Abstract
The use of aspirin has been widely accepted for the secondary prevention of atherosclerotic cardiovascular disease (ASCVD) in all patient populations, as the benefits linked to the reduction of clinical events outweigh the risk of major bleeding. However, despite the undisputable, though modest, potential of aspirin to reduce atherothrombotic events, its overall efficacy and safety in primary ASCVD prevention remains debatable, despite being used for this purpose for decades. The net clinical benefit of aspirin was brought into question by three recent large contemporary randomized controlled trials evaluating its role in various primary prevention populations (individuals with diabetes [ASCEND], an elderly population [ASPREE], and middle-aged adults at high estimated cardiovascular risk [ARRIVE]) and numerous large meta-analyses published during the past year. As a result, the usual generalized recommendations for the use of aspirin in patients with estimated intermediate to high ASCVD risk but without overt ASCVD have already been removed from most international guidelines. Since the primary prevention framework encompasses heterogenous groups of subjects with variable absolute ASCVD risk, a more individualized approach based on the best possible estimated ratio between the potential health benefits from fewer atherothrombotic events and harms because of potential increases in major bleeding is warranted in clinical practice. With this compromise, clinicians can better decide on the personalized use of aspirin in patients at high risk of major adverse cardiovascular events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Results from recent large randomized controlled trials of aspirin in the primary prevention of atherosclerotic cardiovascular disease (ASCVD) have contributed to discussions on the risks vs. benefits in patients at increased risk of but without clinically manifest ASCVD (subjects with multiple risk factors, patients with diabetes, and the elderly). |
There is an increased need for personalized approaches in everyday clinical practice that allow comprehensive assessment of a patient’s risk profile, consistent use of available risk assessment tools, and imaging methods to detect subclinical atherosclerosis. |
Aspirin should not be recommended as a “one-size-fits-all” prevention for primary ASCVD, and its use should involve thoughtful discussion between clinician and patient that weighs benefits against bleeding risks, patient preferences, and other factors. |
1 Introduction
Acetylsalicylic acid (aspirin) has been manufactured and marketed since the 1890's and remains among the most widely used medications worldwide [1,2,3], but it took approximately 60 years more for its antithrombotic potential to be appreciated [4]. A low dose (typically 75–100 mg daily) seems sufficient to inhibit the cyclooxygenase (COX)-1 activity of the prostaglandin synthase and block the production of thromboxane A2 [5, 6]. Its antiplatelet effect is prolonged because of its irreversible mechanism of action (blocking the exposed platelet for its entire lifespan of 7–10 days) that can only be reversed through generation of new platelets [5].
When considering daily recommended doses of aspirin, it is also worth noting its effect on the second COX isoenzyme (COX-2), which is induced in response to inflammatory stimuli and primarily responsible for the synthesis of the platelet inhibitor prostaglandin I2 by endothelial cells. Aspirin is up to 170-fold less effective at inhibiting COX-2 than at inhibiting COX-1 [7, 8]. As such, a low dose is generally used as antiplatelet therapy, and a high dose is usually considered as anti-inflammatory therapy. Body mass and size also affect the systemic bioavailability of aspirin and therefore circulating platelets when used at low doses. Low doses of aspirin have been demonstrated as effective in the prevention of vascular events almost solely in patients weighing < 70 kg and as having almost no benefit in the 80% of men and nearly 50% of women weighing ≥ 70 kg [9].
The benefits of low-dose aspirin in the secondary prevention of atherosclerotic cardiovascular diseases (ASCVDs), resulting in reduced rates of myocardial infarction (MI), stroke, and cardiovascular and all-cause mortality, have been known for more than three decades and clearly outweigh the associated risk of bleeding [10, 11]. Unlike in secondary prevention, the net value of aspirin in primary ASCVD prevention is uncertain despite its widespread use in this setting [12, 13]. On one hand, aspirin trials in apparently healthy subjects have not consistently shown a significant reduction in cardiovascular or all-cause mortality despite reducing the rates of ischemic atherothrombotic events such as MI and stroke [14]. In parallel with a similarly proportional magnitude reduction in major adverse cardiovascular events (MACE), sex-related differences in primary ASCVD prevention benefits have been reported: fewer ischemic strokes in women and fewer nonfatal MIs in men [12, 15]. Although evidence indicates that aspirin may be less effective in women as they are more likely to be resistant to aspirin, these findings of sex-related differences should be interpreted with caution, since the results were of borderline statistical significance and mainly driven by a single trial [12, 16,17,18].
In addition, not only were the absolute beneficial effects in primary prevention very low, but also serious concerns related to the increased incidence of adverse effects as a consequence of bleeding (mainly gastrointestinal) were raised [12, 13, 19]. Therefore, it has been suggested that aspirin should be administered only in seemingly healthy patients with significantly increased ASCVD risk but a low risk of bleeding. Because of the strong link between ASCVD and bleeding (mainly related to age), few patients fulfill such criteria. Furthermore, recent randomized controlled trials (RCTs) have evaluated the benefits and risks of aspirin in the primary prevention of ASCVD [20, 21].
2 Short Review of Recent Large Randomized Controlled Trials
Three large RCTs including more than 47,000 patients further evaluating the efficacy and safety of aspirin 100 mg/day for the primary prevention of ASCVD were published in 2018 [22,23,24]. In summary, these studies found a small cardiovascular benefit for individuals with diabetes mellitus (DM) but no benefit in elderly and estimated high-risk middle-aged populations. In addition, all three demonstrated a clear increase in the risk of bleeding events (Table 1).
The ARRIVE (see Tables 1 and 2 for the full names of trials cited in this article) trial included 12,546 patients with a moderately high ASCVD risk (multiple risk factors, no history of DM) and had a median follow-up duration of 5 years [22]. The results indicated that low-dose oral aspirin had no effect on rates of major cardiovascular events (including cardiovascular death, MI, unstable angina, stroke, and transient ischemic attack [TIA]) (hazard ratio [HR] 0.96; 95% confidence interval [CI] 0.81–1.13; p = 0.6038) but significantly increased the risk of gastrointestinal bleeding (HR 2.11; 95% CI 1.36–3.28; p = 0.0007). It is important to note that these results come from an analysis of the intention-to-treat population. Because of the high dropout rate independent of adverse drug reactions, the so-called per-protocol analysis (of patients who were at least 60% compliant) demonstrated significant reductions of the selected endpoints, e.g., major cardiovascular events (HR 0.81; 95% CI 0.64–1.02) and MI (HR 0.53; 95% CI 0.36–0.79).
The ASCEND study included 15,480 patients aged ≥ 40 years with DM but no known cardiovascular disease and had a follow-up period of 7.4 years [23]. The results confirmed a 12% (8.5 vs. 9.6%; HR 0.88; 95% CI 0.79–0.97; p = 0.01) reduction of the incidence of severe vascular events, including MI, stroke, TIA, or vascular death with aspirin (vs. placebo), but a 29% increase in risk of major bleeding (4.1 vs. 3.2%; HR 1.29; 95% CI 1.09–1.52; p = 0.003). Unfortunately, in terms of initial absolute cardiovascular risk of the study population, this trial remained in the range of other primary prevention trials in low-risk patients, since it did not meet the initially planned annual rate of elevated vascular risk of > 2%, remaining at only 1.2–1.3%. Possible explanations for the limited expression of the cardioprotective effects of aspirin included a healthy lifestyle and improved cardiovascular protection with concomitant medications (particularly statins and/or antihypertensive agents, mainly inhibitors of the renin–angiotensin–aldosterone system) and greater use of proton pump inhibitors, which might modify an otherwise increased risk of bleeding.
The ASPREE trial included 19,114 elderly patients (exclusively healthy adults aged ≥ 70 years or Black and Hispanic patients aged ≥ 65 years) without previously manifested cardiovascular disease and had a median follow-up duration of 4.7 years [24]. The rate of cardiovascular disease was 10.7 and 11.3 events per 1000 person-years with aspirin and placebo, respectively (HR 0.95; 95% CI 0.83–1.08). Low-dose aspirin did not prolong disease-free survival (12.7 and 11.1 deaths from any cause per 1000 person-years with aspirin and placebo, respectively; HR 1.14; 95% CI 1.01–1.29) [25]. However, a significantly increased risk of major bleeding with aspirin therapy was reconfirmed: the rate of major hemorrhage was 8.6 versus 6.2 events per 1000 person-years, respectively (3.8 vs. 2.8%; HR 1.38; 95% CI 1.18–1.62; p < 0.001) [24, 25]. Increasing age was the most important factor for increased bleeding risk, with an approximately 50% increase in the risk of hemorrhagic stroke and nearly twice the risk of major extracranial bleeding with each decade of age, regardless of aspirin use.
3 Recent Meta-Analyses Following Large Clinical Trials
Several meta-analyses have analyzed the results of these new studies within broader contexts, including some older large RCTs [26,27,28,29]. These pooled analyses suggested low-dose aspirin had relatively modest protective effects against atherothrombosis (significantly reduced HRs for atherosclerotic ischemic events such as MIs) and incurred a higher risk of major bleeding. Together, they indicated a lack of significant net clinical benefit from aspirin within the primary ASCVD prevention framework (Table 2).
The results of these older studies, published between 1988 and 2005 and included in the landmark ATTC meta-analysis [12], reflected the best preventive practices of the time, which differ significantly from current standards of care. Since the management of CVD risk factors (e.g., with greater smoking cessation, tighter blood pressure control, and widespread statin use) changed considerably from the 1980s to 2005 and later, newer studies failed to find evidence for a reduced risk of nonfatal MI, which was considered the most prominent potential benefit of aspirin. In the late 1990s, the most cited guidelines developed by recognized scientific societies started to strongly recommend the use of statins. These agents decrease low-density lipoprotein cholesterol (LDL-C) levels and primarily reduce the risk of nonfatal MI in primary prevention but may also exert important pleiotropic effects. Among these, their anti-inflammatory effects prevail [31], which could also have the same kind of effect as aspirin. On the other hand, with more sophisticated diagnostics, there is greater potential for more small ischemic events to be defined as MI (mainly nonfatal) within the endpoints of the newer trials.
In a systematic review, Moriarty and Ethell [32] compared contemporary and older research, with 95,456 patients from older studies and 61,604 patients from the four newer studies (ARRIVE, ASCEND, ASPREE, and JPPP) [32]. The use of aspirin in primary prevention had no significant influence on “hard” endpoints, such as fatal MI and stroke, and cardiovascular and all-cause mortality (relative risks [RRs] for vascular outcomes with older vs. newer studies: MACE 0.89 vs. 0.93; fatal hemorrhagic stroke 1.73 vs. 1.06; any ischemic stroke 0.86 vs. 0.86; any MI 0.84 vs. 0.88; and nonfatal MI 0.79 vs. 0.94). Major hemorrhage was significantly increased in both time periods (RR 1.48 vs. 1.37 in older vs. newer studies, respectively) [32].
Zheng and Roddick [27] reported that, in studies published since the year 2000, aspirin use compared with no aspirin was associated with reductions in the composite cardiovascular outcome and total and ischemic stroke, but no significant difference was found for all-cause and cardiovascular mortality or MI [27]. The use of aspirin for primary prevention of ASCVD was associated with no benefit for the risk of stroke or death but a very modest 0.3% per year reduction in the absolute risk of MI that disappeared when only studies published after 2008 were analyzed [19]. On the other hand, aspirin use in primary prevention is consistently associated with an absolute increase in the rates of intracranial bleeding and major bleeding (0.1 and 0.2% per year, respectively). Overall, the use of aspirin appears harmful when prescribed for primary prevention of ASCVD events in lower-risk patients without diabetes and unselected healthy elderly populations (age > 70 years) [19].
An important aspect related to the potential prophylactic benefits of low-dose aspirin is also the treatment (follow-up) durations of the trials. In a prespecified sensitivity analysis of outcomes in patients with a follow-up of > 5 years, Abdelaziz et al. [28] found a lower rate of all-cause death (RR 0.95; p = 0.032), likely derived from consistent but nonsignificant effects on non-cardiovascular death (RR 0.95; p = 0.08) and cardiovascular death (RR 0.95; p = 0.3). At the same time, the overall analysis of effects in populations with a high (> 7.5%) estimated 10-year ASCVD risk showed only a trend toward lower rates of cardiovascular death, whereas the rates of all-cause death remained similar [28].
4 Aspirin Use in Primary Atherosclerotic Cardiovascular Disease Prevention in Patients with Diabetes Mellitus
It is widely accepted that individuals with DM are at substantially higher absolute risk for both nonvascular and vascular death. A study from the 1980s showed that the annual MI and cardiovascular mortality risk was increased six- to eightfold in patients with noninsulin-dependent DM, suggesting that patients with DM without previous MI have the same high risk of an MI as patients without DM but with previous MI [33]. Given this, aspirin was also expected to have huge potential for benefits in primary cardiovascular prevention in patients with DM. Comparing subjects with and without DM, the ATTC meta-analysis demonstrated similar relative reductions (13 vs. 12%) but larger absolute reductions (0.24 vs. 0.06% per year) for primary prevention of important vascular events with aspirin [12]. No impact on mortality was shown, the effect on stroke was minor, and a larger reduction for nonfatal strokes was reported.
Nonetheless, data from more recent trials and contemporary meta-analyses have shown that the net clinical efficacy of aspirin use in primary ASCVD prevention in patients with DM is rather low [23, 34,35,36]. ASCEND, by far the largest randomized, placebo-controlled primary prevention trial using aspirin in patients with DM, demonstrated only a small significant reduction of serious cardiovascular events, with a concomitant increase in major bleeding [23]. Since ASCEND did not study patients with a high cardiovascular risk (as initially planned) but only patients with DM with unexpectedly low absolute cardiovascular risk, the results were largely confirmatory of earlier primary prevention trials. Apart from potential problems with compliance, possible explanations for the rather small cardioprotective effect with antiplatelet treatment in patients with DM included the adoption of a much healthier lifestyle and markedly improved pharmacological cardiovascular prevention using anti-inflammatory and vasoactive drugs, such as statins or antihypertensive agents [35]. A recent population-based cohort study in patients with DM with high usage of statins (75–88%), aspirin (66–84%), and other vasculoprotective treatments (e.g., angiotensin-converting enzyme inhibitors [42–50%] or angiotensin receptor blockers [19–20%]) found only a small increase in mortality for patients with DM but no change in the incidence of MIs in the absence of angiographically significant coronary artery disease, suggesting that patients with DM without CVD had the same risk of MI as patients without DM [37]. In the same context, greater use of proton pump inhibitors might modify bleeding, specifically and most frequently in the upper gastrointestinal tract. In addition, the low event rate may be at least partly explained by the introduction of new antidiabetic drugs with more favorable cardiovascular effects. The less efficient prophylaxis achieved with aspirin in patients with DM could also be because low-dose aspirin is less efficient at suppressing platelet function. It is possible that the faster resynthesis of platelets and therefore COX isoenzymes enables sufficient recovery of COX-1 activity with once-daily dosing (particularly between 12 and 24 h) and thus overcomes the antiplatelet effects of aspirin [38].
The most recent meta-analysis of patients with DM (including the ASCEND trial) demonstrated a significant 11% relative risk reduction for MACE (absolute risk reduction [ARR] 1.1%), with a number needed to treat (NNT) of 95 to prevent one MACE over 5 years’ average follow-up [36]. In addition, a significant 25% relative reduction in stroke (NNT 101, ARR 0.99%) with aspirin ≤ 100 mg/day but no effect on other endpoints, including all-cause mortality, was found [36]. In summary, it appears that the role of aspirin as a primary CVD prevention strategy in patients with DM remains unresolved. However, it also means that the use of low-dose aspirin may need to be individualized and tailored according to baseline CVD and bleeding risk in this notoriously high-risk group of patients.
5 Aspirin Use and Cancer Prevention
The well-known association between aspirin use and a reduced risk of mainly colorectal and possibly a few other gastrointestinal cancers is supported by a large number of observational studies and a pooled analysis of RCTs [39,40,41]. A meta-analysis of observational studies published up to March 2019 reported a significantly reduced risk (by almost 30% or RR 0.73 on average) of colorectal cancer (CRC) and of other gastrointestinal cancers (esophagus, stomach, hepato-biliary tract, and pancreas; up to 40%) [41].
The risk of CRC reduced linearly with increasing doses of aspirin: 75–100 mg/day conveyed a risk reduction of 10%; 325 mg/day reduced the risk by 35%, and 500 mg/day almost halved the CRC risk. This beneficial effect also increased with duration of use, meaning long-term therapy is required for a protective effect: the risk reduction was 20% for 5 years and 30% for 10 years of aspirin use. The chemopreventive effect of aspirin is not yet entirely known; it can be attributed to both the inhibition of platelet activation triggered by gastrointestinal mucosal lesions (through inactivation of platelet COX-1) and by inhibition of COX-2, which is abnormally expressed in many cancer cell lines and implicated in carcinogenesis, tumor growth, apoptosis, and angiogenesis [40].
Additional evidence for the chemoprotective effects of aspirin is being sought prospectively from a few ongoing primary prevention trials and several adjuvant trials of various low-dose aspirin regimens in patients with newly diagnosed cancers. An important field of clinical research is focused on the discovery of biomarkers to identify subjects who will respond to the antineoplastic effects of aspirin. In addition, it is thought that a systems biology approach to analyzing heterogeneous datasets (genomics, epigenomics, proteomics, lipidomics, and clinical) would allow dynamic systems modeling of candidate pathways involved in the antineoplastic effects of aspirin [40]. This strategy would also allow the identification and use of susceptibility profiles for CRC in the development of new biomarkers to predict its occurrence and recurrence.
The 2016 European Society of Cardiology (ESC) guidelines on cardiovascular prevention [42] did not recommend the use of aspirin as primary prevention for CVD because of the potentially serious risk of increasing major bleeding; however, the ESC Working Group on Thrombosis suggested that a family history of gastrointestinal cancer (mainly CRC) should be included in physician–patient discussions if the estimated 10-year CVD risk is between 10 and 20% [43]. The 2016 US Preventive Services Task Force (USPSTF) recommendation (grade B) for the use of low-dose aspirin stated, “for the primary prevention of CVD and CRC in adults 50–59 years of age who have a 10% or greater 10-year CVD risk, are not at increased risk of bleeding, have a life expectancy of at least 10 years, and are willing to take low-dose aspirin daily for at least 10 years” [44]. This recommendation is not an absolute endorsement of low-dose aspirin for the regular chemoprevention of CRC but suggests that lowering the long-term risk for developing CRC may represent an additional benefit of antiplatelet prophylaxis in primary CVD prevention.
6 Low-Dose Aspirin in Primary Cardiovascular Disease Prevention: Current Guidelines
The inconclusive and uncertain results from major clinical trials and large meta-analyses evaluating daily low-dose aspirin for primary prevention are also reflected in relatively inconsistent recommendations in major evidence-based guidelines [42,43,44,45,46].
The 2016 ESC guidelines on cardiovascular prevention in Europe do not recommend aspirin as primary prevention for CVD because of the potentially serious risk of increased major bleeding [42]. On the other hand, the 2019 ESC guidelines recommend aspirin 75–100 mg/day for primary prevention in patients with DM with high or very high ASCVD risk and low estimated bleeding risk (which must be assessed regularly) [45]. The use of aspirin is no longer recommended for primary prevention in patients with DM at moderate cardiovascular risk, who are indeed very rarely seen in clinical practice (young, no cardiovascular risk factors, and short disease duration) [45]. The IIb class of recommendation (“May be considered”) reflects the overall inconclusiveness of the available evidence and the remaining knowledge gaps. In addition, these guidelines included a short discussion on the need to assess the potential effects of body mass, particularly moderate-to-severe obesity, on antiplatelet drug responsiveness and effectiveness in patients with DM and to investigate higher-dose strategies [9, 45, 47].
In the USA, the USPSTF recommends low-dose aspirin in individuals aged 50–59 years with high 10-year ASCVD risk but without increased risk for bleeding [44]. However, these guidelines were developed in 2016, well before the 2018 publication and subsequent meta-analyses of the three large RCTs on the use of low-dose aspirin in primary CVD prevention. The most recent American College of Cardiology/American Heart Association guidelines recommend considering oral aspirin 75–100 mg daily among adults aged 40–70 years who are at a higher risk of ASCVD and withholding aspirin for primary prevention of ASCVD in adults aged > 70 years and in anyone at increased risk of bleeding [46]. Again, the recommendation is class IIb (weak), meaning that aspirin may be reasonably considered, since its usefulness/effectiveness is not unequivocally well-established.
Nevertheless, despite all these supposedly “refined” recommendations, important questions remain: should aspirin be stopped in individuals who are already taking aspirin but have reached the age of 70 years without adverse effects, and should higher aspirin doses be considered in obese patients with DM? These decisions must still be based on the estimated balance between the overall CVD and bleeding risks of a particular patient and their personal preferences. Future studies should certainly also address these and other important questions.
7 A Call for a More Personalized Approach in Clinical Decision Making
It is obvious that aspirin should not be prescribed for most patients without established, clinically manifest ASCVD. Instead, more aggressive management of major behavioral, lifestyle, and biological cardiovascular risk factors and comorbidities, tailored to the expected ASCVD risk, should be emphasized. Informed shared decision making between clinicians and patients is undoubtedly also a suitable approach to creating individual treatment paths [20, 48]. This also means that, ultimately, the initiation or withdrawal of aspirin therapy must involve discussion of the patient’s wishes and treatment expectations [48, 49].
To properly guide the adjustment of everyday clinical practice, several important points must be discussed in more detail, all with the aim of emphasizing the need for an individualized approach to decision making. First, the consistent use of widely available ASCVD risk charts and/or calculators is paramount. The decision over which tool (Framingham Risk Score, pooled cohort equation, SCORE Risk Chart, etc.) to use is probably not the most critical, since almost all are constructed to estimate an initial 10-year ASCVD risk and help guide and customize therapeutic plans [50]. However, concern has been raised about the tendency of these kinds of calculators to overestimate the real-world ASCVD risk [51, 52]. Therefore, we must continually seek to refine and/or better calibrate existing calculators and to develop more accurate risk assessment tools that can also better estimate individual-level prognosis and the treatment effects of improved short-term and lifetime risk and life expectancy free of ASCVD [53, 54].
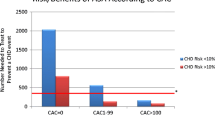
In addition to using risk assessment charts and/or calculators, and before the final decision on whether to use aspirin for primary prevention, it has been recommended that as much supplemental information on the individual patient should be used as possible. This relates to the presence and magnitude of so-called risk-enhancing factors and the use of imaging to ascertain the presence of subclinical atherosclerosis. In combination, these can be extremely useful in further stratifying the overall absolute ASCVD risk. Risk-enhancing factors include (1) family history of premature ASCVD, (2) high-risk ethnicity groups (e.g., South Asian), (3) metabolic syndrome, (4) persistently elevated lipid levels (LDL-C and/or triglycerides), (5) increase in additional biomarkers (e.g., high-sensitivity C-reactive protein, lipoprotein(a), apolipoprotein B, lipoprotein phospholipase A2, etc.), (6) decreased ankle-brachial index, and (7) chronic inflammatory disorders [46, 55, 56]. The coronary artery calcium (CAC) score, carotid ultrasound, and echocardiography may be useful in assessing the atherosclerotic process [46, 57,58,59]. However, universal screening with these supplementary imaging methods would enormously increase healthcare costs so they should be used cautiously and on an individual basis.
Patients can obtain a net clinical benefit when the positive effect of preventing an ASCVD event significantly exceeds the risk of bleeding [60, 61]. It has been demonstrated that general ASCVD risk factors, such as increased age, particular race, sex, presence of DM, high blood pressure, or smoking, could also be associated with an increased risk of bleeding. In short, the greater the benefit of aspirin therapy, the greater the risk of bleeding. Given this strong link between ASCVD and bleeding, and the major role of age, few patients match the eligibility criteria. In the elderly, multiple factors can determine bleeding risk, including prior history of gastrointestinal bleeding, liver or renal disease, fall risk, frailty, and concomitant use of anticoagulants.
Box 1 presents real-world patient cases to demonstrate the clinical reasoning around the use of aspirin within the framework of primary ASCVD prevention.
9 Conclusions
Clinical decisions about the use of aspirin in primary ASCVD prevention should be individualized, and decision making should be shared. Despite the undisputable and highly convincing results of recent clinical trials and meta-analyses showing a clear absence of net clinical benefit in various populations within the primary ASCVD prevention framework, personalized advice is more than warranted, simply to ensure individuals are given the opportunity to benefit. As much as possible, the overall absolute ASCVD risk versus the risk of bleeding should be comprehensively assessed and firmly remain the sole base of everyday clinical practice judgments and interventions.
References
Sneader W. The discovery of aspirin: a reappraisal. BMJ. 2000;321:1591–4.
Raber I, McCarthy CP, Vaduganathan M, Bhatt DL, Wood DA, Cleland JGF, et al. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet. 2019;393:2155–67.
Kolte D, Aronow WS, Banach M. Polypills for the prevention of Cardiovascular diseases. Expert Opin Investig Drugs. 2016;25:1255–64.
Craven L. Coronary thrombosis can be prevented. J Insur Med. 1950;5:47–8.
Patrono C, Baigent C. Low-dose aspirin, coxibs, and other NSAIDS: a clinical mosaic emerges. Mol Interv. 2009;9:31–9.
Paseban M, Marjaneh RM, Banach M, Riahi MM, Bo S, Sahebkar A. Modulation of microRNAs by aspirin in cardiovascular disease. Trends Cardiovasc Med. 2019;30:249–54.
Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest. 2006;116:1391–9.
Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120.
Rothwell PM, Cook NR, Gaziano JM, Price JF, Belch JFF, Roncaglioni MC, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387–99.
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;2(8607):349–60.
Antithrombotic Trialists Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
Baigent C, Blackwell L, Antithrombotic Trialists Collaboration (ATTC), et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60.
Raju N, Sobieraj-Teague M, Hirsh J, O’Donnell M, Eikelboom J. Effect of aspirin on mortality in the primary prevention of cardiovascular disease. Am J Med. 2011;124:621–9.
Brotons C, Robert Benamouzig R, Filipiak KJ, Limmroth V, Borghi C. A systematic review of aspirin in primary prevention: is it time for a new approach? Am J Cardiovasc Drugs. 2015;15:113–33.
Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–13.
Gum PA, Kottke-Marchant K, Poggio ED, Gurm H, Welsh PA, Brooks L, Sapp SK, Topol EJ. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001;88:230–5.
Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304.
Yerman T, Gan WQ, Sin DD. The in uence of gender on the effects of aspirin in preventing myocardial infarction. BMC Med. 2007;5:29.
Mahmoud A, Gad M, Elgendy A, Elgendy I, Bavry A. Efficacy and safety of aspirin for primary prevention of cardiovascular events: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur Heart J. 2019;40:607–17.
Marquis-Gravel G, Roe MT, Harrington RA, Muñoz D, Hernandez AF, Jones WS. Revisiting the role of aspirin for the primary prevention of cardiovascular disease. Circulation. 2019;140:1115–24.
Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16:675–86.
Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036–46.
Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, ASCEND Study Collaborative Group, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–39.
McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–18.
McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379:1499–508.
Gelbenegger G, Postula M, Pecen L, Halvorsen S, Lesiak M, Schoergenhofer C, et al. Aspirin for primary prevention of cardiovascular disease: a meta-analysis with a particular focus on subgroups. BMC Med. 2019;17:198.
Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321:277–87.
Abdelaziz HK, Saad M, Pothineni NVK, Megaly M, Potluri R, Saleh M, et al. Aspirin for primary prevention of cardiovascular events. J Am Coll Cardiol. 2019;73:2915–29.
Xie W, Luo Y, Liang X, Lin Z, Wang Z, Liu M. The Efficacy and safety of aspirin as the primary prevention of cardiovascular disease: an updated meta-analysis. Ther Clin Risk Manag. 2019;15:1129–40.
Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510–20.
Albert MA, Danielson E, Rifai N, Ridker PM, for the PRINCE Investigators. Effect of statin therapy on c-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70.
Moriarty F, Ebell MH. A comparison of contemporary versus older studies of aspirin for primary prevention. Fam Pract. 2019. https://doi.org/10.1093/fampra/cmz080.
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34.
Capodanno D, Angiolillo DJ. Aspirin for primary cardiovascular risk prevention and beyond in diabetes mellitus. Circulation. 2016;134:1579–94.
Schrör K, Kristensen SD, Storey RF, Verheugt FWA. Aspirin and primary prevention in patients with diabetes—a critical evaluation of available randomized trials and meta-analyses. Thromb Haemost. 2019;119:1573–82.
Seidu S, Kunutsor SK, Sesso HD, et al. Aspirin has potential benefits for primary prevention of cardiovascular outcomes in diabetes: updated literature-based and individual participant data meta-analyses of randomized controlled trials. Cardiovasc Diabetol. 2019;18:70.
Olesen KKW, Madsen M, Egholm G, et al. Patients with diabetes without significant angiographic coronary artery disease have the same risk of myocardial infarction as patients without diabetes in a real-world population receiving appropriate prophylactic treatment. Diabetes Care. 2017;40:1103–10.
Rocca B, Santilli F, Pitocco D, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost. 2012;10:1220–30.
Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomized trials. Lancet Oncol. 2012;13:518–27.
Patrignani P, Patrono C. Aspirin and Cancer. J Am Coll Cardiol. 2016;68:967–76.
Bosetti C, Santucci C, Gallus S, Martinetti MM, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol. 2020;31:558–68.
Piepoli M, Hoes A, Agewall S, Albus C, Brotons C, Catapano A, ESC Scientific Document Group, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Eur Heart J. 2016;37:2315–81.
Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology Writing Group on Thrombosis. J Am Coll Cardiol. 2014;64:319–24.
Bibbins-Domingo K; Force USPST. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836–45.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, The Task Force for diabetes, pre- diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD), et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–646.
Rocca B, Fox KAA, Ajjan RA, Andreotti F, Baigent C, Collet JP, et al. Antithrombotic therapy and body mass: an expert position paper of the ESC Working Group on Thrombosis. Eur Heart J. 2018;39:1672–86.
Martin S, Sperling L, Blaha M, Wilson P, Gluckman T, Blumenthal R, Stone N. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol. 2015;65:1361–8.
Thobani A, Dhindsa DS, DeMoss BD, Raad M, Sandesara PB, Sperling LS, Baer JT. Usefulness of aspirin for primary prevention of atherosclerotic cardiovascular disease. Am J Cardiol. 2019;124:1785–9.
Rossello X, Dorresteijn JA, Janssen A, Lambrinou E, Scherrenberg M, Bonnefoy-Cudraz E, et al. Risk prediction tools in cardiovascular disease prevention: a report from the ESC Prevention of CVD Programme led by the European Association of Preventive Cardiology (EAPC) in collaboration with the Acute Cardiovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP). Eur J Prev Cardiol. 2019;26:1534–44.
Rana JS, Tabada GH, Solomon MD, Lo JC, Jaffe MG, Hee Sung S, Ballantyne CM. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic real-world population. J Am Coll Cardiol. 2016;67:2118–30.
DeFilippis AP, Young R, McEvoy JW, Michos ED, Sandfort V, Kronmal RA, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J. 2017;38:598–608.
Ko DT, Sivaswamy A, Sud M, Kotrri G, Azizi P, Koh M, et al. Calibration and discrimination of the Framingham Risk Score and the Pooled Cohort Equations. CMAJ. 2020;192:E442–9.
Jaspers NEM, Blaha MJ, Matsushita K, van der Schouw YT, Wareham NJ, Khaw K-T, et al. Prediction of individualized lifetime benefit from cholesterol lowering, blood pressure lowering, antithrombotic therapy, and smoking cessation in apparently healthy people. Eur Heart J. 2020;41:1190–9.
Plazak ME, Mouradjian MT, Watson K, Reed BN, Noel ZR, Devabhakthuni S, Gale SE. An aspirin a day? Clinical utility of aspirin therapy for the primary prevention of cardiovascular disease. Expert Rev Cardiovasc Ther. 2019;17:561–73.
Krasinska B, Osińska A, Osinski M, Krasinska A, Rzymski P, Tykarski A, Krasiński Z. Standardised tomato extract as an alternative to acetylsalicylic acid in patients with primary hypertension and high cardiovascular risk - a randomised, controlled trial. Arch Med Sci. 2018;14:773–80.
Miedema M, Duprez D, Misialek J, Blaha M, Nasir K, Silverman M, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–60.
Blankstein R, Chandrashekhar Y. Extensive coronary artery calcifications. No longer primary prevention! JACC Cardiovasc Imaging. 2020;13:183–5.
Gresele P, Paciullo F, Migliacci R. Antithrombotic treatment of asymptomatic carotid atherosclerosis: a medical dilemma. Intern Emerg Med. 2020. https://doi.org/10.1007/s11739-020-02347-7.
Whitlock EP, Burda BU, Williams SB, Guirguis-Blake JM, Evans CV. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. preventive services task force. Ann Intern Med. 2016;164:826–35.
Schenone AL, Lincoff AM. Aspirin for primary prevention of atherosclerotic cardiovascular events. Clev Clin J Med. 2020;87:300–11.
Selak V, Jackson R, Poppe K, Wu B, Harwood M, Grey C, et al. Personalized prediction of cardiovascular benefits and bleeding harms from aspirin for primary prevention: a benefit-harm analysis. Ann Intern Med. 2019;171:529–39.
Nudy M, Cooper J, Ghahramani M, Ruzieh M, Mandrola J, Foy AJ. Aspirin for primary atherosclerotic cardiovascular disease prevention as baseline risk increases: a meta-regression analysis. Am J Med. 2020;S0002–9343(20):30432.
Mora S, Manson J. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195–204.
Lanas A, Polo-Tomás M, Casado-Arroyo R. The aspirin cardiovascular/gastrointestinal risk calculator - a tool to aid clinicians in practice. Aliment Pharmacol Ther. 2013;37:738–48.
Hall KT, Kessler T, Buring JE, Passow D, Sesso HD, Zee RYL, et al. Genetic variation at the coronary artery disease risk locus GUCY1A3 modifies cardiovascular disease prevention effects of aspirin. Eur Heart J. 2019;40:3385–92.
Santilli F, Simeone P. Aspirin in primary prevention: the triumph of clinical judgement over complex equations. Intern Emerg Med. 2019;14:1217–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Zlatko Fras, Amirhossein Sahebkar, and Maciej Banach have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Funding
No external funding was used in the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Fras, Z., Sahebkar, A. & Banach, M. The Use of Aspirin in Contemporary Primary Prevention of Atherosclerotic Cardiovascular Diseases Revisited: The Increasing Need and Call for a Personalized Therapeutic Approach. Am J Cardiovasc Drugs 21, 139–151 (2021). https://doi.org/10.1007/s40256-020-00424-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-020-00424-y