Abstract
Cholesteryl ester transfer protein (CETP) plays an important role in lipid metabolism and has presented an attractive target for drug development, primarily resting on the hope that CETP inhibition would reduce cardiovascular events through its ability to increase levels of high-density lipoprotein cholesterol (HDL-C). However, clinical development of CETP inhibitors has proven disappointing, with a spectrum of results spanning from evidence of harm, to futility, to only modest benefit in large-scale cardiovascular outcomes trials. A number of additional insights from genomic studies have suggested potential benefits from these agents in specific clinical settings. We review the current state of CETP inhibitors as an approach to targeting cardiovascular risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cholesteryl ester transfer protein (CETP) inhibitors raise HDL and lower LDL cholesterol. |
Clinical outcome trials of CETP inhibitors have been disappointing in recent years. |
Genetic insights may still suggest potential benefit of these agents, although this requires validation in large scale clinical trials. |
1 Introduction
Statins are widely used to reduce cardiovascular risk in a range of clinical settings, but many patients continue to experience clinical events [1]. This residual risk highlights the need to develop novel therapeutic approaches to achieve more effective prevention of cardiovascular disease. According to population [2,3,4] and animal [5] studies suggesting that high-density lipoproteins (HDLs) are atheroprotective, agents that can increase HDL cholesterol (HDL-C) ought to reduce cardiovascular risk. However, to date, the field has been littered with underwhelming results, from fibrates through to niacin, largely driven by agents with only modest, specific HDL-raising capacity. Over the last decade, interest has increased in developing new therapies that directly target this function. One pharmacological method receiving considerable focus in efforts to substantially raise HDL-C has been the inhibition of cholesteryl ester transfer protein (CETP).
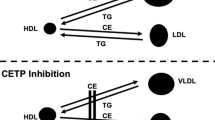
2 Cholesteryl Ester Transfer Protein (CETP) and Lipid Metabolism
CETP is a plasma-based factor that is synthesized in the liver and adipose tissue. It facilitates the transfer of esterified cholesterol from HDL to apolipoprotein B (ApoB)-containing lipoproteins, mainly very low-density lipoprotein and low-density lipoprotein (LDL), in exchange for triglycerides. The precise mechanism underlying this exchange of lipid species remains uncertain but is likely to involve CETP forming a bridge to link lipoproteins or shuttle lipid species between particles [6]. The fundamental reason for the presence of CETP in humans is unknown; several species (e.g., mice) do not endogenously express CETP. Moreover, humans with homozygous CETP deficiency, despite elevated HDL, appear otherwise physiologically normal [7]. While CETP activity theoretically results in cholesterol depletion of LDL particles, the fact that LDL is taken up by the liver suggests that CETP-mediated transfer may play an additional role in reverse cholesterol transport. With their capacity to enrich HDL particles, agents that inhibit CETP have been shown to raise HDL to a much greater degree than any other lipid-modifying agent currently used in clinical practice [8].
3 Evidence Supporting Development of CETP Inhibitors
A number of lines of evidence have suggested that low levels of CETP activity are associated with cardiovascular protection. Population studies have demonstrated that low CETP activity, when associated with elevated HDL-C levels, are associated with less prospective cardiovascular events [9]. Large genome-wide association studies have also reported that polymorphisms associated with low CETP activity similarly had a lower prevalence of cardiovascular disease [10]. Inhibiting CETP via small molecules, vaccines, or antisense oligonucleotides had favorable effects on atherosclerotic plaque burden in rabbit models [11,12,13]. On the basis of these findings, a number of programs have evaluated the impact of small-molecule CETP inhibitors.
4 CETP Inhibitors
4.1 Torcetrapib
Torcetrapib was the first CETP inhibitor to reach an advanced stage of clinical development [14]. Early studies demonstrated dose-dependent elevation of HDL-C > 70% and lowering of LDL cholesterol (LDL-C) by 20%, when administered as monotherapy or in combination with statins [15]. Despite these profound lipid changes, development of torcetrapib was stopped prematurely after adverse clinical effects were observed in a large outcomes trial [16]. When administered in patients at high cardiovascular risk, torcetrapib increased the primary cardiovascular endpoint by 25% and all-cause mortality by 58%. This increase in mortality involved both cardiovascular and non-cardiovascular (cancer, sepsis) events (Table 1). In parallel, three imaging studies failed to demonstrate any benefit of torcetrapib administration on progression of either carotid intima-medial thickness [17, 18] or coronary atherosclerosis [19].
This surprising result provided support for critics of CETP inhibition, suggesting that this strategy would have an adverse effect on HDL function and reverse cholesterol transport. A number of studies provided evidence to suggest that HDL function remained intact in the setting of CETP inhibition. HDL isolated from the plasma of individuals with either CETP deficiency or receiving torcetrapib treatment demonstrated retained capacity to promote cellular cholesterol efflux. In fact, cholesterol efflux activity increased with higher torcetrapib doses [20]. This was supported by observations that torcetrapib was associated with regression of coronary atherosclerosis in a further analysis of patients with the highest HDL-C levels [21]. Parallel studies demonstrated that torcetrapib possessed off-target effects, including blood pressure (BP) elevations (mean 5 mmHg) [22], stimulated adrenal synthesis of cortisol and aldosterone [23], and increased artery wall expression of endothelin [24] and was associated with a modest but statistically significant increase in C-reactive protein (+ 0.04 mg/dL; P = 0.01). Given that patients with aldosterone and bicarbonate levels above the median appeared to have greater mortality, these off-target effects may have contributed to the harm observed with torcetrapib.
4.2 Dalcetrapib
Dalcetrapib is a modest CETP inhibitor, raising HDL-C by up to 30% but with no effect on LDL-C levels [8]. Clinical development of this agent progressed in the post-torcetrapib era on the basis of reassuring findings that demonstrated no adverse effects of dalcetrapib on either endothelial function [25] or plaque inflammation [26]. However, a large clinical outcomes trial in patients with a recent acute coronary syndrome was stopped because of clinical futility, with no evidence of an association between on-treatment HDL-C levels and cardiovascular events [27]. Post hoc pharmacogenomic analyses demonstrated that patients harboring the AA genotype of the ADCY9 gene on chromosome 16 treated with dalcetrapib had a 39% reduction in cardiovascular events and regressed atheroma in their carotids [28]. Conversely, those with the GG phenotype, and particularly GG homozygotes, experienced a 27% increase in cardiovascular events (hazard ratio [HR] 1.27; 95% confidence interval [CI] 1.02–1.58), which was directionally supported by the imaging substudies with either an absence of regression or mild progression. This observation led to the initiation of a new trial to compare the effects of dalcetrapib or placebo on cardiovascular outcomes exclusively in high-risk patients with the ADCY9 AA phenotype.
4.3 Evacetrapib
Evacetrapib is a more potent CETP inhibitor with dose-dependent increases in HDL-C by up to 125% and lowering of LDL-C by 25–30% [29]. While this agent similarly lacked any such torcetrapib off-target effects in early studies, the lipid effects did not translate to clinical benefit, with the large cardiovascular outcomes trial terminated early because of futility [30]. Pharmacogenomic analyses of this trial failed to demonstrate a clear relationship between ADCY9 genotypes and cardiovascular benefit with evacetrapib [31]. Whether this reflects a dalcetrapib-specific effect or the play of chance is unknown. Of note, evaluation of the Kaplan–Meier event curves of the anacetrapib trial (Sect. 4.4) revealed divergence of the curves at 2 years. When applying this late effect to the evacetrapib trial, it is conceivable that any potential benefits, even if modest, from this agent were yet to emerge given the trial was terminated for futility at a mean follow-up of only 2 years.
4.4 Anacetrapib
Anacetrapib is also a potent CETP inhibitor, with dose-dependent HDL-C increasing by up to 138% and LDL-C lowering by 30–40% [32]. A large safety study provided reassuring data, again failing to demonstrate any torcetrapib-like off-target effects [33]. Importantly, it ruled out with 94% certainty that a torcetrapib-like clinical effect would be observed. In fact, a reduction in need for coronary revascularization was observed in this relatively small study. This ultimately translated to demonstration of a modest yet significant reduction in cardiovascular events in a larger trial in which patients were treated for longer than in other CETP-inhibitor programs [34]. The degree of benefit was associated with reductions in levels of non-HDL-C (but not LDL) and had no relationship with HDL-C raising; a finding that is at least partially explained by mendelian randomization data (Sect. 6). In parallel, it became increasingly apparent that, as a lipophilic molecule, anacetrapib demonstrated considerable adipose tissue accumulation, with subsequent slow release back into the circulation [35]. This ultimately resulted in a very long terminal half-life of the drug. When combined with the relatively modest clinical benefit observed in the large outcomes trial, the decision was taken to not pursue regulatory approval, and therefore anacetrapib will not come to clinical practice.
4.5 TA-8995
A third potent CETP inhibitor, TA-8995, underwent early clinical evaluation [36]. At much smaller doses than studied with the other agents, TA-8995 produced dose-dependent increases in HDL-C by up to 179% and LDL-C-lowering by up to 45%, complemented by a lack of apparent torcetrapib-associated safety signals [37]. To date, the agent has not been further developed, but these results give some sense that robust lipid changes can be observed with very small doses.
5 Safety
After the termination of the torcetrapib program, preclinical studies attempted to delineate the cause for harm, not only for clarity of the ILLUMINATE result but also for the entire CETP-inhibitor field. The aldosterone and hypertension effect appeared to be largely CETP independent: not only did rodents lacking CETP become hypertensive when exposed to torcetrapib [23], but also torcetrapib placed in the tissue culture of adrenal cells stimulated the synthesis of aldosterone and cortisol [38]; findings that were not replicated with the chemically dissimilar dalcetrapib or anacetrapib [39]. Nonetheless, the subsequent trials of the remaining agents found a consistent but very modest increase in BP (~ 1 mmHg; one-fifth of the effect seen with torcetrapib). Similarly, the two trials that reported C-reactive protein found a small but statistically significant increase in those treated with CETP inhibitors (~ 0.2 ng/dL). Class effect or not, it seems unlikely these individual phenomena will independently lead to harm (e.g., no signal for intracranial hemorrhage or infection, etc.) but may instead be mitigating potential additional efficacy. If further agents come to trial, these effects may be an ongoing challenge, and, if consistent, the degree of net clinical benefit may dictate whether the drug goes to market.
6 Evidence from Mendelian Randomization
Genome-wide association studies consistently demonstrated a relationship between polymorphisms associated with low CETP activity and lower rates of incident cardiovascular disease [40,41,42]. Mendelian randomization subsequently permitted more extensive investigation of this relationship. This approach uses genotype as a natural randomization tool and demonstrated that polymorphisms associated with low CETP activity resulted in lower rates of cardiovascular disease [10, 42], with the degree of protection correlating with lower levels of ApoB [43]. This provided further evidence to suggest that it is the reduction in atherogenic lipoproteins, not HDL raising, that is likely to underscore any potential benefits of this therapeutic strategy. Further analysis demonstrated that the protection associated with genetically low CETP activity was observed in the presence of functional HMG-CoA reductase, but not in the setting of less HMG-CoA reductase activity; a phenomenon that appears proportional to ApoB rather than LDL levels. Although levels of LDL-C and ApoB tend to be highly correlated, reduction of LDL by CETP inhibition in the setting of a statin produces a discordant, attenuated reduction in ApoB level for a given LDL reduction. While this provides some plausibility to the lack of correlation between LDL reduction and events in both ACCELERATE and REVEAL, it also poses the provocative concept that CETP inhibition may be far more effective when administered as monotherapy and less effective when used in combination with statins [44]. Given that all large trials performed to date have been conducted in patients at high cardiovascular risk, background statin therapy has been expected. Whether this approach would be useful as monotherapy in lower-risk primary prevention or in patients with documented statin intolerance remains to be tested.
7 CETP Inhibition, Diabetes, and Lipoprotein(a)
The era of clinical development of CETP inhibitors has witnessed a transition of focus from increasing HDL-C to reducing atherogenic lipoprotein levels. Additional factors should also be considered with regard to their potential clinical utility. Potent CETP inhibitors have been demonstrated to lower levels of Lp(a) and therefore provide a novel approach to reducing levels of these difficult-to-treat lipid parameters [33]. No studies have evaluated the impact of CETP inhibitors specifically in patients with elevated Lp(a) levels. The trials have also consistently demonstrated that administration of CETP inhibitors appears to have a favorable impact on glycemic control. This was evidenced by reports of lower rates of new-onset diabetes [45] and improved glycemic control in patients with established diabetes at baseline [46]. It is uncertain whether this reflects a specific antidiabetic effect of CETP inhibition or the documented beneficial effect of HDL on a variety of diabetes-relevant pathways, including protection from beta-cell apoptosis, stimulation of beta-cell function, and increasing cellular glucose uptake (thereby reducing insulin resistance) [47, 48]. Whether this suggests that administration in patients with prediabetes or other settings of dysglycemia before the development of fulminant diabetes would be a more optimal cohort for future clinical trials remains to be tested.
8 Summary
After nearly two decades of clinical development, the early failures and subsequent lessons from both outcomes trials and genetic studies suggest that CETP inhibition may still present an alternative approach to reducing cardiovascular risk. Over the course of this era, the likely factor that may produce any clinical benefit has transitioned from the ability to raise HDL-C to lowering a range of atherogenic lipid parameters and potential benefits on glycemic control. Whether this will result in another large clinical outcomes trial, learning from the lessons provided by prior studies, is unknown. For now, the door for CETP inhibition remains slightly open; the question remains, will we walk through one more time?
References
Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46(7):1225–8.
Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(Suppl):11–20.
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15.
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62(5):707–14.
Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–72.
Barter P, Rye KA. Cholesteryl ester transfer protein: its role in plasma lipid transport. Clin Exp Pharmacol Physiol. 1994;21(9):663–72.
Inazu A, Brown ML, Hesler CB, Agellon LB, Koizumi J, Takata K, et al. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med. 1990;323(18):1234–8.
de Grooth GJ, Kuivenhoven JA, Stalenhoef AF, de Graaf J, Zwinderman AH, Posma JL, et al. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response study. Circulation. 2002;105(18):2159–65.
Vasan RS, Pencina MJ, Robins SJ, Zachariah JP, Kaur G, D’Agostino RB, et al. Association of circulating cholesteryl ester transfer protein activity with incidence of cardiovascular disease in the community. Circulation. 2009;120(24):2414–20.
Johannsen TH, Frikke-Schmidt R, Schou J, Nordestgaard BG, Tybjaerg-Hansen A. Genetic inhibition of CETP, ischemic vascular disease and mortality, and possible adverse effects. J Am Coll Cardiol. 2012;60(20):2041–8.
Morehouse LA, Sugarman ED, Bourassa PA, Sand TM, Zimetti F, Gao F, et al. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J Lipid Res. 2007;48(6):1263–72.
Okamoto H, Yonemori F, Wakitani K, Minowa T, Maeda K, Shinkai H. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 2000;406(6792):203–7.
Rittershaus CW, Miller DP, Thomas LJ, Picard MD, Honan CM, Emmett CD, et al. Vaccine-induced antibodies inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20(9):2106–12.
Clark RW, Sutfin TA, Ruggeri RB, Willauer AT, Sugarman ED, Magnus-Aryitey G, et al. Raising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapib. Arterioscler Thromb Vasc Biol. 2004;24(3):490–7.
Brousseau ME, Schaefer EJ, Wolfe ML, Bloedon LT, Digenio AG, Clark RW, et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med. 2004;350(15):1505–15.
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–22.
Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370(9582):153–60.
Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356(16):1620–30.
Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356(13):1304–16.
Yvan-Charvet L, Matsuura F, Wang N, Bamberger MJ, Nguyen T, Rinninger F, et al. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler Thromb Vasc Biol. 2007;27(5):1132–8.
Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation. 2008;118(24):2506–14.
Hermann M, Ruschitzka FT. The hypertension peril: lessons from CETP inhibitors. Curr Hypertens Rep. 2009;11(1):76–80.
Forrest MJ, Bloomfield D, Briscoe RJ, Brown PN, Cumiskey AM, Ehrhart J, et al. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br J Pharmacol. 2008;154(7):1465–73.
Simic B, Hermann M, Shaw SG, Bigler L, Stalder U, Dorries C, et al. Torcetrapib impairs endothelial function in hypertension. Eur Heart J. 2012;33(13):1615–24.
Luscher TF, Taddei S, Kaski JC, Jukema JW, Kallend D, Munzel T, et al. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur Heart J. 2012;33(7):857–65.
Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378(9802):1547–59.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99.
Tardif JC, Rheaume E, Lemieux Perreault LP, Gregoire JC, Feroz Zada Y, Asselin G, et al. Pharmacogenomic determinants of the cardiovascular effects of dalcetrapib. Circ Cardiovasc Genet. 2015;8(2):372–82.
Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA. 2011;306(19):2099–109.
Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376(20):1933–42.
Nissen SE, Pillai SG, Nicholls SJ, Wolski K, Riesmeyer JS, Weerakkody GJ, et al. ADCY9 genetic variants and cardiovascular outcomes with evacetrapib in patients with high-risk vascular disease: a nested case-control study. JAMA Cardiol. 2018;3(5):401–8.
Bloomfield D, Carlson GL, Sapre A, Tribble D, McKenney JM, Littlejohn TW 3rd, et al. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am Heart J. 2009;157(2):352–360 e2.
Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363(25):2406–15.
Group HTRC, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217–27.
Gotto AM Jr, Kher U, Chatterjee MS, Liu Y, Li XS, Vaidya S, et al. Lipids, safety parameters, and drug concentrations after an additional 2 years of treatment with anacetrapib in the DEFINE study. J Cardiovasc Pharmacol Ther. 2014;19(6):543–9.
Ford J, Lawson M, Fowler D, Maruyama N, Mito S, Tomiyasu K, et al. Tolerability, pharmacokinetics and pharmacodynamics of TA-8995, a selective cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br J Clin Pharmacol. 2014;78(3):498–508.
Hovingh GK, Kastelein JJ, van Deventer SJ, Round P, Ford J, Saleheen D, et al. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2015;386(9992):452–60.
Hu X, Dietz JD, Xia C, Knight DR, Loging WT, Smith AH, et al. Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology. 2009;150(5):2211–9.
Clerc RG, Stauffer A, Weibel F, Hainaut E, Perez A, Hoflack JC, et al. Mechanisms underlying off-target effects of the cholesteryl ester transfer protein inhibitor torcetrapib involve L-type calcium channels. J Hypertens. 2010;28(8):1676–86.
Brousseau ME, O’Connor JJ Jr, Ordovas JM, Collins D, Otvos JD, Massov T, et al. Cholesteryl ester transfer protein TaqI B2B2 genotype is associated with higher HDL cholesterol levels and lower risk of coronary heart disease end points in men with HDL deficiency: Veterans Affairs HDL Cholesterol Intervention Trial. Arterioscler Thromb Vasc Biol. 2002;22(7):1148–54.
Ridker PM, Pare G, Parker AN, Zee RY, Miletich JP, Chasman DI. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: genomewide analysis among 18,245 initially healthy women from the Women’s Genome Health Study. Circ Cardiovasc Genet. 2009;2(1):26–33.
Thompson A, Di Angelantonio E, Sarwar N, Erqou S, Saleheen D, Dullaart RP, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 2008;299(23):2777–88.
Ference BA, Kastelein JJP, Ginsberg HN, Chapman MJ, Nicholls SJ, Ray KK, et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA. 2017;318(10):947–56.
Nicholls SJ. CETP-inhibition and HDL-cholesterol: a story of CV risk or CV benefit, or both. Clin Pharmacol Ther. 2018;104(2):297–300.
Masson W, Lobo M, Siniawski D, Huerin M, Molinero G, Valero R, et al. Therapy with cholesteryl ester transfer protein (CETP) inhibitors and diabetes risk. Diabetes Metab. 2018;44(6):508–18.
Barter PJ, Rye KA, Tardif JC, Waters DD, Boekholdt SM, Breazna A, et al. Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation. 2011;124(5):555–62.
Barter PJ, Cochran BJ, Rye KA. CETP inhibition, statins and diabetes. Atherosclerosis. 2018;278:143–6.
von Eckardstein A, Widmann C. High-density lipoprotein, beta cells, and diabetes. Cardiovasc Res. 2014;103(3):384–94.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funds were used to prepare this manuscript.
Conflict of interest
SJN has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Esperion, Resverlogix, Novartis, InfraReDx, and Sanofi-Regeneron and is a consultant for Amgen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi-Regeneron and Novo Nordisk. AJN has no conflicts of interest that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Nicholls, S.J., Nelson, A.J. Do Cholesteryl Ester Transfer Protein Inhibitors Have a Role in the Treatment of Cardiovascular Disease?. Am J Cardiovasc Drugs 19, 229–235 (2019). https://doi.org/10.1007/s40256-018-00323-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-018-00323-3