Abstract
Topical photodynamic therapy (PDT) using daylight is effective in the treatment of actinic keratoses (AKs), offering the potential for treatment of large fields such as full face and balding scalp, but with minimal therapy-associated pain. Comparison with conventional PDT indicates similar efficacy for thin and moderate-thickness AKs, but with significantly less discomfort/pain, driving a patient preference for daylight-mediated PDT (DL-PDT) compared with conventional PDT using high-intensity office/hospital-based light sources. Treatment protocol involves the application of a photosensitizing agent without occlusion and subsequent exposure to ambient daylight within 30 min, with patients exposed to daylight for 1.5–2.0 h. Pivotal randomized controlled trials in Europe and Australia have confirmed the efficacy of methyl aminolevulinic acid (MAL) DL-PDT in comparison with conventional MAL-PDT for mild and moderate-thickness lesions on the face and scalp. Initial clearance rates of 70–89% are reported. DL-PDT using a nanoemulsion aminolevulinic acid (ALA) has recently been shown to be at least as effective as MAL DL-PDT in treating mild and moderate-thickness AKs. DL-PDT may offer a better-tolerated method for treating patients with extensive AK disease. There is emerging literature on the potential for field PDT to reduce the number of new AKs developing, potentially preventing/slowing skin cancer development. Conventional PDT remains established as a therapy for Bowen’s disease (squamous cell carcinoma in situ), superficial and certain thin basal cell carcinomas (BCCs), and AKs. The evidence for the use of DL-PDT beyond AK is limited, although has been reported in actinic cheilitis, superficial BCC, and acne and cutaneous leishmaniasis. There is emerging interest in combination therapy for AK, using one or more field therapies such as DL-PDT as an option to complement with localized treatment for residual lesions. We review current recommendations and consider the appropriate place for DL-PDT in our treatment armamentarium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Daylight photodynamic therapy (DL-PDT) is an effective alternative to conventional PDT, with equivalent efficacy for thin and moderate-thickness actinic keratoses (AKs) on the face and scalp. |
Tolerance of DL-PDT is high, with minimal or no treatment-associated pain. |
DL-PDT is especially suitable for patients with multiple clustered AKs and field disease, and has additional potential in delaying/preventing new lesion development. |
1 Introduction
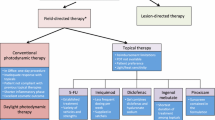
Photodynamic therapy (PDT) involves the activation of a photosensitizing drug by visible light to produce activated oxygen species within target cells, resulting in their destruction. Strong evidence is identified in therapy guidelines to indicate that topical PDT is effective for actinic keratoses (AKs), Bowen’s disease (squamous cell carcinoma [SCC] in situ), superficial basal cell carcinomas (BCCs) and thin nodular BCCs [1,2,3,4].
Three photosensitizing agents are licensed and marketed, but availability differs by country: a formulation of 5-aminolevulinic acid (5-ALA; Levulan®, DUSA Pharmaceuticals, Wilmington, MA, USA), for AKs; a nanoemulsion formulation of 5-ALA (Ameluz®, (Biofrontera Pharma, Leverkusen, Germany) for AKs, including field cancerization and superficial and/or nodular BCC; and an esterified formulation, methyl aminolevulinate (MAL; Metvix®/Metvixia®, Galderma, Paris, France), for AKs, Bowen’s disease, and superficial and nodular BCC. Levulan® was approved by the US FDA in 1999 for the treatment of AKs on the face/scalp. Although the label states a 14- to 18-h interval between application and illumination, in practice, 1 h short-contact, full-face therapy has been shown to be as efficacious as longer incubation [5]. Metvixia® (MAL hydrochloride) was approved by the FDA in 2004 for the treatment of thin and moderately thick AKs of the face and scalp by conventional PDT but was withdrawn in 2012 for commercial reasons (FDA label only).
Topical PDT using daylight has more recently been approved with licence extensions for Metvix/Metvixia in Europe, Australia, Canada and Latin America, and in March 2018 the European Medicines Agency approved daylight DT using Ameluz® (nanoemulsion aminolevulinic acid hydrochloride) gel to treat AKs and field cancerizaton
5-ALA is a precursor in the heme biosynthesis pathway of protoporphyrin IX (PpIX), an endogenous photosensitizer not normally present within tissue in therapeutically useful concentrations. Exogenous administration of each 5-ALA/MAL formulation increases the intracellular concentration of PpIX. The rationale for PDT is based on the cytotoxic action of products generated by excited photosensitizers, including singlet oxygen, which is highly reactive in biological systems [6]. For ALA/MAL-PDT, inhibition of mitochondrial enzymes is likely to be the key event in PDT cell death.
The development of energy-efficient LEDs has facilitated the production of large area, yet portable, red-light sources, which have become the most frequently used lights in clinical practice for delivering PDT for all its indications by standard conventional protocol, although a blue-light source is approved for the treatment of AKs by Levulan® 5-ALA [1,2,3]. Light of appropriate wavelength for activation of the photosensitizer is required in the target tissue. 5-ALA-induced photosensitivity has a porphyrin-like spectrum with maximum excitation at 410 nm and additional smaller peaks at 510, 545, 580 and 635 nm. In Europe, most clinical applications of PDT have used red light around 630–635 nm to achieve adequate penetration, although, in the US, blue light that activates the 410 nm peak is commonly used when treating thin/moderate-thickness AKs [1,2,3].
Topical PDT offers the potential of a practical, non-surgical, clinic/office therapy. PDT may prove advantageous where large size, site, or number of lesions limit the efficacy and/or acceptability of conventional therapies [1,2,3,4]. Fluorescence emitted following conversion of absorbed ALA to the endogenous photosensitizer PpIX can be visualized to assist delineation of surface tumour margins or recurrent disease, and offers particular advantages in using conventional PDT where extent of disease is uncertain [7]. However, discomfort/pain is a common adverse effect with conventional red-light PDT, especially where larger lesions/fields are to be treated [2, 8].
Topical PDT using natural daylight is associated with minimal discomfort, and evidence reviewed below indicates it to be as effective as conventional PDT in AK. Patients expose sites for treatment within 30 min of photosensitizer application, without occlusion, to daylight for 2 h [9]. During conventional red-light PDT, PpIX accumulates during the 3-h occlusion, resulting in a high concentration of reactive oxygen species immediately following illumination, the primary cause of significantly greater pain than during DL-PDT, when exposure to daylight commences within 30 min after MAL application, just after initial synthesis of PpIX, permitting constant photobleaching which results in almost no pain [2, 9]. Although all PpIX absorption peaks are within the visible spectrum, 87% of daylight PpIX activation is due to blue light (380–495 nm).
In this review, the protocol for DL-PDT is assessed, results of studies reviewed, and the potential place of DL-PDT in practice is considered.
2 Treatment Protocol
DL-PDT is indicated for patients with grade I or II AKs, or fields of actinic damage on the face and scalp. A chemical sunscreen with a sun protection factor (SPF) ≥ 20 should be used to block UV, hence preventing sunburn during the 2 h of daylight exposure, but products containing physical filters such as zinc or iron oxide or titanium dioxide must not be used [9]. No interaction between photosensitizing agent and sunscreen has been shown [10].
Sunscreen application is recommended 15 min before skin preparation, although lesion identification can be easier if it is left until after preparation. Sunscreen must be applied on all areas that will be exposed to the sun. Scales and crusts can be removed and skin surface roughened to enhance cream penetration using gentle curettage, mildly abrasive pads or microdermabrasion. Keratolytic pretreatment (salicylic acid or urea) is an alternative to curettage, although it can also be associated with increased pain [11]. Laser pretreatment might also be used to enhance cream delivery.
For DL-PDT, MAL or nanoemulsion ALA should be applied as a thin layer to the entire treatment field, without occlusion, and daylight exposure should begin within 30 min. Beyond this time, there is a risk of increased pain on initial light exposure. Two hours of daylight is recommended, with no benefit observed in longer exposure, but erythema may be greater [9]. The patient should remain exposed to full daylight, avoiding dark shade, with similar light intensity during the entire period of exposure. Consideration of patient comfort is important on hot, cloudless days, where shaded light is preferred and will reduce sweating and sunburn. After daylight exposure, the cream should be washed off and the patient should cover the treatment area from sun for the rest of the day to reduce inflammation. The use of a topical corticosteroid can also be considered to reduce inflammation without affecting efficacy [12].
DL-PDT can be performed in all weather conditions, regardless of sun or cloud, but is not usually perfomed in rain, and, for patient comfort, should be undertaken where the temperature is > 10 °C [9]. Several studies have closely observed for the impact of light dose and weather conditions on the efficacy of DL-PDT, with implications considered in a consensus review [9].
Providing a threshold light dose is achieved, there is no association of lesion response with light dose or weather conditions, as demonstrated in a multicentre study in three Nordic countries [13].
Although extensive use was made of electronic dosimeters during clinical studies, both the observation of lack of light dose-response correlation and subsequent meteorological modelling concluded that protocols for delivery of PDT could be simplified as long as each centre considered whether local weather conditions restricted the period of the year during which daylight PDT could be effectively performed.
Based on calculations of a PpIX light dose above 8 J/cm2 and, for patient comfort, maximum temperature of at least 10°C, in a study of six european locations it has been demonstrated that DL-PDT can be performed most of the year, with the exception of the winter months in northern countries, with data covering latitudes 20°N–70°N [14]. Assessment of weather conditions confirmed the feasibility for DL-PDT throughout the year in Central and South America, as well as in Australia [15, 16].
2.1 Efficacy of Daylight Photodynamic Therapy (PDT)
Conventional PDT with licensed formulations of 5-ALA, nanoemulsion 5-ALA and MAL have been widely studied for non-hyperkeratotic AKs of the face and scalp, with typical clearance rates of 81–92% of lesions 3 months after treatment [3, 4]. In a comparison of conventional protocols using red light, PDT using the nanoemulsion gel formulation of ALA achieved patient complete clearance rates of 78%, compared with 64% with MAL-PDT, when the treatments were compared in a study of 600 patients, each with four to eight mild to moderate AKs on the face/scalp, in 26 European centres [17].
Three initial trials of DL-PDT from the same centre cleared 79, 78, and 76% of AKs after a single treatment as the investigators sought to refine the treatment protocol [13, 18, 19]. In the first study, sunlight-mediated PDT was performed in a split-face protocol, where a 3-month clearance rate of 79% was reported, compared with 71% for lesions treated with conventional red-light PDT [18]. In the second study, daylight, but not necessarily sunlight, was used in a home-based setting, and 16 and 8% concentrations of MAL cream were also compared in a split face/scalp design [19]. At 3 months, 78% of lesions overall had cleared, with no difference between photosensitizing agent concentrations but with a reduction in efficacy for grade II AKs (64%) and thick grade III AKs (39%) compared with thin grade I lesions (80%). In the third study, daylight exposure times of 1.5 versus 2.5 h were compared and were shown to be equivalent, with 76% overall response of thin AKs in a multicentre, randomized trial [13]. Reduced efficacy of thicker lesions was demonstrated in a trial, with 3-month clearance rates for types I, II, and III AKs of 76%, 61% and 49%, respectively, after a single treatment of DL-PDT, with considerable variation in response between centres [20].
Two pivotal intraindividual multicentre comparative studies were performed in a total of 231 patients in Australia and Europe, both observing that MAL-PDT using daylight was non-inferior to conventional PDT, with the Australian study reporting lesion clearance rates of mild AK of 89% and 93%, respectively, 12 weeks after one treatment session [21, 22]. The European study observed equivalent responses of 70% and 74%, with both values being lower as this study included patients with mild and moderate-thickness lesions. Daylight PDT was virtually pain free in comparison with conventional PDT, and was as effective, whether performed in sunny or cloudy conditions.
Both high efficacy and patient satisfaction were demonstrated in a further multicentre study conducted over six European countries in 325 patients receiving a single treatment of MAL DL-PDT for face and/or scalp AKs. This study demonstrated efficacy at 3 months was at least much improved in 83.5% of patients, with 45.9% of patients requiring no retreatment. Moreover, the proportion of patients and physicians who were overall satisfied to very satisfied with the MAL DL-PDT treatment was 80.4 and 90.3%, respectively [23].
Reflecting the need for suitable climate, several additional trials have examined the efficacy of DL-PDT in specific countries, including Italy, Switzerland and Brazil. In the study from Italy, where initial equivalent efficacy for AK type I was shown for DL-PDT (87%) and conventional PDT (91%), poorer efficacy was shown for type II (36 vs. 61% by conventional PDT) and type III (25 vs. 46% by conventional PDT) AKs [24].
A retrospective study of DL-PDT in a private practice setting in Switzerland observed clearance of 77% of all lesions, with most patients reporting no pain, along with local skin reactions that settled within 10 days [25]. A strong patient preference for daylight over conventional PDT was expressed, with the one patient who experienced pain having misunderstood instructions and remaining indoors for 2.5 h before sitting out in the sun.
In a study of 14 patients from Sao Paulo, Brazil, DL-PDT using MAL cleared 86% of AKs (grades I and II), with no recurrences over 3 months [26]. Another small study demonstrated the efficacy and tolerability of DL-PDT in a low-latitude city in Brazil, known for its intense sun, with 16/20 patients reporting no discomfort due to the procedure [27].
In a randomized, split-face trial, 13 patients with 177 grade I–III AKs received DL-PDT, with nanoemulsion ALA compared with MAL, with two treatments for grade II and III AKs [28]. Nanoemulsion ALA DL-PDT cleared 85% of AKs, compared with 74% treated with MAL. The per patient half-face analysis showed ALA to have a significantly higher clearance rate for grade I AKs than MAL, but, for thicker grades, clearance was equal. A recent multicentre intraindividual comparison trial compared DL-PDT using nanoemulsion ALA with MAL in 52 patients with three to nine mild to moderate-thickness AKs on the face/scalp [29]. Equivalent efficacy was demonstrated at 3 months, with total lesion clearance rates of 79.8% with Ameluz® and 76.5% with Metvix® formulations.
2.2 Long-Term Efficacy of Daylight-PDT
Data on sustainability of response to DL-PDT are limited beyond 12 weeks, although the multicentre Australian study that treated only thin grade I AKs observed that 96% of lesions responding at week 12 maintained complete response at 6 months [21]. Although similar recurrence rates of 13% and 10% for DL-PDT and conventional PDT, respectively, were observed during follow-up in another study, the 12-month clearance rate overall indicated a significantly high efficacy of conventional PDT versus DL-PDT of 76 and 66%, respectively [30]. Differences in efficacy for this study, also predominantly of grade I AKs, were noted for lesions located on the face (scalp lesions had equivalent clearance/recurrence rates). Despite the better outcome of conventional PDT, almost 70% of AK I lesions treated with DL-PDT were in complete remission at 12 months, with improved tolerability. The superior clearance rate for scalp lesions compared with facial lesions treated with DL-PDT may be due to more direct exposure of bald scalp to daylight.
2.3 Efficacy of Daylight-PDT for Actinic Keratoses (AKs) in Immunosuppressed Patients
Conventional ALA-PDT and MAL-PDT have both been shown to be effective in clearing AKs in organ transplant recipients (OTRs). Although initial clinical response rates appear similar to those in immunocompetent patients, it has been noted that recurrence rates were higher in OTRs during a 48-week follow-up [31]. Reduced efficacy of PDT in OTRs may result from the large number of intraepithelial lesions, more prominent hyperkeratosis, higher proportion of lesions on sites, e.g. dorsum of hands, where response rates are typically poorer, and an altered, secondary local immune response. Data comparing PDT with other therapies in OTRs are limited, although MAL-PDT has been shown to be superior in efficacy to topical 5-fluorouracil, even after 6 months, for the treatment of epidermal dysplasia in OTRs [32].
DL-PDT using ablative fractional laser (AFL) resurfacing pretreatment has been used to treat AKs in OTRs with the aim of overcoming the issues of poorer efficacy of PDT in OTRs and treatment intolerance due to pain in conventional PDT [33]. In this randomized controlled trial (RCT), four areas were randomized in each patient. Three-month complete clearance rates for the patches treated were superior with the pretreatment followed by DL-PDT, with a median clearance rate of 74% compared with comparable complete clearance rates of 46% and 50% for DL-PDT and conventional PDT, respectively, without prior treatment.
3 PDT for AK: Therapy Guideline Recommendations
Guideline recommendations and systematic reviews to inform treatment choice remain largely limited to publications reviewing conventional PDT. A Cochrane Library systematic review searched databases up to March 2011 and identified 83 RCTs that studied the effect of 18 topical treatments for AK [34]. The primary outcome ‘participant complete clearance’ significantly favoured field treatments of 3% diclofenac in 2.5% hyaluronic acid, 0.5% 5-fluorouracil, 5% imiquimod, and 0.025–0.05% ingenol mebutate, compared with vehicle or placebo. It significantly favoured the treatment of individual AK lesions with PDT as early studies of PDT were designed towards individual lesion response. ALA-PDT was also significantly favoured compared with cryotherapy. Based on investigator and participant evaluation, imiquimod treatment and PDT resulted in better cosmetic outcomes than cryotherapy and 5-fluorouracil. A more recent systematic review performed in 2013 undertook to compare the evidence of the effectiveness of PDT compared with other therapies, restricted to RCTs with at least 10 participants [35]. Thirteen studies were included in the final analysis, of which four were eligible for final meta-analysis. PDT was concluded to offer a 14% better chance of complete lesion clearance at 3 months after treatment than cryotherapy for thin AKs on the face and scalp.
Therapy-specific guidelines from the British Association of Dermatology list PDT alongside topical therapies as suitable for isolated or scattered AKs, as a suitable choice for patients wishing to manage background actinic changes, and as part of maintenance treatment for low-grade AKs in sun-damaged skin [36]. In that evidence-based review, PDT is favoured where there are a large number of AKs (field therapy) and for confluent recalcitrant AKs not responding to other treatments, with strong evidence for use on the scalp and face. This will assist practitioners in deciding where best to deploy PDT within their clinical practice.
There is limited direct comparison evidence of DL-PDT with standard therapies. DL-PDT has been compared with ingenol mebutate in the treatment of 27 patients with 323 grade I and II AKs. [37] Both 25 cm2 target areas achieved complete response in 40.47% of cases, and the average AK lesion clearance rate was similar. Areas treated with DL-PDT had a lower reduction in mean grade II AK clearance rate compared with grade I AK, with reduction similar for both types in the ingenol-treated areas. The tolerability profile was superior for DL-PDT, with mean local skin responses and pain higher in areas treated with ingenol mebutate. Reflecting on the excellent tolerability, there is a strong case for considering DL-PDT for large fields of AKs, expecially where thin AKs are predominant, with a balding scalp displaying multiple lesions particularly appropriate for considering this modality.
4 Use of Daylight PDT in Combination with Other Therapy
There is potential for combining PDT with other modalities to improve efficacy or extend the reach of PDT. Data regarding combination use of DL-PDT are sparse, but pretreatment of AKs with topical 5-fluorouracil or diclofenac has been reported, as well as several combinations of using topical imiquimod and conventional PDT [38]. A recent randomized intraindividual study investigated whether 7 days of pretreatment twice daily with topical 5% 5-fluorouracil enhanced the treatment efficacy of DL-PDT in 24 patients with AKs on the hands [39]. At 3-month follow-up, the overall lesion response rate was significantly higher for the combination (62.7 vs. 51.8%), while pain and erythema were similar. Combination treatment could offer a very effective practical solution for patients with extensive AK, including on limb sites, where we would anticipate a poorer efficacy with monotherapy.
5 PDT: Evidence for Prevention of AK
Although the exact rate of malignant transformation to SCC is unknown, the majority of SCCs appear to arise from within AKs [40]. Therapies that can treat large fields of actinically damaged skin may offer benefit in reducing the development of new lesions and potentially new cancers, although there remains a lack of evidence of specific prevention of SCC to date. As a field-directed therapy, PDT appears suitable for use for the treatment of areas of extensive actinic damage, offering the potential to treat subclinical lesions, and even potentially delay/prevent new lesions, with a decreased expression of p53 demonstrated. The preventive potential of PDT has been studied in immunosuppressed (see below) and immunocompetent individuals; a single treatment of ALA-PDT demonstrated a delay of approximately 6 months in the development of new AKs [41].
Nanoemulsion ALA by either the conventional or, recently, daylight protocol is licensed for the treatment of entire fields with cancerization (areas of skin where multiple AK lesions are surrounded by an area of actinic and sun-induced damage within a limited field). Following a single treatment by the conventional protocol, nanoemulsion ALA-PDT achieved a patient complete clearance rate of 91% after a maximum of two treatments [42]. DL-PDT has been compared with conventional PDT as a preventive treatment for nonmelanoma skin cancer (NMSC) in patients with actinic field damage [43]. Patients all had previous NMSC on the face/scalp and AK on the same fields. They received one cycle of treatment and were evaluated for the development of new lesions over 1 year. The total number of new AKs and the mean time to development did not significantly differ between sides in the 26 patients treated, but local adverse events were more intense with conventional PDT. An open intrapatient randomized study of 27 renal OTRs reported a significant delay in the development of new lesions at sites treated with conventional MAL-PDT [44]. By 12 months, 62% of treated areas were free from new lesions, compared with 35% of control areas. In a further study of topical PDT in 81 OTRs, conventional MAL-PDT was administered twice, 1 week apart, then at 3, 9 and 15 months [45]. Compared with control sites, the occurrence of new lesions was reduced during the initial months of the study, but lost by 27 months, suggesting additional treatments after 15 months might be required.
In a recent randomized split-face study to determine the impact of field PDT on the development of new lesions, a single treatment of conventional (lesional) MAL-PDT was compared with PDT over the entire field in patients with a maximum of 10 AKs on each side (all lesions on the face or scalp). After 9 months, significantly fewer new lesions were observed compared with the side receiving lesion-only therapy [46].
An international consensus recommended PDT as a good therapy option to treat field cancerization, but recognized the tolerability issues of field treatment using conventional PDT [47]. Reflecting on the superior tolerability of DL-PDT over conventional PDT, prophylactic PDT using daylight in a 6-monthly cycle appears more feasible than by conventional protocol, but this requires further study and consideration of cost effectiveness, as well as comparison with other modalities.
6 Daylight PDT for Photorejuvenation
Several studies have reported on the rejuvenating effects of PDT, such as reduced fine wrinkles, mottled hyperpigmentation, tactile roughness, skin texture, telangiectasias, facial erythema, and sallowness [48,49,50,51]. The cosmetic effects of topical PDT are supported by immunohistochemical analysis that showed both upregulation of collagen production and increased epidermal proliferation [52, 53]. These molecular effects, together with the disappearance of Tp53, a marker for epidermal carcinogenesis, may explain why PDT is able to reverse the signs of photoaging [54]. Chronically sun-damaged skin is also characterized by sallowness, telangiectasias, hyperpigmentations, roughness, and wrinkles. Therefore, simultaneous treatment of AKs and photodamage is highly desirable.
An expert group met to develop recommendations for the use of daylight MAL-PDT in patients with large-scale photodamaged skin, and concluded that actinic field damage can be treated as effectively with daylight MAL-PDT as conventional PDT, but with the advantage that treatment is nearly pain free, facilitating its use over large areas of actinic field damage [55]. A recent parallel-group, double-blind, randomized placebo-controlled trial from Columbia assessed daylight MAL-PDT in 60 patients with facial photodamage [56], and reported that treatment was well tolerated, without pain, and showed significant improvement in photodamage, although Herpes simplex prophylaxis was recommended before sessions. A trial is currently underway assessing the efficacy of repetitive DL-PDT in preventing AK and investigating the possible rejuvenating effects of this treatment; results are awaited [57].
DL-PDT using Levulan® has been used to treat 80 patients from Southern California with multiple AKs related to chronic photodamage, with ALA applied 1 h prior to light exposure, and with a chemical sunscreen applied 30 min into incubation [58]. Patients sat outside for 2.5 h and were requested to seek shaded or direct sunlight for 15–30 extra minutes the following day (after further sunscreen application). They were advised to protect themselves from further sunlight and stay indoors for 48 h. Patients tolerated treatment well, with no pain during the first exposure, although patients described a mild burning sensation on the second day of exposure. Significant clinical improvement was observed, with a reduction in AKs, as well as a reduction in lentigines and other signs of ageing.
7 Adverse Events
Guidelines indicate that the most common adverse event of ‘pain/discomfort’ restricted to the illuminated area, is commonly experienced during PDT [1,2,3,4]. It usually peaks within minutes of commencing light exposure and probably reflects nerve stimulation and/or tissue damage by reactive oxygen species, possibly aggravated by hyperthermia. Although most patients will tolerate conventional PDT without anaesthesia/analgesia, as the face and scalp may be more susceptible to pain, there is strong interest in refining the method of delivery of PDT so as to minimize pain and increase the scope for treatment of large fields, e.g. face, balding scalp.
Several reports have tried to compare the pain associated with conventional ALA and MAL-PDT, but differences in disease indication, protocol and whether compounded or branded agents were used has limited direct comparison. In a comparison of AKs treated with either conventional ALA-PDT or MAL-PDT, less pain was associated with MAL-PDT, but compounded ALA cream was utilized and a longer drug incubation time period than standard US therapy was used; comparison should be with 1-h drug incubation with Levulan® and 3-h drug incubation under occlusion with MAL [59].
Immediately following treatment, erythema and oedema were common, with erosion, crust formation and healing over 2–6 weeks, but ulceration is very rare. Swelling can be locally marked, giving rise to occasional observations of an urticated reaction at the treatment site.
Hyper- or hypopigmentation can occasionally be seen in treated areas but usually resolves within 6 months. Evidence would indicate the risk of cancer associated with PDT to be low, but in view of the latent period for carcinogenesis, guidelines advise careful reporting of malignancies in sites of prior PDT [1,2,3,4]. A few case reports are noted in guidelines where skin cancer developed at sites where PDT had been performed, but these lesions may either represent evolution of a partially treated precancer by PDT, or the coincidental development of a skin cancer in a sun-damaged field receiving PDT to treat lesions within the field.
8 Cost Effectiveness of DL-PDT for AK
Cost effectivenss of PDT delivered by different methods is hard to compare with multiple lesion and patient-specific factors, as well as clinic set-up considerations. The cost effectiveness of DL-PDT compared with conventional PDT for AK was assessed in a ranndomized trial, with inclusion of societal and private costs, including the time patients spent in treatment [60]. The total costs per patient were significantly lower for DL-PDT (€132) compared with conventional PDT (€170), giving a cost saving of €38. The estimated probabilities for patients’ complete response were 0.429 for DL-PDT and 0.686 for conventional PDT, a difference in probability of being healed of 0.257. The incremental cost-effectiveness ratio showed a monetary gain of €147 per unit of effectiveness lost. DL-PDT was therefore less costly but also less effective than conventional PDT, providing lower value for money. Further studies are required and the improved tolerance of DL-PDT should be recalled, as well as the cost impact of different clinic set-ups for delivery of DL-PDT.
9 Simulated Alternatives to Daylight PDT
As climatic conditions may not always permit daylight PDT outdoors, the spectrum of five different lamps for simulated indoor ‘daylight PDT’ were investigated for their ability to photobleach PpIX [61]. The lamps investigated were halogen, white light-emitting diode (LED), red LED panel, and lamps used for conventional PDT. Four of the five light sources were able to photobleach PpIX completely (intensity of light from red LED panel insufficient) and offer an alternative light source for simulated daylight PDT. The amount of PpIX activating daylight available in a glass greenhouse was also assessed as an alternative to daylight PDT, as originally described. The greenhouse was suitable for DL-PDT since the effect of solar light is lowered by only 25%, and, as minimal UVB radiation passes through the greenhouse glass, sun protection is not needed.
Simulated daylight PDT has also been evaluated using nanoemulsion ALA and paired illumination sources installed at the ceiling of the treatment room, with patients sitting in the room for 2 h after a short 30-min period of occlusion of the ALA preparation [62]. The patient complete clearance rate 3 months after two sessions, 1 week apart, was 75% (82% in patients with only grade I lesions and 67% in patients with at least one grade II lesion), with a lesion clearance rate of 93%. Simulated DL-PDT is well tolerated with no/minimal pain.
A study has compared the effectiveness of DL-PDT and artificial white light (AWL) LED PDT for the treatment of AKs on the forehead and scalp [63]. DL-PDT involved using MAL and standard protocol, while the AWL-mediated MAL-PDT used an operating light source chosen for the light spectrum of the LEDs. There was a 62% reduction in AKs after one treatment with DL-PDT and 68% for AWL-PDT at 1 month (48% and 64%, respectively, at 9 months). Both treatments were rated highly tolerable by patients with no significant difference in pain scores.
10 Daylight PDT: Additional Indications
The concepts of daylight PDT have stimulated fresh interest in the therapeutic scope of this therapy for dermatological indications. Patients with lower-lip actinic cheilitis have been treated with DL-PDT, receiving two treatments 7–14 days apart [64]. Complete clinical response was achieved in 7/10 patients at 3 months. A pilot study of previously untreated superficial and small nodular BCCs using two DL-PDT treatments, 1 week apart, cleared 94% of lesions at 3 months, dropping to 74% complete response at 12 months [65].
Daylight PDT using a novel variant of 5-aminolevulinate ester, 1.5% 3-butenyl ALA-bu gel, was used to treat facial acne in a placebo-controlled trial [66]. At 12 weeks, both inflammatory and non-inflammatory acne lesions had decreased significantly by 58.0% and 34.1%, respectively, in the PDT group.
Cutaneous leishmaniasis (CL) has been shown to respond to DL-PDT, in an open study of 31 patients with CL, with patients either treated in the hospital garden under supervision or at home [67]. The overall cure rate was 89%, and was similar between the hospital and home treatment groups, increasing the ease of use of DL-PDT, even in technologically deprived countries where the majority of Leishmania infections are encountered.
11 Conclusion
DL-PDT has a substantial evidence base, demonstrating equivalence to conventional PDT, although efficacy for thicker AKs may be inferior to alternative therapies. Consensus recommendations from national experts have concluded that DL-PDT has a role in the treatment of AKs in Australia, South America, and Europe, including the UK and the Iberian peninsula [68,69,70,71,72]. The absence or near absence of pain during treatment has overcome the principal concern about using topical PDT over larger areas. The opportunity for field DL-PDT to the face or scalp is practical and can reduce costs associated with delivery of the treatment. There are restrictions on when PDT can be administered in many countries, depending on climatic considerations, but alternative light sources that simulate PDT are under study. Additional indications for DL-PDT are being explored and seek to extend the potential of PDT as a useful platform for the treatment of several dermatoses. Additional data comparing DL-PDT with other therapies for AK are required, along with relevant cost-effectiveness assessment. Nevertheless, current evidence indicates DL-PDT to be an effective, well-tolerated therapy where field treatment may reduce new lesion development, as well as offer photorejuvenation.
References
Braathen LR, Szeimies RM, Basset-Seguin N, Bissonnette R, Foley P, Pariser D, et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. J Am Acad Dermatol. 2007;56:125–43.
Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy. Br J Dermatol. 2008;159:1245–66.
Morton CA, Szeimies RM, Sidoroff A, Braathen LR. European guidelines for topical photodynamic therapy part 1: treatment delivery and current indications—actinic keratoses, Bowen’s disease, basal cell carcinoma. J Eur Acad Dermatol Venereol. 2013;27:536–44.
Morton C, Szeimies RM, Sidoroff A, Wennberg AM, Basset-Seguin N, Calzavara-Pinton P, et al. European Dermatology Forum European Dermatology Forum Guidelines on topical photodynamic therapy. Eur J Dermatol. 2015;25:296–311.
Touma D, Yaar M, Whitehead S, Konnikov N, Gilchrest BA. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol. 2004;140(1):33–40.
Kim M, Jung HY, Park HJ. Topical PDT in the treatment of benign skin diseases: principles and new applications. Int J Mol Sci. 2015;16:23259–78.
Fritsch C, Lang K, Schulte KW, Neuse W, Ruzicka T, Lehman P. Fluorescence diagnosis and photodynamic therapy in dermatology: an overview. In: Patrice T, editor. photodynamic therapy. Cambridge: Royal Society of Chemistry; 2003. p. 177–212.
Grapengiesser S, Ericson M, Gudmundsson F, Larkö O, Rosén A, Wennberg AM. Pain caused by photodynamic therapy of skin cancer. Clin Exp Dermatol. 2002;27:493–7.
Wiegell SR, Wulf HC, Szeimies RM, Basset-Seguin N, Bissonnette R, Gerritsen MJ, et al. Daylight photodynamic therapy for actinic keratosis: an international consensus: International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:673–9.
Osman-Ponchet H, Sevin K, Gaborit A. Lack of effect of selected sunscreens applied on ex vivo human skin for 5-methyl-aminolevulinic acid penetration and protoporphyrin IX photoactivation. Photodiagnosis Photodyn Ther. 2017;17:75–81.
Gholam P, Fink C, Bosselmann I, Enk AH. Retrospective analysis evaluating the effect of a keratolytic and physical pretreatment with salicylic acid, urea and curettage on the efficacy and safety of photodynamic therapy of actinic keratoses with methylaminolaevulinate. J Eur Acad Dermatol Venereol. 2016;30:619–23.
Wiegell SR, Petersen B, Wulf HC. Topical corticosteroid reduces inflammation without compromising the efficacy of photodynamic therapy for actinic keratoses: a randomized clinical trial. Br J Dermatol. 2014;171:1487–92.
Wiegell SR, Fabricius S, Stender IM, Berne B, Kroon S, Andersen BL, et al. A randomized, multicentre study of directed daylight exposure times of 1(1/2) vs. 2(1/2) h in daylight-mediated photodynamic therapy with methyl aminolaevulinate in patients with multiple thin actinic keratoses of the face and scalp. Br J Dermatol. 2011;164:1083–90.
Wiegell SR, Fabricius S, Heydenreich J, Enk CD, Rosso S, Bäumler W, et al. Weather conditions and daylight-mediated photodynamic therapy: protoporphyrin IX-weighted daylight doses measured in six geographical locations. Br J Dermatol. 2013;168:186–91.
Grinblat B, Galimberti G, Pantoja G, et al. Feasibility of daylight-mediated photodynamic therapy for actinic keratosis throughout the year in Central and South America: a meteorological study. Int J Dermatol. 2016;55:e488–93.
Spelman L, Rubel D, Murrell DF, et al. Treatment of face and scalp solar (actinic) keratosis with daylight-mediated photodynamic therapy is possible throughout the year in Australia: Evidence from a clinical and meteorological study. Australas J Dermatol. 2016;57:24–8.
Dirschka T, Radny P, Dominicus R, Mensing H, Brüning H, Jenne L, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br J Dermatol. 2012;166:137–46.
Wiegell SR, Haedersdal M, Philipsen PA, Eriksen P, Enk CD, Wulf HC. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study. Br J Dermatol. 2008;158:740–6.
Wiegell SR, Haedersdal M, Eriksen P, Wulf HC. Photodynamic therapy of actinic keratoses with 8% and 16% methyl aminolaevulinate and home-based daylight exposure: a double-blinded randomized clinical trial. Br J Dermatol. 2009;160:1308–14.
Wiegell SR, Fabricius S, Gniadecka M, Stender IM, Berne B, Kroon S, et al. Daylight-mediated photodynamic therapy of moderate to thick actinic keratoses of the face and scalp: a randomized multicentre study. Br J Dermatol. 2012;166:1327–32.
Rubel DM, Spelman L, Murrell DF, See JA, Hewitt D, Foley P, et al. Daylight PDT with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional PDT in actinic keratosis treatment: a randomised controlled trial. Br J Dermatol. 2014;171:1164–71.
Lacour JP, Ulrich C, Gilaberte Y, Von Felbert V, Basset-Seguin N, Dreno B, et al. Daylight photodynamic therapy with methyl aminolevulinate cream is effective and nearly painless in treating actinic keratoses: a randomised, investigator-blinded, controlled, phase III study throughout Europe. J Eur Acad Dermatol Venereol. 2015;29:2342–8.
Fargnoli MC, Ibbotson SH, Hunger RE, Rostain G, Gaastra MTW, Eibenschutz L, et al. Patient and physician satisfaction in an observational study with methyl aminolevulinate daylight-photodynamic therapy in the treatment of multiple actinic keratoses of the face and scalp in 6 European countries. J Eur Acad Dermatol Venereol. https://doi.org/10.1111/jdv.14691 (Epub 14 Nov 2017).
Fargnoli MC, Piccioni A, Neri L, et al. Conventional vs. daylight methyl aminolevulinate photodynamic therapy for actinic keratosis of the face and scalp: an intra-patient, prospective, comparison study in Italy. J Eur Acad Dermatol Venereol. 2015;29:1926–32.
Braathen LR. Daylight photodynamic therapy in private practice in Switzerland: gain without pain. Acta Derm Venereol. 2012;92:652–3.
Grinblat BM, Festa Neto C, Sanches JA Jr, Szeimies RM, Oliveira AP, Torezan LA. Daylight photodynamic therapy for actinic keratoses in São Paulo. Brazil. Photodermatol Photoimmunol Photomed. 2015;31:54–6.
Galvão LEG, Gonçalves HS, Botelho KP, Caldas JC. Daylight photodynamic therapy - Experience and safety in treatment of actinic keratoses of the face and scalp in low latitude and high brightness region. Anais Brasileiros de Dermatologia. 2017;92:142–4.
Neittaanmäki-Perttu N, Karppinen TT, Grönroos M, Tani TT, Snellman E. Daylight photodynamic therapy for actinic keratoses: a randomized double-blinded nonsponsored prospective study comparing 5-aminolaevulinic acid nanoemulsion (BF-200) with methyl-5-aminolaevulinate. Br J Dermatol. 2014;171:1172–80.
EU Clinical Trials Register. EudraCT number 2015-004382-83. https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-004382-83/results. Accessed 22 Apr 2018.
Fargnoli MC, Piccioni A, Neri L, Tambone S, Pellegrini C, Peris K. Long-term efficacy and safety of daylight photodynamic therapy with methyl amninolevulinate for actinic keratosis of the face and scalp. Eur J Dermatol. 2017;27:89–91.
Dragieva G, Hafner J, Dummer R, Schmid-Grendelmeier P, Roos M, Prinz BM, et al. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen’s disease in transplant recipients. Transplantation. 2004;77:115–21.
Perrett CM, McGregor JM, Warwick J, Karran P, Leigh IM, Proby CM, et al. Treatment of posttransplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320–8.
Togsverd-Bo K, Lei U, Erlendsson AM, Taudorf EH, Philipsen PA, Wulf HC, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients: a randomized controlled trial. Br J Dermatol. 2015;172:467–74.
Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev 2012;(12):CD004415.
Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta - analysis. JAMA Dermatol. 2014;150:1281–8.
de Berker D, McGregor JM, Mohd Mustapa MF, Exton LS, Hughes BR. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176:20–43.
Genovese G, Fai D, Fai C, Mavilia L, Mercuri SR. Daylight methyl-aminolevulinate photodynamic therapy versus ingenol mebutate for the treatment of actinic keratoses: an intraindividual comparative analysis. Dermatol Ther. 2016;29:191–6.
Lucena SR, Salazar N, Gracia-Cazaña T, Zamarrón A, González S, Juarranz A, et al. Combined treatments with photodynamic therapy for non-melanoma skin cancer. Int J Mol Sci. 2015;16:25912–33.
Nissen CV, Heerfordt IM, Wiegell SR, Mikkelsen CS, Wulf HC. Pretreatment with 5-fluorouracil cream enhances the efficacy of daylight-mediated photodynamic therapy for actinic keratosis. Acta Derm Venereol. 2017;97:617–21.
Czarnecki D, Meehan CJ, Bruce F, Culjak G. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207–9.
Apalla Z, Sotiriou E, Chovarda E, Lefaki I, Devliotou-Panagiotidou D, Ioannides D. Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo-controlled study. Br J Dermatol. 2010;162:171–5.
Reinhold U, Dirschka T, Ostendorf R, Aschoff R, Berking C, Philipp-Dormston WG, et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz®) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy when using the BF-RhodoLED® lamp. Br J Dermatol. 2016;175:696–705.
Sotiriou E, Apalla Z, Vrani F, et al. Daylight photodynamic therapy vs Conventional photodynamic therapy as skin cancer preventive treatment in patients with face and scalp cancerization: an intra-individual comparison study. J Eur Acad Dermatol Venereol. 2017;31:1303–7.
Wulf HC, Pavel S, Stender I, Bakker-Wensveen CA. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25–8.
Wennberg AM, Stenquist B, Stockfleth E, et al. Photodynamic therapy with methyl aminolevulinate for prevention of new skin lesions in transplant recipients: a randomized study. Transplantation. 2008;86:423–9.
Seubring I, Groenewoud JMM, Gerritsen MP. Comparison of “Lesion-by-Lesion” and field photodynamic therapy in the prevention of actinic keratoses: a randomized, split-face, Single-Blind Pilot Study. Dermatology. 2016;232:708–14.
Braathen LR, Morton CA, Basset-Seguin N, Bissonnette R, Gerritsen MJP, Gilaberte Y, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063–6.
Ruiz-Rodriguez R, Sanz-Sanchez T, Cordoba S, et al. Photodynamic photorejuvenation. Dermatol Surg. 2002;28:742–4.
Issa MC, Pineiro-Maceira J, Vieira MT, Olej B, Mandarim-de-Lacerda CA, Luiz RR, et al. Photorejuvenation with topical methyl aminolevulinate and red light: a randomized, prospective, clinical, histopathologic, and morphometric study. Dermatol Surg. 2010;36:39–48.
Piccioni A, Fargnoli MC, Schoinas S, Suppa M, Frascione P, Ginebri A, et al. Efficacy and tolerability of 5-aminolevulinic acid 0.5% liposomal spray and intense pulsed light in wrinkle reduction of photodamaged skin. J Dermatol Treat. 2011;22:247–53.
Sanclemente G, Medina L, Villa JF, Barrera LM, Garcia HI. A prospective split-face double-blind randomized placebo-controlled trial to assess the efficacy of methyl aminolevulinate + red-light in patients with facial photodamage. J Eur Acad Dermatol Venereol. 2011;25:49–58.
Park MY, Sohn S, Lee ES, Kim YC. Photorejuvenation induced by 5-aminolevulinic acid photodynamic therapy in patients with actinic keratosis: a histologic analysis. J Am Acad Dermatol. 2010;62:85–95.
Szeimies RM, Torezan L, Niwa A, Valente N, Unger P, Kohl E, et al. Clinical, histopathological and immunohistochemical assessment of human skin field cancerization before and after photodynamic therapy. Br J Dermatol. 2012;167:150–9.
Bagazgoitia L, Cuevas Santos J, Juarranz A, Jaen P. Photodynamic therapy reduces the histological features of actinic damage and the expression of early oncogenic markers. Br J Dermatol. 2011;165:144–51.
Philipp-Dormston WG, Sanclemente G, Torezan L, et al. Daylight photodynamic therapy with MAL cream for large-scale photodamaged skin based on the concept of ‘actinic field damage’: recommendations of an international expert group. J Eur Acad Dermatol Venereol. 2016;30:8–15.
Sanclemente G, Mancilla GA, Hernandez G. A double-blind randomized controlled trial to assess the efficacy of daylight photodynamic therapy with methyl-aminolevulinate vs. Placebo and daylight in patients with facial photodamage. Actas Dermo-Sifiliográficas. 2016;107:224–34.
Kohl E, Koller M, Zeman F, et al. Daylight photodynamic therapy versus cryosurgery for the treatment and prophylaxis of actinic keratoses of the face—protocol of a multicenter, prospective, randomized, controlled, two-armed study. BMC Dermatol. 2017;17:12.
Lane KL, Hovenic W, Ball K, Zachary CB. Daylight photodynamic therapy: the Southern California experience. Lasers Surg Med. 2015;47:168–72.
Kasche A, Luderschmidt S, Ring J, Hein R. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J Drugs Dermatol. 2006;5:353–6.
Neittaanmäki-Perttu N, Grönroos M, Karppinen T, Snellman E, Rissanen P. Photodynamic Therapy for Actinic Keratoses: A Randomized Prospective Non-sponsored Cost-effectiveness Study of Daylight-mediated Treatment Compared with Light-emitting Diode Treatment. Acta Derm Venereol. 2016;96:241–4.
Lerche CM, Heerfordt IM, Heydenreich J, Wulf HC. Alternatives to outdoor daylight illumination for photodynamic therapy: use of greenhouses and artificial light sources. Int J Mol Sci. 2016;17:309.
Kellner C, Bauriedl S, Hollstein S, Reinhold U. Simulated-daylight photodynamic therapy with BF-200 aminolaevulinic acid for actinic keratosis: assessment of the efficacy and tolerability in a retrospective study. Br J Dermatol. 2015;172:1146–8.
O’Gorman SM, Clowry J, Manley M, McCavana J, Gray L, Kavanagh A, et al. Artificial white light vs daylight photodynamic therapy for actinic keratoses: a randomized clinical trial. JAMA Dermatol. 2016;152:638–44.
Fai D, Romanello E, Brumana MB, et al. Daylight photodynamic therapy with methyl-aminolevulinate for the treatment of actinic cheilitis. Dermatol Ther. 2015;28:355–68.
Wiegell SR, Skødt V, Wulf HC. Daylight-mediated photodynamic therapy of basal cell carcinomas—an explorative study. J Eur Acad Dermatol Venereol. 2014;28:169–75.
Kwon HH, Moon KR, Park SY, Yoon JY, Suh DH, Lee JB. Daylight photodynamic therapy with 1.5% 3-butenyl 5-aminolevulinate gel as a convenient, effective and safe therapy in acne treatment: A double-blind randomized controlled trial. J Dermatol. 2016;43:515–21.
Enk CD, Nasereddin A, Alper R, Dan-Goor M, Jaffe CL, Wulf HC. Cutaneous leishmaniasis responds to daylight-activated photodynamic therapy: proof of concept for a novel self-administered therapeutic modality. Br J Dermatol. 2015;172:1364–70.
See JA, Shumack S, Murrell DF, Rubel DM, Fernández-Peñas P, Salmon R, et al. Consensus recommendations on the use of daylight photodynamic therapy with methyl aminolevulinate cream for actinic keratoses in Australia. Australas J Dermatol. 2016;57:167–74.
Grinblat B, Galimberti G, Chouela E, Sanclemente G, Lopez M, Alcala D, et al. Daylight-mediated photodynamic therapy for actinic damage in Latin America: consensus recommendations. Photodermatol Photoimmunol Photomed. 2016;32:81–7.
Morton CA, Wulf HC, Szeimies RM, Gilaberte Y, Basset-Seguin N, Sotiriou E, et al. Practical approach to the use of daylight photodynamic therapy with topical methylaminolevulinate for actinic keratosis: a European consensus. J Eur Acad Dermatol Venereol. 2015;29:1718–23.
Ibbotson S, Stones R, Bowling J, Campbell S, Kownacki S, Sivaramakrishnan M, et al. A consensus on the use of daylight photodynamic therapy in the UK. J Dermatolog Treat. 2017;28:360–7.
Gilaberte Y, Aguilar M, Almagro M, Correia O, Guillén C, Harto A, et al. Spanish-Portuguese consensus statement on use of daylight-mediated photodynamic therapy with methyl aminolevulinate in the treatment of actinic keratosis. Actas Dermosifiliogr. 2015;106:623–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript
Conflicts of interest
Colin A. Morton has been a member of advisory boards for Almirall, Biofrontera, Galderma, and Leo Pharma, and has received speaker honoraria from Biofrontera and Galderma. Lasse R. Braathen has received congress/travel support, and fees for lectures and advisory board participation from Galderma.
Rights and permissions
About this article
Cite this article
Morton, C.A., Braathen, L.R. Daylight Photodynamic Therapy for Actinic Keratoses. Am J Clin Dermatol 19, 647–656 (2018). https://doi.org/10.1007/s40257-018-0360-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-018-0360-y