Abstract
Objective
To evaluate the comparative effects of dapagliflozin versus placebo in patients with heart failure (HF), focusing on functional capacity, symptoms, and safety outcomes.
Background
Despite advancements in heart failure (HF) therapy, HF is still a significant cause of recurrent hospitalization and death worldwide. Dapagliflozin has demonstrated potential in lowering hospitalizations and mortality associated with heart failure; however, its impact on functional capacity, particularly the 6-min walk distance (6MWD), and the comprehensive assessment of safety outcomes in diverse HF populations, including those with preserved or reduced ejection fraction (HFpEF and HFrEF, respectively), requires further investigation.
Methods
PubMed, Web of Science, Cochrane Library, and Scopus databases were comprehensively searched to identify randomized controlled trials (RCTs) investigating the efficacy of dapagliflozin in comparison with control interventions for heart failure. The primary outcome was a change in the 6MWD, KCCQ score, and safety measures included hospitalization, all-cause mortality, and adverse events.
Results
In our meta-analysis of ten studies involving 12,695 patients with heart failure, dapagliflozin showed significantly improved Kansas City Cardiomyopathy Questionnaire (KCCQ) scores [risk ratio (RR) of 2.75, 95% confidence interval (CI) (1.95–3.569), p < 0.00001] and no significant differences in 6-min walk distance [6MWD; RR of 3.59, 95% CI (− 1.44 to 8.63), p = 0.16]. Dapagliflozin demonstrated a notable reduction in hospitalization for heart failure [RR of 0.76, 95% CI (0.68–0.84), p < 0.00001], significant overall reduction on the effect of any cause mortality [RR of 0.90, 95% CI (0.83–0.99), p = 0.03). There was, however, no significant effect on adverse events [RR of 0.96, 95% CI (0.98–1.03), p = 0.39).
Conclusions
Our meta-analysis of ten trials concluded that dapagliflozin significantly improved KCCQ scores in both HFrEF and HFpEF. The improvement in 6MWD was not statistically significant but trended toward dapagliflozin. Dapagliflozin also showed a mortality benefit in patients with reduced ejection fraction; however, in patients with preserved ejection fraction, the result was not statistically significant. There was also a statistically significant reduction in heart failure hospitalizations across all classes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dapagliflozin improved the functional capacity in patients with heart failure across all classes, with improvement in KCCQ scores in these patients. |
Dapagliflozin showed a trend toward improving 6MWD; however, the results were not statistically significant. |
Dapagliflozin showed a reduction in mortality in patients with HFrEF. |
Dapagliflozin significantly reduced hospitalizations in all classes of patients with heart failure. |
1 Introduction
Despite the implementation of established therapies, heart failure (HF) remains a primary cause of recurring hospital admissions and mortality worldwide [1]. The possibility of developing HF and its consequences, including mortality, increases in the presence of type 2 diabetes mellitus (T2DM) [2, 3].
SGLT-2 inhibitors have been demonstrated to lower the likelihood of heart failure-related hospitalization in patients with T2DM [4,5,6,7].
SGLT2 inhibitors have been identified in two large trials that included ambulatory patients with heart failure with reduced ejection fraction (HFrEF) to have been effective in reducing hospitalizations for worsening heart failure (and other episodes of heart failure exacerbation), as well as the risk of death from cardiovascular events [8, 9].
Two more trials have revealed a parallel benefit associated with SGLT2 inhibitors in patients suffering from heart failure with preserved ejection fraction (HFpEF). The combined analysis of these four trials consistently demonstrates favorable outcomes across the whole range of ejection fractions [10,11,12,13]. The results of these studies led to the integration of SGLT-2 inhibitors as recommended medications for heart failure by the ACC/AHA/HFSA throughout the spectrum [14].
Improving symptoms and functional capacity are additional objectives in therapeutic interventions for heart failure, which are considered necessary by both patients and regulatory agencies [15, 16]. Symptom improvement has been observed in patients with HFrEF and HFpEF treated with SGLT2 inhibitors, as measured by the patient-reported Kansas City Cardiomyopathy Questionnaire (KCCQ). However, the persistent beneficial effects on objective markers of physical function have not been commonly recognized [8,9,10,11, 17,18,19,20].
Regarding physical activity, particularly the 6-min walk distance (6MWD), different studies reported diverse outcomes. The EMPERIAL-Reduced, EMPERIAL-Preserved found no benefit of empagliflozin with regards to improvement in symptoms as assessed by the 6MWD and KCCQ scores. [18]. In contrast, the PRESERVED-HF trial demonstrated a significant increase in 6MWD and improvement in KCCQ scores with dapagliflozin over the same period in patients with HFpEF [19]. Furthermore, 6MWD increase was not reproduced in the smaller HFrEF trial, DEFINE-HF [17].
These findings highlight conflicting results in 6 MWD and KCCQ. A recent large randomized controlled trial (RCT) on the effect of dapagliflozin with regards to symptoms in heart failure casts a new spotlight on this. In addressing a notable gap in the existing research and emphasizing the need to reconcile conflicting findings related to the impact of SGLT2 inhibitors on 6MWD and KCCQ scores, it is appropriate to have an updated systematic review. This systematic review and meta-analysis aimed to assess the comparative effects of dapagliflozin versus placebo in patients with heart failure, with a primary focus on functional capacity and physical activity including 6MWD and KCCQ score. Additionally, we will examine the safety outcomes, including all-cause mortality and HF hospitalization.
2 Methods
2.1 Eligibility Criteria
This meta-analysis included all randomized clinical trials that met our PICO criteria: population participants (P): individuals with heart failure, including those with either preserved or reduced ejection fraction; intervention (I): dapagliflozin plus standard therapy; comparator (C): standard therapy; outcomes (O): change in 6MWD, KCCQ score, any-cause mortality, hospitalization for heart failure, and adverse events.
2.2 Information Sources and Search Strategy
The PubMed, Scopus, Web of Science, and Cochrane Library databases were searched until January 2024. The primary search terms were as follows (Dapagliflozin OR “Farxiga” OR “BMS512148” OR “BMS-512148”) AND (“Heart failure” OR “Cardiac Failure” OR “Heart Decompensation” OR “Systolic heart failure” OR “Diastolic heart failure” OR “Myocardial Failure” OR “Heart Insufficiency” OR HFrEF or “Heart failure with reduced ejection fraction” OR HFpEF OR “heart failure with preserved ejection fraction”).
2.3 Selection Process:
Duplicates were eliminated using the EndNote software (Clarivate Analytics, PA). The obtained references were evaluated using two screening steps: the first involved a relevance assessment of the titles and abstracts and the second involved a full-text article screening of the selected abstracts to establish final eligibility for quantitative analysis. Two reviewers (B.A. and S.I.) independently performed title and abstract screening. Conflicts were resolved through a third author (W.A.). The selection procedure was conducted using Rayyan website, an online software that helps to expedite the initial screening of abstracts and titles and synthesize multiple RCTs [22].
2.4 Data Extraction
A predesigned extraction sheet was used to collect the following data: baseline characteristics [age, gender status (male), body mass index, and medical comorbidities such as hypertension and diabetes mellitus]; summary characteristics (study design, country, number of participants in each group, inclusion criteria, and duration of follow-up); outcome data (change in 6MDW, KCCQ score, any cause mortality, hospitalization for heart failure, any adverse events)
2.5 Risk of Bias and Quality Assessment
The revised Cochrane risk-of-bias tool for RCTs (ROB2) was used to evaluate the risk of bias in the included clinical trials was used [23]. This evaluation consistently assessed the randomization process, concealment of the allocation sequence, deviations from the intended interventions, utilization of appropriate analysis to estimate the effect of assignment to the intervention, measurement of the outcome, selection of the reported results, and overall risk of bias. Assessment of the methodological quality of the studies was classified as low risk, some concerns, or high risk of bias.
2.6 Statistical Analysis
RevMan v5.3 was used to conduct the statistical analysis [24]. The risk ratio (RR) was used to synthesize dichotomous outcomes, and the mean difference (MD) with 95% confidence interval (CI) was used to pool continuous outcomes. Chi-square and I2 tests were both used in the assessment of heterogeneity. The I2 test measured the degree of heterogeneity, while the chi-square test was used to determine whether heterogeneity existed. The Cochrane Handbook’s (chapter nine) [25] interpretation of the I2 is as follows: heterogeneity is not significant for percentages 0–40, moderate for percentages 30–60, significant for percentages 50–90, and significant for percentages 75–100. For the Chi-square test to identify significant heterogeneity, the alpha level must be less than 0.1. Leave-one-out sensitivity analysis was used to resolve heterogeneity by methodically removing each study from the pooled analysis.
3 Results
3.1 Literature Search Results
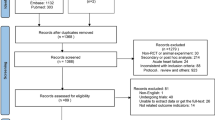
Our literature search resulted in 1201 records After removing duplicates using EndNote (Clarivate Analytics, Philadelphia, PA), we refined the dataset to 1047 records for further screening. Following a thorough full-text screening, we identified ten randomized controlled trials (RCTs) that matched the inclusion criteria for our meta-analysis, as shown in (Fig. 1). This meta-analysis was reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta- Analysis (PRISMA) statement [26].
3.2 Characteristics of the Included Studies
In our comprehensive meta-analysis, we incorporated ten studies [8, 11, 17, 19, 21, 25, 27,28,29,30,31], encompassing a sizable population of 12,695 individuals presenting with heart failure. This study included patients with both reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF), all of whom received dapagliflozin in comparison with a control group receiving either placebo or standard therapy. The dapagliflozin group comprised a large number of participants (n = 6350), whereas the control group consisted of 6345 participants. Two studies [8, 31] initially focused on patients with chronic kidney disease (CKD) or type 2 diabetes but were incorporated based on their preplanned analysis of outcomes concerning the presence of heart failure at baseline. The primary outcomes were changes in 6-min walk distance (6MWD) and Kansas City Cardiomyopathy Questionnaire (KCCQ) scores. The secondary outcomes were all-cause mortality, hospitalization for heart failure, and adverse events. A summary of the included studies and the baseline characteristics of the participants are provided in Table 1 and Table 2, respectively.
3.3 Risk of Bias Assessment
According to the Cochrane RoB2, nine randomized clinical trials had an overall some concern risk of bias and two studies had a low risk of bias, as shown in the risk of bias graph (Fig. 2)
3.4 Primary Outcome:
3.4.1 Changes in 6-min walk distance (6MWD)
The overall analysis did not show a significant difference in changes in 6-min walk distance (6MWD) between the dapagliflozin and control groups [RR of 3.59, 95% confidence interval (CI) (− 1.44 to 8.63), p = 0.16). Pooled analysis demonstrated homogeneity (I2 = 37%, p = 0.17). Regarding subgroup analysis, no significant difference in changes in 6MWD was observed between the dapagliflozin and control groups for patients with HFpEF and HFrEF [RR = 0.87, 95% CI (− 5.68 to 7.42), p = 0.79] and [RR of 7.52, 95% CI (− 0.35 to 15.39), p = 0.06, respectively] (Fig. 3).
3.4.2 Changes in Kansas City Cardiomyopathy Questionnaire (KCCQ) Scores
The overall analysis demonstrated a statistically significant improvement in (KCCQ) scores for patients receiving dapagliflozin compared with the control group [RR of 2.75, 95% CI (1.95–3.56), p < 0.00001]. Pooled analysis demonstrated homogeneity (I2 = 0%, p = 0.81). In the subgroup analysis, the improvement in the KCCQ score was noticed in both heart failure groups. In the HFpEF group, the improvement in KCCQ score was associated with a RR of 1.76, 95% CI (0.18–3.33), p = 0.03, while in the HFrEF group when compared with placebo, RR of 3.10, 95% CI (2.17–4.04), p < 0.00001 (Fig. 4).
3.5 Secondary Outcome
3.5.1 Hospitalization for HF
The analysis revealed a significant reduction in the risk of hospitalization for heart failure in patients receiving dapagliflozin compared with that in the control group [RR = 0.76, 95% CI (0.68–0.84), p < 0.00001). Pooled analysis demonstrated homogeneity (I2 = 0%, p = 0.86) (Fig. 5).
3.5.2 Any Cause of Mortality
The overall analysis also revealed a statistically significant difference in the all-cause mortality between dapagliflozin and control groups [RR = 0.90, 95% CI (0.83–0.99), p = 0.03). Pooled analysis demonstrated homogeneity (I2 = 0%, p = 0.87). In the subgroup analysis, the reduction in all-cause mortality was also observed in the HFrEF group. In the HFrEF subgroup, when compared with placebo, RR of 0.84, 95% CI (0.72–0.97), p = 0.02. This mortality benefit was not seen in the HFpEF group, when compared with placebo [RR = 0.94, 95% CI (0.84–1.06), p = 0.31) (Fig. 6).
3.5.3 Any Adverse Events
The overall analysis demonstrated no statistically significant reduction in the risk of adverse events in patients receiving dapagliflozin compared with the control group [RR of 0.98, 95% CI (0.93–1.03), p = 0.39]. Pooled analysis demonstrated homogeneity (I2 = 6.9%, p = 0.30). The subgroup analysis did not reveal a significant difference between the two groups for patients with HFrEF and HFpEF ([RR = 1.03, 95% CI (0.92–1.16), p = 0.61] and [RR = 0.96, 95% CI (0.91–1.02), p = 0.17], respectively) (Fig. 7).
4 Discussion
4.1 Summary of Main Results
This meta-analysis aims to assess the effect of dapagliflozin on functional capacity in patients with heart failure, considering both reduced and preserved ejection fractions. The findings suggest dapagliflozin was associated with a notable improvement in KCCQ scores across all heart failure classes. With regards to 6MWD, though not statistically significant in either subgroup, there was a trend toward the dapagliflozin group. The mortality benefit of dapagliflozin was only seen in the heart failure with reduced EF subclass. The was no statistically significant reduction in adverse events in either subgroup. Consistency prevailed in the results across all the studies included, and there was statistical homogeneity in the analysis of various outcomes.
4.2 Justification of Results
4.2.1 Importance of Using Dapagliflozin for Patients with Heart Failure and Related Adverse Events
The effect of dapagliflozin in individuals with heart failure with and without diabetes has been widely acknowledged.
By targeting SGLT-2 receptors in the kidney, dapagliflozin induces osmotic diuresis of glucose, along with natriuresis and water loss. This process contributes to a decreased preload on the heart in patients with heart failure [32]. An additional potential mechanism is the impact of dapagliflozin on afterload through its interaction with the endothelium, which primarily leads to a decrease in vascular resistance, ultimately lowering afterload [33]. The optimization of myocardial substrate utilization, which leads to an increase in cardiac output, is facilitated by SGLT2 inhibitors. This is achieved through the reduction of cardiac carbohydrate uptake and augmentation in the uptake of β-hydroxybutyrate and ketone bodies [34]. In addition to its anti-inflammatory effects, this drug class also plays a role in preventing myocardial fibrosis [35]. In clinical applications, dapagliflozin is associated with various adverse effects such as mycotic infections in the genital tract and urinary tract infections [36]. Other side effects associated with dapagliflozin include hypotension, hypoglycemia, and reduction in serum uric acid levels [37]. An association between SGLT2 inhibitors and diabetic ketoacidosis (DKA) was noted in the DAPA-HF trial, where every case of DKA occurred exclusively in patients with T2DM. The incidence of DKA was reported to be 0.1% with dapagliflozin and 0% with placebo [38].
4.2.2 Understanding the Variations in 6MWD in Patients with Heart Failure using Dapagliflozin
Contrary to the recognized benefits of dapagliflozin in various aspects of heart failure such as mortality, hospitalization, and adverse events, our analysis did not reveal a significant difference in 6MWD between the dapagliflozin and control groups.
For many years, the 6MWD has been used as a standard to evaluate capacity for function in heart failure (HF). Nevertheless, using this measure to evaluate the implications of SGLT2 inhibitors resulted in interesting results.
The uncertainty of established successful medicines, such as sacubitril/valsartan, and the failure to correlate 6MWD improvements with survival benefits represents a comparative issue. This pattern remains in the HFpEF studies (DETERMINE-Preserved and EMPERIAL-Preserved) emphasizing 6MWD’s limitations of [18, 21, 39]. In contrast, the PRESERVED-HF trial demonstrated a significant increase in the 6MWD with dapagliflozin [19]. However, this improvement is contrasted by lower baseline limitations in the KCCQ and 6MWD compared with the DETERMINE and EMPERIAL trials [18, 21], highlighting the potential influence of baseline characteristics on responsiveness to SGLT2 inhibitors.
4.2.3 Improvement of Kansas City Cardiomyopathy Questionnaire (KCCQ) Score
Our meta-analysis revealed that dapagliflozin was associated with an improvement in the KCCQ score compared with the control group. Additionally, a subgroup analysis based on HF type of heart failure was conducted.
In the HFrEF subgroup, self-reported symptoms, as assessed by the KCCQ total symptom score (TSS), showed a median improvement in the DETERMINE-Reduced Trials [21]. This improvement aligns with findings from other trials, including DAPA-HF and smaller trials [17]. Collective evidence suggests that dapagliflozin leads to improvement in patient-reported symptoms, which persist for at least 12 months and beyond. There was an improvement in KCCQ physical limitation score (PLS) in the DEFINE-HF and DAPA-HF trials. This score assesses limitations in routine activities such as dressing, showering, walking, and housework [8, 17]. Although KCCQ-PLS has not been consistently demonstrated, in comparison with KCCQ-TSS, the cumulative data suggest a modest enhancement of KCCQ-PLS by SGLT2 inhibitors in individuals with (HFrEF).
In the HFpEF subgroup, no significant effect on KCCQ-TSS was observed in DETERMINE-Preserved, aligning with DELIVER and PRESERVED-HF. This is similar to the finding seen with empagliflozin in the EMPEROR-Preserved, and EMPERIAL-Preserved [10,11,18,19,21,]. The KCCQ-PLS score indicated no significant difference between the two groups in DETERMINE-Preserved [21], consistent with the findings in EMPEROR-Preserved [10]. However, this contrasts with the PRESERVED-HF [19] trial where dapagliflozin significantly improved the KCCQ-PLS score. This systematic review shows a trend toward improvement in KCCQ score in patients with HFpEF.
To sum up, these findings clearly suggested dapagliflozin was associated with improvement in KCCQ score in two heart failure types but with a pronounced effect in HFrEF. It remains uncertain whether these findings truly indicate a lesser impact of dapagliflozin on KCCQ scores in patients with HFpEF or if the study’s power was constrained by the relatively small sample size.
4.2.4 Safety Outcomes of Dapagliflozin in Heart Failure
Regarding hospitalization for heart failure, the findings from our meta-analysis underscore the demonstrated impact of dapagliflozin on safety outcomes in patients with HF. The observed significant reduction in the risk of hospitalization for heart failure aligns with previous evidence [6, 8, 11] suggesting the efficacy of dapagliflozin in preventing HF-related events.
Regarding the any-cause mortality, a thorough assessment within the subgroup analysis showed a notable reduction in the risk of all-cause mortality in patients with HFrEF. Despite not obtaining a similar finding in the HFpEF group, our analysis found a statistically significant difference in overall any-cause mortality between the dapagliflozin and control groups. The favorable response of a specific subgroup to dapagliflozin indicated that the drug may be useful in reducing mortality risks in a well-defined population.
Regarding the risk for adverse events, there were no significant difference in adverse events between the patients with HFrEF and HFpEF according to the subgroup analyses and the overall comparison with placebo.
4.3 Agreement and Disagreement with Previous Studies
4.3.1 Agreement
Our comprehensive study and the most recent meta-analysis [40] revealed a consistently favorable impact of dapagliflozin. Specifically, both highlighted a noteworthy reduction in all-cause mortality, a significant decrease in the overall risk of adverse events, and a substantial reduction in hospitalization for heart failure among patients receiving dapagliflozin. This systematic review went a step further to illustrate important symptom benefits of dapagliflozin.
4.3.2 Disagreement
Our study comprehensively assessed the safety profile of dapagliflozin in patients with heart failure, examining not only its safety but also its effectiveness in enhancing both the functional capacity of the heart and overall physical function.
In comparison to past meta-analyses [40], For the change in 6MWD, our study provides the most updated evidence regarding the effect of dapagliflozin on 6MWD, which previous meta-analysis did not provide. Moreover, while analyzing the differences in KCCQ scores, it is important to keep in mind that although both studies show improvement, there are variations concerning the degree of improvement shown in the HFpEF and HFrEF. Furthermore, it is essential to take into consideration the particular scales that are utilized for measurement, such as KCCQ-TSS and KCCQ-PLS. In our study, each of these measurements was covered in detail.
4.4 Strength Points and Limitations
Our meta-analysis has various advantages: the study provided a comprehensive assessment and thorough evaluation of dapagliflozin’s impact on heart failure, considering both HFrEF and HFpEF, and explores various outcomes, including symptoms, functional capacity, hospitalizations, mortality, and adverse events. Symptoms and functional capacity of our patients gets overlooked and this meta-analysis suggest that despite the mortality benefits of dapagliflozin, there is also a symptom improvement as assessed by the KCCQ score. In addition, all clinical trials in which patients with heart failure were the initial study population or whose population randomization was predetermined based on whether or not heart failure was present at baseline were included.
Furthermore, the study conducted subgroup analyses based on heart failure type, providing valuable insights into the differential effects of dapagliflozin in patients with HFrEF and HFpEF.
This meta-analysis has various limitations, and certain included studies exhibited incomplete information concerning the baseline characteristics of patients. Some of the studies included in the analysis had limited sample sizes, particularly in the context of HFpEF, potentially limit the generalizability of findings for this specific subgroup. The fact that there is a statistical benefit in KCCQ scores in all classes of heart failure and that significance is not seen in the 6MWD does bring into question the importance of this metric in assessing patients with heart failure.
Moreover, the primary emphasis of the study was on outcomes over the short to medium term, indicating that investigations with prolonged follow-up periods would be valuable for understanding the sustained effects of dapagliflozin.
4.5 Implications for Clinical Practice
The study suggests that dapagliflozin is valuable addition to the management of heart failure, offering benefits in symptom improvement, reduced hospitalizations, and favorable safety profiles. Furthermore, the differential response observed between HFrEF and HFpEF suggest these benefits are better seen in the HFrEF subtype.
4.6 Recommendations for Future Researchers
To overcome the limitation of diversity in trial outcomes emphasizes the importance of considering patient characteristics and baseline limitations when evaluating the efficacy of a treatment. Larger trials can be carried out to especially focus on the benefit of dapagliflozin in the subtype of patients with preserved EF.
4.7 Conclusions
Our comprehensive meta-analysis, which included ten randomized controlled trials with patients treated with dapagliflozin and diagnosed with heart failure, concludes that dapagliflozin shows significant improvements in KCCQ scores, a notable decrease in hospitalizations, and any cause of mortality across all ranges of heart failure. Consistent benefits for both HFrEF and HFpEF are emphasized in the study. However, it is crucial to acknowledge that no significant difference was noted between the two groups in terms of changes in 6MWD.
References
Metra M, Carubelli V, Ravera A, Stewart Coats AJ. Heart failure 2016: still more questions than answers. Int J Cardiol. 2016;227:766–77. https://doi.org/10.1016/j.ijcard.2016.10.060.
Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64(21):2281–93. https://doi.org/10.1016/j.jacc.2014.08.036.
Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29–34. https://doi.org/10.1016/0002-9149(74)90089-7.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. https://doi.org/10.1056/NEJMoa1611925.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. https://doi.org/10.1056/NEJMoa1812389.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. https://doi.org/10.1056/NEJMoa1811744.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, DAPA-HF Trial Committees and Investigators, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. https://doi.org/10.1056/NEJMoa1911303.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, EMPEROR-Reduced Trial Investigators, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. https://doi.org/10.1056/NEJMoa2022190.
Anker SD, Butler J, Filippatos G, Shahzeb Khan M, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, EMPEROR-Preserved Trial Committees and Investigators, et al. Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR-Preserved trial. Eur J Heart Fail. 2020;22:2383–92. https://doi.org/10.1002/ejhf.2064.
Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, DELIVER Trial Committees and Investigators, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–98. https://doi.org/10.1056/NEJMoa2206286.
Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, Vaduganathan M, Gasparyan SB, Bengtsson O, Lindholm D, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022;28:1956–64. https://doi.org/10.1038/s41591-022-01971-4.
Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive metaanalysis of five randomised controlled trials. Lancet. 2022;400:757–67. https://doi.org/10.1016/S0140-6736(22)01429-5.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 ACC/AHA/HFSA Guideline for the Management of Heart Failure. J Cardiac Fail. 2022;28(5):e1–167. https://doi.org/10.1016/j.cardfail.2022.02.009.
Fiuzat M, Lowy N, Stockbridge N, Sbolli M, Latta F, Lindenfeld J, Lewis EF, Abraham WT, Teerlink J, Walsh M, et al. Endpoints in heart failure drug development: history and future. JACC Heart Fail. 2020;8:429–40. https://doi.org/10.1016/j.jchf.2019.12.011.
Zannad F, Garcia AA, Anker SD, Armstrong PW, Calvo G, Cleland JG, Cohn JN, Dickstein K, Domanski MJ, Ekman I, et al. Clinical outcome endpoints in heart failure trials: a European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail. 2013;15:1082–94. https://doi.org/10.1093/eurjhf/hft095.
Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. 2019;140:1463–76. https://doi.org/10.1161/1161/CIRCULATIONAHA.119.042929.
Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42:700–10. https://doi.org/10.1093/eurheartj/ehaa943.
Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–60. https://doi.org/10.1038/s41591-021-01536-x.
Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J, Lanfear DE, Lingvay I, Kosiborod MN, Januzzi JL. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28:809–13. https://doi.org/10.1038/s41591-022-01703-8.
McMurray JJV, Docherty KF, de Boer RA, Hammarstedt A, Kitzman DW, Kosiborod MN, Maria Langkilde A, Reicher B, Senni M, Shah SJ, Wilderäng U, Verma S, Solomon SD. Effect of dapagliflozin versus placebo on symptoms and 6-minute walk distance in patients with heart failure: the DETERMINE Randomized Clinical Trials. Circulation. 2023. https://doi.org/10.1161/CIRCULATIONAHA.123.065061(Epub ahead of print. PMID: 38059368).
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:4l4898. https://doi.org/10.1136/bmj.l4898.
Collaboration TCC. RevMan. Oxford, UK: The Cochrane Collaboration; 2014.
Chapter 9: Summarizing study characteristics and preparing for synthesis | Cochrane Training. Available at: https://training.cochrane.org/handbook/current/chapter-09. Accessed 25 Dec 2023.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Borlaug BA, Reddy YNV, Braun A, Sorimachi H, Omar M, Popovic D, Alogna A, Jensen MD, Carter R. Cardiac and metabolic effects of dapagliflozin in heart failure with preserved ejection fraction: the CAMEO-DAPA Trial. Circulation. 2023;148(10):834–44. https://doi.org/10.1161/CIRCULATIONAHA.123.065134(Epub 2023 Aug 3. PMID: 37534453; PMCID: PMC10529848).
Ibrahim A, Ghaleb R, Mansour H, Hanafy A, Mahmoud NM, Abdelfatah Elsharef M, Kamal Salama M, Elsaughier SM, Abdel-Wahid L, Embarek Mohamed M, Ibrahim AK, Abdel-Galeel A. Safety and efficacy of adding dapagliflozin to furosemide in type 2 diabetic patients with decompensated heart failure and reduced ejection fraction. Front Cardiovasc Med. 2020;7(7):602251. https://doi.org/10.3389/fcvm.2020.602251(PMID:33426003;PMCID:PMC7793915).
Palau P, Amiguet M, Domínguez E, Sastre C, Mollar A, Seller J, Garcia Pinilla JM, Larumbe A, Valle A, Gómez Doblas JJ, de la Espriella R, Miñana G, Mezcua AR, Santas E, Bodí V, Sanchis J, Pascual-Figal D, Górriz JL, Baýes-Genís A, Núñez J, DAPA-VO2 Investigators (see Appendix). Short-term effects of dapagliflozin on maximal functional capacity in heart failure with reduced ejection fraction (DAPA-VO2): a randomized clinical trial. Eur J Heart Fail. 2022;24(10):1816–26. https://doi.org/10.1002/ejhf.2560(Epub 2022 Jun 6. PMID: 35604416).
Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, Choy AMJ, Gandy S, George J, Khan F, Pearson ER, Houston JG, Struthers AD, Lang CC. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care. 2020;43(6):1356–9. https://doi.org/10.2337/dc19-2187(Epub 2020 Apr 3. PMID: 32245746; PMCID: PMC7245350).
McMurray JJV, Wheeler DC, Stefánsson BV, Jongs N, Postmus D, Correa-Rotter R, Chertow GM, Hou FF, Rossing P, Sjöström CD, Solomon SD, Toto RD, Langkilde AM, Heerspink HJL, DAPA-CKD Trial Committees and Investigators. Effects of dapagliflozin in patients with kidney disease, with and without heart failure. JACC Heart Fail. 2021;9(11):807–20. https://doi.org/10.1016/j.jchf.2021.06.017(Epub 2021 Aug 23. Erratum in: JACC Heart Fail. 2022 Jun;10(6):446-447. PMID: 34446370).
Pabel S, Hamdani N, Luedde M, Sossalla S. SGLT2 Inhibitors and Their Mode of Action in Heart Failure-Has the Mystery Been Unravelled? Curr Heart Fail Rep. 2021;18(5):315–28. https://doi.org/10.1007/s11897-021-00529-8.
Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310. https://doi.org/10.1016/j.freeradbiomed.2017.01.035.
Zhai M, Du X, Liu C, Xu H. The effects of dapagliflozin in patients with heart failure complicated with type 2 diabetes: a meta-analysis of placebo-controlled randomized trials. Front Clin Diabetes Healthc. 2021;2: 703937. https://doi.org/10.3389/fcdhc.2021.703937.
Anderson SL. Dapagliflozin efficacy and safety: a perspective review. Ther Adv Drug Saf. 2014;5(6):242–54. https://doi.org/10.1177/2042098614551938.
Anitha AP, Balasubramanian S, Ramalingam AG, Samuel Kennady SR, Ganamurali N, Dhanasekaran D, et al. An exploration of the experience of dapagliflozin in clinical practice. Future Sci OA. 2022;8(8):Fso816. https://doi.org/10.2144/fsoa-2022-0038.
Blair HA. Dapagliflozin: A review in symptomatic heart failure with reduced ejection fraction. Am J Cardiovasc Drugs. 2021;21(6):701–10. https://doi.org/10.1007/s40256-021-00503-8.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. https://doi.org/10.1056/NEJMoa2024816.
Piepoli MF, Hussain RI, Comin-Colet J, Dosantos R, Ferber P, Jaarsma T, Edelmann F. OUTSTEP-HF: randomised controlled trial comparing shortterm effects of sacubitril/valsartan versus enalapril on daily physical activity in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. 2021;23:127–35. https://doi.org/10.1002/ejhf.2076.
Ali AE, Mazroua MS, ElSaban M, Najam N, Kothari AS, Mansoor T, Amal T, Lee J, Kashyap R. Effect of dapagliflozin in patients with heart failure: a systematic review and meta-analysis. Glob Heart. 2023;18(1):45. https://doi.org/10.5334/gh.1258.PMID:37636033;PMCID:PMC10453961.
Acknowledgements
We acknowledge the assistance of Hanaa Ismail in drafting and proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of Interest
B.A., W.A., S.I., and P.B. declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Data Availability
The datasets generated during and /or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author’s Contributions
B.A.: conceptualization, supervision, validation, writing, and reviewing. W.A.: writing, reviewing, and methodology. S.I.: analysis and investigation. P.B.: writing and reviewing.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Addo, B., Agyeman, W., Ibrahim, S. et al. Dapagliflozin in Heart Failure: A Comprehensive Meta-analysis on Functional Capacity, Symptoms, and Safety Outcomes. Am J Cardiovasc Drugs (2024). https://doi.org/10.1007/s40256-024-00669-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s40256-024-00669-x