Abstract
Background
Improving health-related quality of life (HRQoL) is essential in treating heart failure (HF). Evidence of sodium-glucose cotransporter-2 (SGLT-2) inhibitors on HRQoL and exercise capacity needs to be systematically analyzed.
Aim
This meta-analysis aimed to summarize the effects of SGLT-2 inhibitors on HRQoL, exercise capacity, and volume depletion in patients with HF.
Method
Randomized controlled trials were searched from PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials. The intervention arm was the SGLT-2 inhibitor group, and the control group was the placebo group. HRQoL outcomes were the Kansas City Cardiomyopathy Questionnaires (KCCQ)-OSS (Overall Summary Score), KCCQ-CSS (Clinical Summary Score), and KCCQ-TSS (Total Symptom Score). Exercise capacity was a 6-min walk test distance (6MWTD). The last search was conducted in May 2022. Two researchers independently screened articles, extracted data, and evaluated the quality of included trials. The Cochrane risk-of-bias tool was used to assess the quality of each study. Random or fixed-effect models were used in statistical methods. I2 statistics were used to assess heterogeneity.
Results
Eight studies (6,213 patients) were included. Compared to the placebo group, SGLT-2 inhibitors significantly improved HRQoL parameters of the KCCQ-CSS score [mean difference (MD) 5.17, 95% confidence interval (95% CI) 4.61–5.73, P < 0.01] and the KCCQ-OSS score (MD 4.00, 95% CI 3.44–4.56, P < 0.01). SGLT-2 inhibitors also significantly improved exercise capacity 6MWTD (MD 21.90, 95% CI 6.54–37.25, P = 0.005). There were no significant differences in KCCQ-TSS (MD 1.95, 95% CI − 1.10 to 5.01, P = 0.21) and volume depletion [odds ratio (OR) 1.15, 95% CI 0.94–1.42, P = 0.18] between the treatment and placebo groups.
Conclusion
SGLT-2 inhibitors could improve HRQoL and exercise capacity in patients with chronic HF. SGLT-2 inhibitors did not have an impact on volume depletion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
Improving health-related quality of life (HRQoL) is an important outcome for treating heart failure.
-
This meta-analysis of randomized controlled trials indicates that SGLT-2 inhibitors have benefits in improving HRQoL in patients with heart failure.
-
The meta-analysis also shows that SGLT-2 inhibitors can improve exercise capacity, as measured by the 6-min walk test distance in patients with heart failure.
Introduction
Heart failure (HF) is a clinical syndrome with signs or symptoms caused by a cardiac abnormality with elevated levels of natriuretic peptides or objective evidence of cardiogenic congestion. HF is associated with other cardiovascular diseases [1, 2]. More than 33.7 million people suffer from heart failure worldwide. HF has become a global public health problem characterized by high morbidity, mortality, and hospitalization rates [3, 4]. HF patients have markedly altered quality of life (QoL) compared to other chronic diseases and healthy populations [1, 4]. Poor health-related quality of life (HRQoL) is associated with adverse long-term prognoses, such as hospitalization and mortality, in patients with HF [5,6,7,8]. Maintaining a good QoL is as important as survival for most patients living with HF. Therefore, it is critical to improving the QoL in patients with HF.
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are novel hypoglycemic agents that block renal reabsorption of glucose. Studies have shown that SGLT-2 inhibitors improve the outcome of HF. The protective effect of SGLT-2 inhibitors on HF is independent of the reduction in blood glucose [9,10,11,12,13]. Early use of SGLT-2 inhibitors reduces hospitalization and mortality, and improves QoL and health status [14,15,16,17]. A systematic review and meta-analysis evaluated the effects of SGL-2 inhibitors on health-related QoL (HRQoL) in patients with HF and reduced ejection fraction (HFrEF) [18]. The meta-analysis included nine studies (n = 9,428) up to March 20, 2021. HRQoL was evaluated using Kansas City Cardiomyopathy Questionnaires (KCCQ), the rate of improvement of KCCQ ≥ 5 points, the rate of deterioration of KCCQ ≥ 5 points, and the exercise capacity of the 6-min walk test distance (6MWTD) [18]. SGLT-2 inhibitors significantly improved HRQoL as measured by KCCQ scores [mean difference (MD) 2.13, 95% confidence interval (95% CI) 1.11–3.14, P < 0.001]. The rate of KCCQ-overall summary score ≥ 5 points [relative risk (RR) 1.15, 95% CI 1.08–1.21, P < 0.001]. No significant differences in 6MWTD (MD 24.45, 95% CI − 22.82 to 71.72, P = 0.31) between the SGLT-2 and placebo groups [18].
SGLT-2 inhibitors can affect hemodynamics and significantly reduce blood pressure [13, 19]. Few studies have reported the effects of SGLT-2 inhibitors on volume depletion in patients with HF [20,21,22]. In patients with HFrEF, dapagliflozin caused volume depletion of 10.1% in patients with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2 and 5.8% in patients with eGFR > 60 mL/min/1.73m2 [21]. The adverse effect of volume depletion occurred in 6.8% of patients with HF with preserved ejection fraction (HFpEF) treated with dapagliflozin [20]. Volume depletion events occurred in 12.1% of patients in the empagliflozin group compared to 6.3% in the placebo group [22]. In one study [18], HFrEF was the main study population, and volume depletion was not discussed. However, no systematic review or meta-analysis has evaluated the effects of SGLT-2 inhibitors on HRQoL and volume depletion in patients with chronic HF regardless of ejection fraction. Therefore, it is necessary to further investigate the impact of SGLT-2 inhibitors on HF with an updated meta-analysis.
Aim
This meta-analysis aimed to summarize the effects of SGLT-2 inhibitors on HRQoL, exercise capacity, and volume depletion in patients with HF.
Method
Protocol and registration
This meta-analysis was performed according to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [23]. The protocol for this meta-analysis was registered with PROSPERO (the International Prospective Register of Systematic Reviews), registration number CRD42022335503.
Search strategy
Data were reviewed from PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials. Detailed search terms are shown in Supplemental Table 1. Reference lists of included studies were manually checked for potentially eligible studies. Google Scholar search engines were used to identify additional references. All included studies were published in English. The last search was performed on May 12, 2022.
Inclusion and exclusion criteria
Studies that met the following criteria were included: (1) patients ≥ 18 years of age with chronic HF (HFrEF or HFpEF), (2) randomized, double-blind, placebo-controlled trials, (3) compared SGLT-2 inhibitors with placebo, and (4) reported any of the following outcomes: KCCQ-OSS (overall summary score), KCCQ-CSS (clinical summary score), KCCQ-TSS (total symptom score), 6MWTD, and volume depletion. Review articles and animal experiments were excluded.
Assessment of risk of bias
The Cochrane risk-of-bias quality assessment tool was used to assess the quality of the included randomized controlled trials. The following seven categories were evaluated: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective outcome reporting, and other biases [24]. The quality of each study was assessed as “low risk of bias,” “high risk of bias,” or “unclear risk of bias.” The quality assessment was completed independently by two researchers (YW and ZG). A third investigator (JY) was invited to resolve any inconsistencies.
Data extraction
Two researchers (LW and ZG) independently screened the literature against the inclusion criteria. A third researcher (CZ) was invited if there was any dispute. Data extracted from each study included author, year, registry number, group, dose, sample size, population, length of follow-up, sex, and age. The HRQoL outcomes (KCCQ-OSS, KCCQ-CSS, KCCQ-TSS, 6MWTD) and volume depletion were also extracted.
Statistical analyses
Mean differences (MD) with 95%CIs were used to measure the effect size for continuous variable outcomes. Odds ratios (OR) with 95%CIs were used to measure the effect size for the outcomes of dichotomous variables. The Higgins I2 test was used for statistical heterogeneity. I2 < 50% indicates low heterogeneity, 50 ≤ I2 ≤ 75% indicates moderate heterogeneity, and I2 ≥ 75% indicates high heterogeneity [25]. A fixed-effect model was used if I2 < 50% otherwise a random-effect model was used. The source of heterogeneity was explored by subgroup or sensitivity analysis when I2 ≥ 50%. Funnel plots were used to assess publication bias [26]. The meta-analysis was performed using RevMan (version 5.3). P < 0.05 was considered statistically significant.
Results
Search results and basic information on the included studies
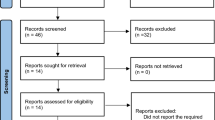
A total of 1,952 articles were obtained in the initial review. After reading the titles, abstract, and full texts according to the inclusion and exclusion criteria, eight studies were included in the analysis. The literature selection process is shown in Fig. 1.
These studies included 6,213 patients. There were three dapagliflozin trials enrolling 5,321 patients [20, 21, 27], four empagliflozin trials enrolling 444 patients [22, 28,29,30], and one canagliflozin trial with 448 patients [31]. The follow-up times ranged from 12 weeks to 27.8 months. The mean age ranged from 59.9 to 71 years. Regarding the patient population, one study was HFpEF patients [20], one was HFrEF (EF 40% or HFpEF) [22], and one was HF (irrespective of EF) [31]. The baseline characteristics of the included studies are shown in Table 1.
Assessment of risk of bias
The risk of bias assessment is shown in Fig. 2. The risk of bias was low to moderate without high risk. Seven RCTs were unclear about allocation concealment (selection bias) and blinding participants and personnel (performance bias). Eight RCTs were unclear in terms of selective reporting (reporting bias).
Health-related quality of life analysis
Five studies reported KCCQ-CSS [20, 22, 27, 28, 31]. There was no statistical heterogeneity between the studies (P = 0.14, I2 = 42%). A fixed-effects model was used for meta-analysis. Compared to the placebo group, the SGLT-2 inhibitor treatment group had a significantly higher KCCQ-CSS score (MD 5.17, 95% CI 4.61–5.73, P < 0.01) (Fig. 3A). Five studies reported KCCQ-OSS [20, 22, 27, 28, 31]. The studies had no statistical heterogeneity (P = 0.42, I2 = 0%). A fixed-effects model was used for meta-analysis. Compared to the placebo group, the SGLT-2 inhibitor treatment group had a significantly higher KCCQ-OSS score (MD 4.00, 95% CI 3.44–4.56, P < 0.01) (Fig. 3B). Three studies reported KCCQ-TSS [28, 30, 31]. The studies had no statistical heterogeneity (P = 0.22, I2 = 33%). A fixed-effects model was used for meta-analysis. There were no significant differences in the KCCQ-TSS scores between the SGLT-2 inhibitor treatment and placebo groups (MD 1.95, 95% CI 1.10–5.01, P = 0.21) (Fig. 3C).
Exercise capacity outcome and impact on volume depletion
Five studies reported 6MWTD [20, 22, 27, 29, 30]. There was statistical heterogeneity among the studies (P < 0.01, I2 = 93%). A random-effects model was used for meta-analysis. Compared to the placebo group, the SGLT-2 inhibitor treatment group significantly improved 6MWTD (MD 21.90, 95% CI 6.54–37.25, P = 0.005) (Fig. 4A).
Three studies examined volume depletion [20, 21, 28]. The studies had no statistical heterogeneity (P = 0.49, I2 = 0%). A fixed-effects model was used for meta-analysis. Compared to the placebo group, there were no significant differences in volume depletion between the SGLT-2 inhibitor treatment and the placebo groups (OR 1.15, 95% CI 0.94–1.42, P = 0.18) (Fig. 4B).
Publication bias
The funnel plots of KCCQ-OSS and 6MWTD showed an asymmetric distribution of the studies (Supplemental Figs. 1A, B). A slight asymmetry was observed in the KCCQ-CSS funnel plot (Supplemental Fig. 1C). The funnel plot analysis was not performed for KCCQ-TSS and volume depletion as there were too few studies.
Additional analysis
There was a high degree of heterogeneity in the 6MWTD analysis, I2 = 93%. Subgroup analysis was performed based on diabetes and ejection fraction. In the subgroup analysis of patients with diabetes, heterogeneity was not substantially reduced (Supplemental Fig. 2A). However, in the subgroup analysis for the ejection fraction, no heterogeneity was observed when EF ≤ 40% (Supplemental Fig. 2B). After excluding one study [27], the result demonstrated that there was no difference compared to the control group (303.7 vs. 301.3, P = 0.79), and the heterogeneity in the sensitivity analysis decreased from 93 to 74% (Supplemental Fig. 2C). Although there still exists high heterogeneity after excluding each study one by one, the results tended to be consistent. The influence of heterogeneity on the reliability of the results was small (Supplemental Table 2). Therefore, the primary source of heterogeneity was due to inconsistency in the category of chronic HF.
Discussion
Several large randomized trials have demonstrated cardiovascular protective effects of SGLT-2 inhibitors, such as reduced hospitalization and mortality in heart failure, which go beyond hypoglycemic effects [32,33,34]. The cardiovascular protective mechanism of SGLT-2 inhibitors involves many aspects, such as inhibiting cardiac Na + /H + exchange activity, reducing preload and afterload, and improving myocardial metabolism [10]. HRQoL is closely related to the prognosis of heart failure. Most meta-analyses analyze the effects of SGLT-2 inhibitors on cardiovascular protection, hospitalization, or mortality in patients with HF [35, 36]. Few trials have evaluated the impact of SGLT-2 inhibitors on QoL in heart failure, which calls for a rethink of endpoints for HF studies [14, 37]. Furthermore, the evidence for the effects of SGLT-2 inhibitors on the improvement of HRQoL and exercise capacity seems to be conflicting [18]. In this meta-analysis, SGLT-2 inhibitors improved HRQoL indicators of KCCQ-OSS and KCCQ-CSS in patients with chronic HF regardless of the ejection fraction. The results are similar to the previous meta-analysis in patients with HF failure with reduced EF [18].
Possible adverse effects should be considered when using SGLT-2 inhibitors for cardioprotection. SGLT-2 inhibitors can reduce volume and blood pressure when therapy starts. Volume depletion is significant in type 1 diabetes. The risk of infection, amputation, volume depletion, and diabetic ketoacidosis is higher in patients with cardiovascular disease [38,39,40]. The present meta-analysis is the first to analyze the impact of SGLT-2 inhibitors on volume depletion in patients with HF. Analysis showed that SGLT-2 inhibitors did not significantly increase the risk of volume depletion in patients with HFrEF or HFpEF. Although volume overload is one of the symptoms of HF, volume reduction is not an important therapeutic goal in chronic HF. Therefore, the adverse effects of volume depletion caused by SGLT-2 inhibitors should be further explored [41].
Unlike the previous meta-analysis [18], this meta-analysis found that SGLT2 inhibitors significantly improved exercise capacity, as measured by 6MWTD, in patients with chronic HF. Inconsistent results could be due to (1) the current analysis included both HFrEF and HFpEF patients instead of just HFrEF patients, and (2) the included trials had longer follow-up periods (up to 6 months) instead of 12 weeks, and the time was updated compared to the previous analysis. The improvement of exercise capacity became evident in HF patients with milder symptoms and over longer periods [18].
To our knowledge, this meta-analysis is the first quantitative analysis of QoL and volume depletion in patients with heart failure. This meta-analysis has the following limitations: (1) there were limited trials for each outcome, the overall quality of the included studies was low to moderate, contributing to publication bias, and (2) three included studies came from the same research group. Therefore, more prospective trials must be conducted to assess the effects of SGLT-2 inhibitors on quality of life, exercise capacity, and volume depletion.
Conclusion
SGLT-2 inhibitors improve the quality of life and exercise capacity in patients with chronic heart failure. SGLT-2 inhibitors have no significant impact on volume depletion in this population.
Change history
10 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11096-023-01572-2
References
Coats A, Forman DE, Haykowsky M, et al. Physical function and exercise training in older patients with heart failure. Nat Rev Cardiol. 2017;14:550–9.
Bauersachs J, de Boer RA, Lindenfeld J, et al. The year in cardiovascular medicine 2021: heart failure and cardiomyopathies. Eur Heart J. 2022;43:367–76.
Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–78.
Taylor RS, Walker S, Smart NA, et al. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol. 2019;73:1430–3.
Leggio M, Tiberti C, Armeni M, et al. Exercise capacity characterization and physical activity intensification should be priorities in heart failure patients. J Am Coll Cardiol. 2019;74:589–90.
White-Williams C, Rossi LP, Bittner VA, et al. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the american heart association. Circulation. 2020;141:e841–63.
Johansson I, Joseph P, Balasubramanian K, et al. Health-related quality of life and mortality in heart failure: the global congestive heart failure study of 23000 patients from 40 countries. Circulation. 2021;143:2129–42.
Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. 2021;128:1421–34.
Udell JA, Yuan Z, Rush T, et al. Cardiovascular outcomes and risks after initiation of a sodium glucose cotransporter 2 inhibitor: results from the EASEL population-based cohort study (evidence for cardiovascular outcomes with sodium glucose cotransporter 2 inhibitors in the real world). Circulation. 2018;137:1450–9.
Ni L, Yuan C, Chen G, et al. SGLT2i: beyond the glucose-lowering effect. Cardiovasc Diabetol. 2020;19:98.
Bhatia K, Jain V, Gupta K, et al. Prevention of heart failure events with sodium-glucose co-transporter 2 inhibitors across a spectrum of cardio-renal-metabolic risk. Eur J Heart Fail. 2021;23:1002–8.
Evans M, Morgan AR, Yousef Z, et al. Optimising the heart failure treatment pathway: the role of SGLT2 inhibitors. Drugs. 2021;81:1243–55.
Zeng Q, Zhou Q, Liu W, et al. Mechanisms and perspectives of sodium-glucose co-transporter 2 inhibitors in heart failure. Front Cardiovasc Med. 2021;8:636152.
Kosiborod MN, Jhund PS, Docherty KF, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: Results from the DAPA-HF trial. Circulation. 2020;141:90–9.
Spertus JA. Quality of life in EMPEROR-reduced: emphasizing what is important to patients while identifying strategies to support more patient-centred care. Eur Heart J. 2021;42:1213–5.
Slomski A. Empagliflozin improves quality of life in HFpEF. JAMA. 2022;327:1539.
Tomasoni D, Fonarow GC, Adamo M, et al. Sodium-glucose co-transporter 2 inhibitors as an early, first-line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2022;24:431–41.
He Z, Yang L, Nie Y, et al. Effects of SGLT-2 inhibitors on health-related quality of life and exercise capacity in heart failure patients with reduced ejection fraction: a systematic review and meta-analysis. Int J Cardiol. 2021;345:83–8.
Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–8.
Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–60.
Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation. 2021;143:298–309.
Nassif ME, Qintar M, Windsor SL, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation. 2021;143:1673–86.
Page MJ, Mckenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. 2019;140:1463–76.
Jensen J, Omar M, Kistorp C, et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: a double-blinded, randomized, and placebo-controlled trial. Am Heart J. 2020;228:47–56.
Santos-Gallego CG, Vargas-Delgado AP, Requena JA, et al. Randomized trial of empagliflozin in non-diabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77(3):243–55.
Lee M, Brooksbank K, Wetherall K, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143:516–25.
Spertus JA, Birmingham MC, Nassif M, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28:809–13.
Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28.
Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323(14):1353–68.
Yousif A, Madhavan MV, Stone GW, et al. Sodium-glucose cotransporter 2 inhibitors in patients with heart failure: a systematic review and meta-analysis of randomized trials. Eur Heart J Qual Care Clin Outcomes. 2021;8(4):383–90.
Shariq UM, Jamal ST, Mustafa MM, et al. Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2020;25(5):495–502.
Fu S, Litwin SE, Tedford RJ. Lessons from SGLT-2 inhibitors: rethinking endpoints for heart failure studies. Nat Med. 2021;27(11):1872–3.
Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17(12):761–72.
Zheng C, Lin M, Chen Y, et al. Effects of sodium-glucose cotransporter type 2 inhibitors on cardiovascular, renal, and safety outcomes in patients with cardiovascular disease: a meta-analysis of randomized controlled trials. BioMed Central. 2021;20(1):83.
Wang W, Zhang L, Pei X, et al. Evaluation of the safety of sodium-glucose co-transporter-2 inhibitors for treating patients with type 1 diabetes. Diabetes Obes Metab. 2020;22(10):1767–76.
Kosiborod MN, Vaduganathan M. SGLT-2 inhibitors in heart failure: volume or value? J Am Coll Cardiol. 2021;77(11):1393–6.
Acknowledgements
None
Funding
This study was funded by the Science and Technology Department of Hebei Province (Grant Number 21377765D) and Medical science research project of Hebei Health Commission (Grant Number 20221440).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhimin Guo, Lingjiao Wang, and Jing Yu are co-first authors.
The original online version of this article was revised: The missing equally contribution statement is included. And the order of authors is corrected as “Zhimin Guo, Lingjiao Wang, Jing Yu, Yiqi Wang, Zhiqiang Yang, Chunhua Zhou.”
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Z., Wang, L., Yu, J. et al. The role of SGLT-2 inhibitors on health-related quality of life, exercise capacity, and volume depletion in patients with chronic heart failure: a meta-analysis of randomized controlled trials. Int J Clin Pharm 45, 547–555 (2023). https://doi.org/10.1007/s11096-022-01504-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01504-6