphingomyelinase bioactivity in human CD4+ T-cell activation and immune responses. Cell Death Dis. 2015;6:e1828.
De Lira MN, Raman SJ, Schulze A, Schneider-Schaulies S, Avota E. Neutral sphingomyelinase-2 (NSM 2) controls T cell metabolic homeostasis and reprogramming during activation. Front Mol Biosci. 2020;7:217.
Reines I, Kietzmann M, Mischke R, Tschernig T, Luth A, Kleuser B, et al. Topical application of sphingosine-1-phosphate and FTY720 attenuate allergic contact dermatitis reaction through inhibition of dendritic cell migration. J Invest Dermatol. 2009;129:1954–62.
Japtok L, Schaper K, Baumer W, Radeke HH, Jeong SK, Kleuser B. Sphingosine 1-phosphate modulates antigen capture by murine langerhans cells via the S1P2 receptor subtype. PLoS ONE. 2012;7:e49427.
Kim YI, Park K, Kim JY, Seo HS, Shin KO, Lee YM, et al. An endoplasmic reticulum stress-initiated sphingolipid metabolite, ceramide-1-phosphate, regulates epithelial innate immunity by stimulating beta-defensin production. Mol Cell Biol. 2014;34:4368–78.
Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011;286:34121–30.
Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101.
Melnik B, Hollmann J, Plewig G. Decreased stratum corneum ceramides in atopic individuals—a pathobiochemical factor in xerosis? Br J Dermatol. 1988;119:547–9.
Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219–23.
Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–6.
Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30.
Boer DEC, van Smeden J, Al-Khakany H, Melnik E, van Dijk R, Absalah S, et al. Skin of atopic dermatitis patients shows disturbed beta-glucocerebrosidase and acid sphingomyelinase activity that relates to changes in stratum corneum lipid composition. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158673.
Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53:2755–66.
Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–78.
McLean WH. Filaggrin failure—from ichthyosis vulgaris to atopic eczema and beyond. Br J Dermatol. 2016;175(Suppl 2):4–7.
Fartasch M, Bassukas ID, Diepgen TL. Disturbed extruding mechanism of lamellar bodies in dry non-eczematous skin of atopics. Br J Dermatol. 1992;127:221–7.
Chermprapai S, Broere F, Gooris G, Schlotter YM, Rutten V, Bouwstra JA. Altered lipid properties of the stratum corneum in canine atopic dermatitis. Biochim Biophys Acta Biomembr. 2018;1860:526–33.
Pilgram GS, Vissers DC, van der Meulen H, Pavel S, Lavrijsen SP, Bouwstra JA, et al. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol. 2001;117:710–7.
Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. J Clin Invest. 2019;129:1463–74.
Hatano Y, Terashi H, Arakawa S, Katagiri K. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol. 2005;124:786–92.
Tawada C, Kanoh H, Nakamura M, Mizutani Y, Fujisawa T, Banno Y, et al. Interferon-gamma decreases ceramides with long-chain fatty acids: possible involvement in atopic dermatitis and psoriasis. J Invest Dermatol. 2014;134:712–8.
Sawada E, Yoshida N, Sugiura A, Imokawa G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: an implication for the disrupted barrier mechanism in atopic dermatitis. J Dermatol Sci. 2012;68:25–35.
Hara J, Higuchi K, Okamoto R, Kawashima M, Imokawa G. High-expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. J Invest Dermatol. 2000;115:406–13.
Oizumi A, Nakayama H, Okino N, Iwahara C, Kina K, Matsumoto R, et al. Pseudomonas-derived ceramidase induces production of inflammatory mediators from human keratinocytes via sphingosine-1-phosphate. PLoS ONE. 2014;9:89402.
van Smeden J, Janssens M, Boiten WA, van Drongelen V, Furio L, Vreeken RJ, et al. Intercellular skin barrier lipid composition and organization in netherton syndrome patients. J Invest Dermatol. 2014;134:1238–45.
van Smeden J, Al-Khakany H, Wang Y, Visscher D, Stephens N, Absalah S, et al. Skin barrier lipid enzyme activity in Netherton patients is associated with protease activity and ceramide abnormalities. J Lipid Res. 2020;61:859–69.
Yokose U, Ishikawa J, Morokuma Y, Naoe A, Inoue Y, Yasuda Y, et al. The ceramide [NP]/[NS] ratio in the stratum corneum is a potential marker for skin properties and epidermal differentiation. BMC Dermatol. 2020;20:6.
Cho Y, Lew BL, Seong K, Kim NI. An inverse relationship between ceramide synthesis and clinical severity in patients with psoriasis. J Korean Med Sci. 2004;19:859–63.
Aldahmesh MA, Mohamed JY, Alkuraya HS, Verma IC, Puri RD, Alaiya AA, et al. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am J Hum Genet. 2011;89:745–50.
Elojeimy S, Liu X, McKillop JC, El-Zawahry AM, Holman DH, Cheng JY, et al. Role of acid ceramidase in resistance to FasL: therapeutic approaches based on acid ceramidase inhibitors and FasL gene therapy. Mol Ther. 2007;15:1259–63.
Carrie L, Virazels M, Dufau C, Montfort A, Levade T, Segui B, et al. New insights into the role of sphingolipid metabolism in melanoma. Cells. 2020;9:1967.
Takeichi T, Torrelo A, Lee JYW, Ohno Y, Lozano ML, Kihara A, et al. Biallelic mutations in KDSR disrupt ceramide synthesis and result in a spectrum of keratinization disorders associated with thrombocytopenia. J Invest Dermatol. 2017;137:2344–53.
Boyden LM, Vincent NG, Zhou J, Hu R, Craiglow BG, Bayliss SJ, et al. Mutations in KDSR cause recessive progressive symmetric erythrokeratoderma. Am J Hum Genet. 2017;100:978–84.
Lin CL, Xu R, Yi JK, Li F, Chen J, Jones EC, et al. Alkaline ceramidase 1 protects mice from premature hair loss by maintaining the homeostasis of hair follicle stem cells. Stem Cell Reports. 2017;9:1488–500.
Sugarman JL, Parish LC. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol. 2009;8:1106–11.
Novotny J, Hrabalek A, Vavrova K. Synthesis and structure-activity relationships of skin ceramides. Curr Med Chem. 2010;17:2301–24.
Kaneko T, Tanaka T, Nagase M. Agent for protecting skin and hair moisture. US Patent 635532. 2002.
Berkers T, Visscher D, Gooris GS, Bouwstra JA. Topically applied ceramides interact with the stratum corneum lipid matrix in compromised ex vivo skin. Pharm Res. 2018;35:48.
Sahle FF, Metz H, Wohlrab J, Neubert RH. Polyglycerol fatty acid ester surfactant-based microemulsions for targeted delivery of ceramide AP into the stratum corneum: formulation, characterisation, in vitro release and penetration investigation. Eur J Pharm Biopharm. 2012;82:139–50.
Tessema EN, Gebre-Mariam T, Frolov A, Wohlrab J, Neubert RHH. Development and validation of LC/APCI-MS method for the quantification of oat ceramides in skin permeation studies. Anal Bioanal Chem. 2018;410:4775–85.
Kovacik A, Pullmannova P, Maixner J, Vavrova K. Effects of ceramide and dihydroceramide stereochemistry at c-3 on the phase behavior and permeability of skin lipid membranes. Langmuir. 2018;34:521–9.
Tessema EN, Gebre-Mariam T, Neubert RHH, Wohlrab J. Potential applications of phyto-derived ceramides in improving epidermal barrier function. Skin Pharmacol Physiol. 2017;30:115–38.
Morifuji M. The beneficial role of functional food components in mitigating ultraviolet-induced skin damage. Exp Dermatol. 2019;28(Suppl 1):28–31.
Vollmer DL, West VA, Lephart ED. Enhancing Skin Health: By Oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–49.
Merrill AH Jr. Characterization of serine palmitoyltransferase activity in chinese hamster ovary cells. Biochim Biophys Acta. 1983;754:284–91.
Hornemann T, Richard S, Rutti MF, Wei Y, von Eckardstein A. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J Biol Chem. 2006;281:37275–81.
Beeler T, Bacikova D, Gable K, Hopkins L, Johnson C, Slife H, et al. The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3-ketosphinganine reductase is identified in a screen for temperature-sensitive suppressors of the Ca2+-sensitive csg2Delta mutant. J Biol Chem. 1998;273:30688–94.
Mizutani Y, Kihara A, Igarashi Y. Identification of the human sphingolipid C4-hydroxylase, hDES2, and its up-regulation during keratinocyte differentiation. FEBS lett. 2004;563:93–7.
Kihara A, Igarashi Y. FVT-1 is a mammalian 3-ketodihydrosphingosine reductase with an active site that faces the cytosolic side of the endoplasmic reticulum membrane. J Biol Chem. 2004;279:49243–50.
Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life. 2010;62:347–56.
Houben E, Holleran WM, Yaginuma T, Mao C, Obeid LM, Rogiers V, et al. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J Lipid Res. 2006;47:1063–70.
Sassa T, Ohno Y, Suzuki S, Nomura T, Nishioka C, Kashiwagi T, et al. Impaired epidermal permeability barrier in mice lacking elovl1, the gene responsible for very-long-chain fatty acid production. Mol Cell Biol. 2013;33:2787–96.
Lin MH, Hsu FF, Crumrine D, Meyer J, Elias PM, Miner JH. Fatty acid transport protein 4 is required for incorporation of saturated ultralong-chain fatty acids into epidermal ceramides and monoacylglycerols. Sci Rep. 2019;9:13254.
Uchida Y, Houben E, Park K, Douangpanya S, Lee YM, Wu BX, et al. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol. 2010;130:2472–80.
Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58). J Biol Chem. 2010;285:7300–11.
Hirabayashi T, Anjo T, Kaneko A, Senoo Y, Shibata A, Takama H, et al. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat Commun. 2017;8:14609.
Zhou J, Saba JD. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem Biophys Res Commun. 1998;242:502–7.
Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, et al. Ceramide kinase, a novel lipid kinase Molecular cloning and functional characterization. J Biol Chem. 2002;277:23294–300.
Acknowledgements
We thank Ms Joan Wakefield for superb editorial assistance (Northern California Institute for Research and Education, San Francisco Veterans Affairs Health Care System, and the University of California San Francisco). We acknowledge the support of the Medical Research Services of the Veterans Affairs Medical Center, San Francisco.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by the National Institutes of Health Grants R01 AR062025 (to YU), and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B7050504), the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program (reference number P0014701) for The Industries of Economic Cooperation Region and Main Research Program (E0210600-01) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT (to KP). This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
Authors state no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Both YU and KP researched, wrote and re-wrote this manuscript.
Rights and permissions
About this article
Cite this article
Uchida, Y., Park, K. Ceramides in Skin Health and Disease: An Update. Am J Clin Dermatol 22, 853–866 (2021). https://doi.org/10.1007/s40257-021-00619-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-021-00619-2