Abstract
Radiodermatitis (radiation dermatitis, radiation-induced skin reactions, or radiation injury) is a significant side effect of ionizing radiation delivered to the skin during cancer treatment as well as a result of nuclear attacks and disasters, such as that which occurred in Fukushima in 2011. More specifically, 95 % of cancer patients receiving radiation therapy will develop some form of radiodermatitis, including erythema, dry desquamation, and moist desquamation. These radiation skin reactions result in a myriad of complications, including delays in treatment, diminished aesthetic appeal, and reduced quality of life. Recent technological advancements and novel treatment regimens have only been successful in partly ameliorating these adverse side effects. This article examines the current knowledge surrounding the pathogenesis, clinical manifestations, differential diagnoses, prevention, and management of radiodermatitis. Future research should examine therapies that incorporate the current understanding of the pathophysiology of radiodermatitis while measuring effectiveness using objective and universal outcome measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Radiodermatitis is a major side effect associated with radiation exposure and exists on a continuum ranging from erythema to moist desquamation. |
Despite recent advancements in technology and the development of new treatments, there is no definitive evidence supporting any one intervention in the prevention or treatment of radiodermatitis. |
1 Introduction
Ionizing radiation is often utilized to treat various forms of cancer. In North America, 50 % of cancer patients will receive radiotherapy during their illness [1]. It is estimated that up to 95 % of these patients will develop some degree of radiodermatitis [2, 3]. Radiodermatitis is also referred to as radiation dermatitis, radiation-induced skin reactions, or radiation injury of the subdermal fat. It may also be caused by a variety of other forms of radiation exposure (i.e., other interventional procedures, environmental factors, or occupation-related exposure, as seen in nuclear power plant workers), even when the skin is not the primary target. These radiation-induced skin changes have been recognized and scientifically reported since the beginning of the 20th century [4, 5].
The effects of radiation injury may affect the patient’s quality of life and well-being, resulting in a potentially detrimental cessation of therapy and consequent inappropriate treatment [1, 5]. Consequently, much research has explored the underlying factors, pathophysiology, and management of radiodermatitis. Novel technologies and treatment schedules have been successful in partly ameliorating, but not eliminating, these adverse side effects [4]. Despite this growing interest, much remains to be explored and discovered with respect to this pervasive condition.
2 Epidemiology
Radiodermatitis is among the most common side effects experienced by patients receiving radiation therapy for sarcoma, breast, anal, vulva, and head and neck cancers [2, 7, 8]. The higher incidence of radiodermatitis in these cancers is due to proximity of the intended radiation target to the skin and hence the inability to spare the skin from higher doses of radiation [9]. Additionally, radiation injury may be more symptomatic in certain skin regions such as the vulva and anus [3]. Radiation dermatitis occurs in up to 95 % of patients receiving radiotherapy [2, 3]. In Canada, the USA, Europe, and Australia, at least 50 % of patients diagnosed with cancer will receive radiation therapy during their illness [1]. Erythema is the first visible manifestation, occurring in more than 90 % of these patients, followed by moist desquamation in more than 30 % of patients (Table 1) [10]. The varying severities of radiation-induced skin reactions in cancers most commonly associated with radiodermatitis are explored in Table 2. This varying degree of severity depends on numerous risk factors that have been classified in the literature as being patient-related (intrinsic), treatment-related (extrinsic), or both (intrinsic and extrinsic) [11, 12]. Patient-related risk factors may include age, sex, smoking, poor nutritional status, high body mass index (BMI), large breast cup size, excessive skin folds, ethnic origin, coexisting disease, hormonal status, ultraviolet (UV) exposure, tumor site, and genetic factors. Treatment-related factors include the total radiation dose, the dose fractionation schedule, the type of external beam employed, radio-sensitizers, concurrent chemotherapy, the site of treatment, and the volume and surface area of irradiated tissue [2, 3, 6, 8, 9, 11]. Three studies have demonstrated reduced incidence, stage, severity, and duration of radiation-induced skin reactions in breast cancer patients receiving intensity-modulated radiotherapy (IMRT) versus conventional radiation therapy [13–15]. IMRT significantly improves dose distribution compared with conventional radiation therapy [14].
3 Pathogenesis
3.1 Acute Effects
The pathogenesis of radiodermatitis involves a combination of direct radiation injury and a subsequent inflammatory response, affecting cellular elements in the epidermis, dermis, and vasculature. The energy from the initial dose of ionizing radiation during radiation therapy produces immediate tissue damage via the production of secondary electrons and reactive oxygen species (ROS) that attack cellular structures (i.e., cell membranes and DNA). Each subsequent fraction of radiation generates greater inflammatory cell recruitment [16–18]. Ionizing radiation causes an acute reaction that causes changes in skin pigmentation through the migration of melanosomes, interrupted hair growth, and damage to the deeper dermis, while sparing the upper epidermal layer. Damage to the dermis disrupts the normal process of skin cell repopulation, initially resulting in erythema due to dermal vessel dilation and histamine-like substance release [12]. At higher doses of radiotherapy, greater damage occurs and the skin attempts to compensate by increasing its rate of mitosis in the basal keratinocyte cell layer. However, as the turnover of novel cells is faster than the shedding of the old cells, this leads to thickened, scaly skin (dry desquamation). At even higher radiation doses, the basal layer is unable to recover and an exudate is released; this is referred to as moist desquamation (see Table 1, [12, 17, 19]). These varying degrees of damage compromise the integrity of the physical barrier produced by the skin and its immune function, resulting in increased risk of infection [3]. Damage to the vascular endothelium induces hypoxia and upregulates transforming growth factor (TGF)-β, a cytokine that plays a central role in mediating radiation-induced fibrosis [20]. Fibrosis and tissue hypoxia resulting from vascular damage result in the generation of ROS [20, 21]. ROS cause significant damage to cellular structures and promote the production of inflammatory cytokines in the skin. During radiation treatment, ROS production increases dramatically and overwhelms the body’s protective antioxidant system [21].
The mechanism of radiation-induced inflammation is not yet completely understood, but keratinocytes, fibroblasts, and endothelial cells stimulate immune cells in the epidermal and dermal layers, as well as those in circulation [3]. These activating signals result in a cascade of cytokines and chemokines (i.e., interleukin [IL]-1α, IL-1β, tumor necrosis factor [TNF]-α, IL-6, IL-8, C-C motif chemokine ligand [CCL]-4, C-X-C motif chemokine ligand [CXCL]-10, and CCL2) that in turn result in skin fibrosis, the production of matrix metalloproteases that degrade dermal components and the basal cell layer, and act on vascular endothelial cells to upregulate adhesion molecules (i.e., intercellular adhesion molecule [ICAM]-1, vascular cell adhesion molecule [VCAM]-1, and E-selectin). These adhesion molecules are important in the facilitation of transendothelial migration of immune cells from circulation to irradiated skin, a hallmark of radiation-induced skin injury [3, 20, 21].
3.2 Late Effects
The development of chronic dermatitis and skin fibrosis appears to be attributable to the activity of dermal fibroblasts. The TGF-β cytokine appears to play an integral role in this process. Irradiation and its effects on the coagulation cascade, i.e., an associated increase in thrombin-induced TGF-β activation. TGF-β binds to its receptor complex and activates Smad3 proteins that initiate the fibrotic process [20, 21]. A study by Müller and Meineke [22] demonstrated that ionizing radiation effects chemokine production by dermal fibroblasts via the release of mast-cell-derived histamine, serotonin, TNF-α, and tryptase. These fibroblast-derived mediators in turn influence the nature and magnitude of inflammatory cell recruitment at the site of irradiation [3, 22].
Bone marrow-derived cells (BMDCs) appear to play an integral role in recovery. Mesenchymal cells, endothelial progenitor cells, and myelomonocytic cells have all been implicated in the healing process. It is believed that these cells are drawn to sites of radiation damage due to the chemotactic effects of stromal cell-derived factor (SDF)-1 and CXCR4 overproduction [20, 21]. Myelomonocytic cells appear to be the predominant BMDC that localize to irradiated tissue and stimulate vessel formation and repair via the release of angiogenic factors. These BMDCs have been shown to be an effective treatment in accelerating the wound-healing process [23]. However, these cells may initiate the inflammatory cascade and cause ischemia reperfusion injury [20, 21].
Adipose tissue, a rich source of mesenchymal stem cells, appears to have wound-healing effects similar to those of BMDCs [24]. Adipose-derived stem cells (ADSCs) are multipotent cells capable of promoting angiogenesis, secreting biochemical messengers (i.e., cytokines and growth factors), and stimulating dermal fibroblast proliferation during the re-epithelialization phase of wound healing [25]. Two-dimensional electrophoretic gel proteomic analysis has demonstrated that the intracellular protein composition of both BMDCs and ADSCs are similar [26]. ADSCs are 100 times more abundant than BMDCs per tissue [27]. Furthermore, ADSCs can be more easily obtained from donor sites than BMDCs through liposuction or solid fat tissue at sites distant from the radiation injury [23]. The regenerative potential of ADSCs was demonstrated in a study examining the use of non-cultured ADSCs in the treatment of chronic radiation injuries. The study concluded that the ADSC treatment was effective in improving the quality of the wounds and did not result in recurrence or new ulceration [25].
4 Clinical Presentation
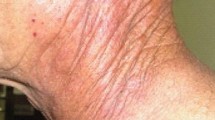
Radiodermatitis is often categorized as either acute or chronic (i.e., late), ranging from acute erythema to chronic skin fibrosis. Acute radiodermatitis, which by definition occurs within the first 90 days of radiation therapy, typically starts to occur after a moderately high dose has been delivered to the skin (e.g., 35–40 Gy in 2 Gy per fraction) [20] (Fig. 1). Severe acute injuries are not a predictor of late injuries [28]. Acute reactions are graded as a spectrum ranging from erythema to dry and eventually moist desquamation (see Table 1). Acute effects begin with erythema, edema, and pigment changes. Patients often report heightened skin sensitivity and tightness. With higher doses of radiation, the patient may develop dry desquamation presenting with dryness, pruritus, and scaling. Finally, with a further increase in dose of ionizing radiation, the patient may develop moist desquamation. The treatment field will appear moist, tender, red, and be accompanied by light or heavy serous exudate and crusting [2, 12]. De Langhe et al. [29] reviewed patients after whole-breast IMRT. Bra cup size ≥D (p < 0.001), BMI (p < 0.001), and smoking during radiotherapy (p = 0.029) were shown as risk factors for radiodermatitis [29].
Chronic radiodermatitis often presents several months to years after radiation therapy has been completed [5, 7] (Fig. 2). Chronic changes can be transient, such as the peau d’orange appearance of the edematous skin [4]. Post-inflammatory hypo- and hyperpigmentation are common chronic changes seen in patients as a result of the dermoepithelial junction being disrupted; depending on patient- and treatment-related factors, it may persist or normalize with time [4]. The patient may also experience a loss of hair follicles, nails, skin appendages, and sebaceous glands in the treatment field, as well as experience textural changes (xerosis, scales, etc.) [4, 9, 20, 21]. Telangiectasia and fibrosis are also common among patients experiencing chronic radiodermatitis, with the latter predisposing patients to ulcers, skin breakdown, tissue retraction and subsequent movement limitation, pain, and thrombosis/obstruction due to the proliferation of small blood vessels [5, 20]. Abnormal fibroblast activity and the deposition of thickened collagen may result in the development of radiation-induced morphea (RIM). RIM is a rare, under-recognized, painful, and disfiguring complication of radiotherapy that is often misdiagnosed as another dermatological condition or recurrent malignancy due to its non-characteristic appearance (erythematous plaques, indurate popular lesions, etc.) [30]. At higher radiation doses, this acute dermatitis and dermal ischemia, as a result of the obstruction of small vessels, may progress into radiation necrosis. This condition is characterized by a marked impairment in healing and increased propensity for infection [2, 5].
These radiation-induced skin changes often occur in the setting of radiotherapy and may result in a process called “field cancerization,” whereby the ionizing radiation used to treat a target neoplasm will often affect adjacent tissue, resulting in radiation dermatitis, mutations to mitochondria and nuclear DNA, and chromosomal instability. Following these subclinical changes, precursor and incipient neoplasms may later develop in the tissue immediately surrounding the primary neoplasm [31].
5 Severity Grading of Radiation Dermatitis
Accurate assessment and classification of radiation dermatitis is essential for appropriate treatment, management, and monitoring in clinical practice. Several assessment tools have been developed to describe the spectrum of radiation dermatitis; however, a gold standard is yet to be established [3]. The most widely used grading scales are (1) the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 for the classification of acute radiation dermatitis and (2) the Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) scale or Late Effects Normal Tissue Task Force/Subjective, Objective, Management, and Analytic (LENT/SOMA) scale for the classification of chronic dermatitis [3, 32]. Both the CTCAE and RTOG/EORTC tools assess acute radiation injury on a scale from 0 to 4, with increments of 1. These large increments often fail to capture subtle yet important skin changes. As a result, a number of newer scales have been developed with smaller increments (i.e., 0.5), including the Oncology Nursing Society (ONS) and Radiation Dermatitis Severity (RDS) scales [3]. Whereas both the CTCAE and the RTOG/EORTC scales measure acute radiation injury, only the RTOG/EORTC tool provides acute grading for skin toxicities and late toxicities [32]. Both the RTOG/EORTC and the LENT/SOMA scales measure late skin and subcutaneous tissue changes (graded 1–4), but only the LENT/SOMA scale incorporates pain intensity [3].
Although these tools allow for the classification and grading of radiation-associated skin toxicities, data examining their reliability and validity are sparse [32]. As a result, a number of “objective” measurement techniques have been developed to assess radiation-induced skin changes, including laser Doppler perfusion imaging, quantitative ultrasound, reflectance colorimetry, digital photography, and spectrophotometry [33, 34]. These non-invasive methods measure parameters directly and indirectly associated with radiation-induced skin changes, including changes in microcirculation (laser Doppler perfusion imaging) [35], skin thickness and Pearson co-efficient (quantitative ultrasound) [33], and melanin and erythema indices of skin discoloration (reflectance colorimetry, digital photography, and spectrophotometry) [34, 36, 37].
It is important to note that certain treatments in combination with radiation therapy can result in an increased incidence and severity of radiation-induced skin reactions by causing increased cellular damage and impaired cellular repair. These drugs, termed “radiosensitizers” are often administered immediately before, during, or <7 days after radiation therapy [4]. For instance, the conjunctive use of paclitaxel or docetaxel with radiotherapy in the treatment of breast cancer has been shown to cause synergistic cutaneous toxicity that is both dose and schedule dependent [38]. Another study has demonstrated that the simultaneous use of tamoxifen with radiation therapy may result in an increased incidence of subcutaneous fibrosis [39].
6 Differential Diagnosis
A number of cutaneous conditions that resemble radiodermatitis may become manifest during or after radiation exposure. One such condition is contact dermatitis, a localized inflammatory skin reaction that occurs in response to both physical and chemical irritants. Contact dermatitis presents clinically along a continuum, ranging from erythema to necrosis [40].
Radiation port dermatophytosis is another condition that may appear similar to radiodermatitis in its clinical manifestation. Radiation port dermatophytosis is the occurrence of tinea corporis within the radiation therapy treatment field. The lesions caused by the condition are often pruritic, erythematous scaling patches that are circular in appearance and spread outwardly. The diagnosis may be confirmed by microscopic examination of a potassium hydroxide preparation, biopsy, or fungal culture taken of the lesion [41].
A number of cutaneous hypersensitivity syndromes may appear similar to radiodermatitis. These include erythema multiforme, Stevens–Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) [4]. SJS and TEN are characterized by varying degrees of epidermal detachment and may or may not occur together. Patients who are HIV positive and receiving radiotherapy are particularly at risk of developing these two conditions [42].
Finally, radiation recall dermatitis (RRD) is a condition that closely resembles radiodermatitis. It is an acute inflammatory reaction confined to previous sites of irradiation after the administration of certain pharmacological agents [43, 44]. RRD is most commonly triggered by chemotherapeutic agents (i.e., doxorubicin, gemcitabine, docetaxel) [43, 44]. Patients with RRD present with a pruritic, maculo-papular eruption with erythema, a reaction that is mild to moderate in severity. Although great variations exist in the time between drug administration and the onset of symptoms, RRD typically manifests after days to weeks of drug exposure [45].
7 Prevention, Management, and Treatment
Recommendations on the use of dermatologic skin-care products and practices for the prevention and management of radiation dermatitis are limited. Most interventions used to ameliorate radiation-induced skin reactions are based on anecdotal evidence or poorly powered studies. As a result, treatment practices among practitioners are often varied, leaving patients confused and with conflicting information [7, 17].
General management of radiodermatitis begins with basic preventive measures, including self-care and the use of prophylactic topical corticosteroids. Self-care includes daily hygiene practices (i.e., washing and the use of soaps and deodorants), clothing (i.e., wearing loose-fitted clothing over the site receiving radiotherapy), and diet (e.g., avoiding tobacco and alcohol, and maintaining adequate hydration) [17]. The use of mild soap and deodorant is now accepted as standard clinical practice despite being a topic of contention in the past [2]. Preventing patients from partaking in socially expected hygiene practices may cause unnecessary distress and social isolation without any proven benefit [2, 7, 32]. Apart from general preventive measures, there is little evidence to date supporting any particular clinical intervention (see Table 3).
The use of IMRT has been shown to reduce skin toxicities [5, 10, 32].
The results illustrated in Table 3 appear to favor the use of steroids and silver sulfadiazine (SSD) in the prophylactic treatment of radiation-induced skin reactions. Several studies have examined the use of prophylactic steroids in the reduction of acute radiation dermatitis and have demonstrated a favorable effect [46–48]. The Miller et al. [48] trial, which compared the use of 0.1 % mometasone furoate versus placebo, provides the strongest evidence in support of prophylactic steroids. This trial demonstrated a significant reduction in the mean grade of discomfort/burning (1.5 vs. 2.1; p = 0.02) and itching (1.5 vs. 2.2; p = 0.02) in the mometasone treatment group versus the placebo control group. There also appears to be some evidence in favor of SSD cream as a prophylactic measure in the prevention of acute radiation dermatitis. In a trial conducted by Hemati et al. [49], breast cancer patients (n = 102) receiving radiation who were treated with SSD had a significantly lower RTOG skin injury score than controls (p < 0.001). There is insufficient evidence to support the use of the other agents outlined in Table 3 in the prophylaxis and treatment of radiation-induced skin injury (e.g., hyaluronic acid, aloe vera). However, it is important to note that the trials examining the use of trolamine, in particular, have demonstrated no benefit [50–53]. Those trials that support the use of trolamine are often plagued by methodological challenges, including small sample sizes [54].
For patients with established radiation-induced telangiectasia and fibrosis, a handful of studies favor the use of pulse dye laser for cosmesis and the use of pentoxifylline for the reduction of fibrosis. Nymann et al. [55] compared the use of long-pulsed dye laser (LPDL) with the use of intense pulse light (IPL) and found that the efficacy of LPDL was superior and that patients preferred LPDL in the treatment of radiation-induced telangiectasia [55]. The trials examining pentoxifylline in the treatment of radiation-induced fibrosis have shown that pentoxifylline (in combination with vitamin E) may lead to continuous clinical regression and functional improvement [56].
8 Conclusions and Future Directions
Radiodermatitis is one of the most common side effects experienced by patients undergoing radiotherapy [2, 7, 8]. Despite its prevalence, a gold standard does not exist for its prevention and management. Many of the currently used interventions are often based upon anecdotal evidence, poorly powered studies, or physician preferences [2, 5]. Furthermore, trials evaluating topical agents have failed to demonstrate effectiveness in the prevention and management of radiation-induced skin injury. These therapies do not account for the underlying pathophysiology (i.e., dermal damage), a process that involves the disruption of the intricate cellular balance between dermis and epidermis [2]. Moreover, it is often difficult to quantify this damage and draw comparisons, as many of the measures are susceptible to inter-rater variability and fail to account for important outcome measures (i.e., patient-reported outcomes [33]).
Future research should be conducted in a more systematic manner and should strive for a more rigorous study design. These studies should incorporate current knowledge regarding the underlying pathophysiology of the condition and include objective and universal outcome measures. These measures should be validated and account for patient-reported outcomes.
References
Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41.
McQuestion M. Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs. 2011;27(2):e1–17.
Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 Pt 2):985–93.
Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28–46.
Feight D, Baney T, Bruce S, McQuestion M. Putting evidence into practice: evidence-based interventions for radiation dermatitis. Clin J Oncol Nurs. 2011;15(5):481–92.
Dendaas N. Toward evidence and theory-based skin care in radiation oncology. Clin J Oncol Nurs. 2012;16(5):520–5.
Salvo N, Barnes E, van Draanen J, Stacey E, Mitera G, Breen D, et al. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol. 2010;17(4):94–112.
Hindley A, Zain Z, Wood L, Whitehead A, Sanneh A, Barber D, et al. Mometasone furoate cream reduces acute radiation dermatitis in patients receiving breast radiation therapy: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2014;90(4):748–55.
Radvansky LJ, Pace MB, Siddiqui A. Prevention and management of radiation-induced dermatitis, mucositis, and xerostomia. Am J Health Syst Pharm. 2013;70(12):1025–32.
Chan RJ, Larsen E, Chan P. Re-examining the evidence in radiation dermatitis management literature: an overview and a critical appraisal of systematic reviews. Int J Radiat Oncol Biol Phys. 2012;84(3):e357–62.
Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14:53.
Morgan K. Radiotherapy-induced skin reactions: prevention and cure. Br J Nurs. 2014;23(16):S24, S26–32.
Freedman GM, Anderson PR, Li J, Eisenberg DF, Hanlon AL, Wang L, et al. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol. 2006;29(1):66–70.
Pignol J-P, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–92.
Freedman GM, Li T, Nicolaou N, Chen Y, Ma CCM, Anderson PR. Breast intensity-modulated radiation therapy reduces time spent with acute dermatitis for women of all breast sizes during radiation. Int J Radiat Oncol Biol Phys. 2009;74(3):689–94.
Vano-Galvan S, Fernandez-Lizarbe E, Truchuelo M, Diaz-Ley B, Grillo E, Sanchez V, et al. Dynamic skin changes of acute radiation dermatitis revealed by in vivo reflectance confocal microscopy. J Eur Acad Dermatol Venereol. 2013;27(9):1143–50.
Glover D, Harmer V. Radiotherapy-induced skin reactions: assessment and management. Br J Nurs. 2014;23(4):S28, S30–5.
Hu SC-S, Hou M-F, Luo K-H, Chuang H-Y, Wei S-Y, Chen G-S. Changes in biophysical properties of the skin following radiotherapy for breast cancer. J Dermatol. 2014;41(12):1087–94.
Trueman E, Taylor L. Using a soft-silicone dressing to treat moist desquamation. Br J Nurs. 2014;23(10):S32, S34–7.
Amber KT, Shiman MI, Badiavas EV. The use of antioxidants in radiotherapy-induced skin toxicity. Integr Cancer Ther. 2014;13(1):38–45.
Kim JH, Kolozsvary AJJ, Jenrow KA, Brown SL. Mechanisms of radiation-induced skin injury and implications for future clinical trials. Int J Radiat Biol. 2013;89(5):311–8.
Müller K, Meineke V. Radiation-induced mast cell mediators differentially modulate chemokine release from dermal fibroblasts. J Dermatol Sci. 2011;61(3):199–205.
Akita S, Yoshimoto H, Akino K, Ohtsuru A, Hayashida K, Hirano A, et al. Early experiences with stem cells in treating chronic wounds. Clin Plast Surg. 2012;39(3):281–92.
Akita S. Treatment of radiation injury. Adv Wound Care. 2014;3(1):1–11.
Akita S, Yoshimoto H, Ohtsuru A, Hirano A, Yamashita S. Autologous adipose-derived regenerative cells are effective for chronic intractable radiation injuries. Radiat Prot Dosimetry. 2012;151(4):656–60.
Roche S, Delorme B, Oostendorp RAJ, Barbet R, Caton D, Noel D, et al. Comparative proteomic analysis of human mesenchymal and embryonic stem cells: Towards the definition of a mesenchymal stem cell proteomic signature. Proteomics. 2009;9(2):223–32.
Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3):132–41.
Meyer F, Fortin A, Wang CS, Liu G, Bairati I. Predictors of severe acute and late toxicities in patients with localized head-and-neck cancer treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82(4):1454–62.
De Langhe S, Mulliez T, Veldeman L, Remouchamps V, van Greveling A, Gilsoul M, et al. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer. 2014;14:711.
Spalek M, Jonska-Gmyrek J, Gałecki J. Radiation-induced morphea: a literature review. J Eur Acad Dermatol Venereol. 2015;29(2):197–202.
Piérard GE, Piérard-Franchimont C, Paquet P, Quatresooz P. Emerging therapies for ionizing radiation-associated skin field carcinogenesis. Expert Opin Pharmacother. 2009;10(5):813–21.
Wong RKS, Bensadoun RJ, Boers-Doets CB, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933–48.
Yoshida EJ, Chen H, Torres M, Andic F, Liu H, Chen Z, et al. Reliability of quantitative ultrasonic assessment of normal-tissue toxicity in breast cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(2):724–31.
Rizza L, D’Agostino A, Girlando A, Puglia C. Evaluation of the effect of topical agents on radiation-induced skin disease by reflectance spectrophotometry. J Pharm Pharmacol. 2010;62(6):779–85.
Simonen P, Hamilton C, Ferguson S, Ostwald P, O’Brien M, O’Brien P, et al. Do inflammatory processes contribute to radiation induced erythema observed in the skin of humans? Radiother Oncol. 1998;46(1):73–82.
Piérard GE. EEMCO guidance for the assessment of skin colour. J Eur Acad Dermatol Venereol. 1998;10(1):1–11.
Wengström Y, Forsberg C, Näslund I, Bergh J. Quantitative assessment of skin erythema due to radiotherapy—evaluation of different measurements. Radiother Oncol. 2004;72(2):191–7.
Coleman CN, Turrisi AT. Radiation and chemotherapy sensitizers and protectors. Crit Rev Oncol Hematol. 1990;10(3):225–52.
Azria D, Gourgou S, Sozzi WJ, Zouhair A, Mirimanoff RO, Kramar A, et al. Concomitant use of tamoxifen with radiotherapy enhances subcutaneous breast fibrosis in hypersensitive patients. Br J Cancer. 2004;91(7):1251–60.
Clark SC, Zirwas MJ. Management of occupational dermatitis. Dermatol Clin. 2009;27(3):365–83.
Casamiquela KM, Cohen PR. Radiation port dermatophytosis: Tinea corporis occurring at the site of irradiated skin. Dermatol Online J. 2012;18(1):5.
Urosevic-Maiwald M, Harr T, French L, Dummer R. Stevens–Johnson syndrome and toxic epidermal necrolysis overlap in a patient receiving cetuximab and radiotherapy for head and neck cancer. Int J Dermatol. 2012;51:864–7.
Haas RLM, de Klerk G. An illustrated case of doxorubicin-induced radiation recall dermatitis and a review of the literature. Neth J Med. 2011;69(2):72–5.
Levy A, Hollebecque A, Bourgier C, Loriot Y, Guigay J, Robert C, et al. Targeted therapy-induced radiation recall. Eur J Cancer. 2013;49(7):1662–8.
Azad A, Maddison C, Stewart J. Radiation recall dermatitis induced by pazopanib. Onkologie. 2013;36(11):674–6.
Shukla PN, Gairola M, Mohanti BKRG. Prophylactic beclomethasone spray to the skin during postoperative radiotherapy of carcinoma breast: a prospective randomized study. Indian J Cancer. 2006;43:180–4.
Schmuth M, Wimmer MA, Hofer S, Sztankay A, Weinlich G, Linder DM, et al. Topical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double-blind study. Br J Dermatol. 2002;146(6):983–91.
Miller RC, Schwartz DJ, Sloan JA, Griffin PC, Deming RL, Anders JC, et al. Mometasone furoate effect on acute skin toxicity in breast cancer patients receiving radiotherapy: a phase III double-blind, randomized trial from the North Central Cancer Treatment Group N06C4. Int J Radiat Oncol Biol Phys. 2011;79(5):1460–6.
Hemati S, Asnaashari O, Sarvizadeh M, Motlagh BN, Akbari M, Tajvidi M, et al. Topical silver sulfadiazine for the prevention of acute dermatitis during irradiation for breast cancer. Support Care Cancer. 2012;20(8):1613–8.
Elliott EA, Wright JR, Swann RS, Nguyen-Tân FTC, et al. Phase III trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Trial 99-13. J Clin Oncol. 2006;24:2092–7.
Pommier P, Gomez F, Sunyach MP, D’Hombres A, Carrie C, Montbarbon X. Phase III randomized trial of Calendula officinalis compared with trolamine for the prevention of acute dermatitis during irradiation for breast cancer. J Clin Oncol. 2004;22(8):1447–53.
Fenig E, Brenner B, Katz A, Sulkes J, Lapidot M, Schachter J, et al. Topical Biafine and Lipiderm for the prevention of radiation dermatitis: a randomized prospective trial. Oncol Rep. 2001;8(2):305–9.
Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: radiation therapy oncology group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48(5):1307–10.
Abbas H, Bensadoun RJ. Trolamine emulsion for the prevention of radiation dermatitis in patients with squamous cell carcinoma of the head and neck. Support Care Cancer. 2012;20(1):185–90.
Nymann P, Hedelund L, Hædersdal M. Intense pulsed light vs. long-pulsed dye laser treatment of telangiectasia after radiotherapy for breast cancer: a randomized split-lesion trial of two different treatments. Br J Dermatol. 2009;160(6):1237–41.
Delanian S, Balla-Mekias S, Lefaix JL. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol. 1999;17(10):3283–90.
Kouvaris JR, Kouloulias VE, Plataniotis GA, Balafouta EJ, Vlahos LJ. Dermatitis during radiation for vulvar carcinoma: prevention and treatment with granulocyte-macrophage colony-stimulating factor impregnated gauze. Wound Repair Regen. 2001;9(3):187–93.
Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, Thomas CR, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299(16):1914–21.
Roy I, Fortin A, Larochelle M. The impact of skin washing with water and soap during breast irradiation: a randomized study. Radiother Oncol. 2001;58(3):333–9.
Westbury C, Hines F, Hawkes E, Ashley S, Brada M. Advice on hair and scalp care during cranial radiotherapy: a prospective randomized trial. Radiother Oncol. 2000;54(2):109–16.
Campbell IR, Illingworth MH. Can patients wash during radiotherapy to the breast or chest wall? A randomized controlled trial. Clin Oncol (R Coll Radiol). 1992;4(2):78–82.
Watson LC, Gies D, Thompson E, Thomas B. Randomized control trial: evaluating aluminum-based antiperspirant use, axilla skin toxicity, and reported quality of life in women receiving external beam radiotherapy for treatment of stage 0, I, and II breast cancer. Int J Radiat Oncol. 2012;83(1):e29–34.
Bennett C. An investigation into the use of a non-metallic deodorant during radiotherapy treatment: a randomised controlled trial. J Radiother Pract. 2009;8(01):3.
Théberge V, Harel F, Dagnault A. Use of Axillary deodorant and effect on acute skin toxicity during radiotherapy for breast cancer: a prospective randomized noninferiority trial. Int J Radiat Oncol Biol Phys. 2009;75(4):1048–52.
Gee A, Moffitt D, Churn M, Errington RD. A randomised controlled trial to test a non-metallic deodorant used during a course of radiotherapy. J Radiother Pract. 2000;1(04):205–12.
Omidvari S, Saboori H, Mohammadianpanah M, Mosalaei A, Ahmadloo N, et al. Topical betamethasone for prevention of radiation dermatitis. Indian J Dermatol Venereol Leprol. 2007;73(3):209.
Boström Å, Lindman H, Swartling C, Berne B, Bergh J. Potent corticosteroid cream (mometasone furoate) significantly reduces acute radiation dermatitis: results from a double-blind, randomized study. Radiother Oncol. 2001;59(3):257–65.
Kirova YM, Fromantin I, De Rycke Y, Fourquet A, Morvan E, Padiglione S, et al. Can we decrease the skin reaction in breast cancer patients using hyaluronic acid during radiation therapy? Results of phase III randomised trial. Radiother Oncol. 2011;100(2):205–9.
Primavera G, Carrera M, Berardesca E, Pinnaró P, Messina MGAG. A double-blind, vehicle-controlled clinical study to evaluate the efficacy of MAS065D (XClair), a hyaluronic acid-based formulation, in the management of radiation-induced dermatitis. Cutan Ocul Toxicol. 2006;25:165–71.
Liguori V, Guillemin C, Pesce GF, Mirimanoff RO, Bernier J. Double-blind, randomized clinical study comparing hyaluronic acid cream to placebo in patients treated with radiotherapy. Radiother Oncol. 1997;42(2):155–61.
Heggie S, Bryant GP, Tripcony L, Keller J, Rose P, Glendenning M, et al. A Phase III study on the efficacy of topical aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002;25(6):442–51.
Williams MS, Burk M, Loprinzi CL, Hill MSP, et al. Phase III double-blind evaluation of an aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int J Radiat Oncol Biol Phys. 1996;36:345–9.
Wells M, Macmillan M, Raab G, MacBride S, Bell N, MacKinnon K, et al. Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? A randomised controlled trial. Radiother Oncol. 2004;73(2):153–62.
Evensen JF, Bjordal K, Jacobsen AB, Løkkevik E, Tausjø JE. Effects of Na-sucrose octasulfate on skin and mucosa reactions during radiotherapy of head and neck cancers—a randomized prospective study. Acta Oncol. 2001;40(6):751–5.
Lievens Y, Haustermans K, Van den Weyngaert D, Van den Bogaert W, Scalliet P, Hutsebaut L, et al. Does sucralfate reduce the acute side-effects in head and neck cancer treated with radiotherapy? A double-blind randomized trial. Radiother Oncol. 1998;47(2):149–53.
Maiche A, Isokangas OP, Gröhn P. Skin protection by sucralfate cream during electron beam therapy. Acta Oncol. 1994;33(2):201–3.
Dale PS, Tamhankar CP, George D, Daftary GV. Co-medication with hydrolytic enzymes in radiation therapy of uterine cervix: evidence of the reduction of acute side effects. Cancer Chemother Pharmacol. 2001;47(Suppl):S29–34.
Gujral MS, Patnaik PM, Kaul R, Parikh HK, Conradt C, Tamhankar CP, et al. Efficacy of hydrolytic enzymes in preventing radiation therapy-induced side effects in patients with head and neck cancers. Cancer Chemother Pharmacol. 2001;47(Suppl):S23–8.
Halperin EC, Gaspar L, George S, Darr DPS. A double-blind, randomized, prospective trial to evaluate topical vitamin C solution for the prevention of radiation dermatitis. Int J Radiat Oncol Biol Phys. 1993;26:413–6.
Röper B, Kaisig D, Auer F, Mergen E, Molls M. Thêta-Cream® versus Bepanthol® Lotion in breast cancer patients under radiotherapy: a new prophylactic agent in skin care? Strahlentherapie und Onkol. 2004;180(5):315–22.
Lin LC, Que J, Lin LK, Lin FC. Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized study. Int J Radiat Oncol Biol Phys. 2006;65(3):745–50.
Aygenc E, Celikkanat S, Kaymakci M, Aksaray F, Ozdem C. Prophylactic effect of pentoxifylline on radiotherapy complications: a clinical study. Otolaryngol Head Neck Surg. 2004;130(3):351–6.
Niazi TM, Vuong T, Azoulay L, Marijnen C, Bujko K, Nasr E, et al. Silver clear nylon dressing is effective in preventing radiation-induced dermatitis in patients with lower gastrointestinal cancer: results from a phase III study. Int J Radiat Oncol Biol Phys. 2012;84(3):e305–10.
Aquino-Parsons C, Lomas S, Smith K, Hayes J, Lew S, Bates AT, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215–21.
Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971–8.
Lanigan SW, Joannides T. Pulsed dye laser treatment of telangiectasia after radiotherapy for carcinoma of the breast. Br J Dermatol. 2003;148(1):77–9.
Løkkevik E, Skovlund E, Reitan JB, Hannisdal E, Tanum G. Skin treatment with bepanthen cream versus no cream during radiotherapy: a randomized controlled trial. Acta Oncol. 1996;35(8):1021–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Manni Singh, Afsaneh Alavi, Rebecca Wong, and Sadanori Akita have no conflicts of interest to disclose related to this manuscript.
Funding
No funding was received for the preparation of this review.
Rights and permissions
About this article
Cite this article
Singh, M., Alavi, A., Wong, R. et al. Radiodermatitis: A Review of Our Current Understanding. Am J Clin Dermatol 17, 277–292 (2016). https://doi.org/10.1007/s40257-016-0186-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-016-0186-4