Abstract
Background
Achievement of glycemic control is an important objective in the management of type 2 diabetes mellitus (T2DM).
Objective
The objective of this study was to evaluate the safety and efficacy of the dipeptidyl peptidase-4 inhibitor saxagliptin versus placebo as add-on therapy in patients with T2DM inadequately controlled with insulin alone or insulin plus metformin.
Methods
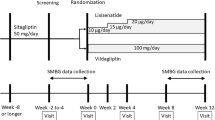
This was a long-term (28-week) extension of a short-term (24-week), randomized, double-blind, parallel-group trial of saxagliptin 5 mg once daily versus placebo as add-on therapy to open-label insulin or insulin plus metformin therapy totaling 52 weeks of treatment. In contrast with the goal of maintaining a stable insulin dosage during the short-term phase, during the extension phase the insulin dosage was flexible and adjusted as deemed appropriate by the investigator. The study was conducted in a clinical practice setting, including family practice and hospital sites. Patients with T2DM aged 18–78 years with glycated hemoglobin (HbA1c) 7.5–11 % on a stable insulin regimen (30–150 U/day with or without metformin) for ≥8 weeks at screening were included in the study. Patients were stratified by metformin use and randomly assigned 2:1 to oral saxagliptin 5 mg (n = 304) or placebo (n = 151) once daily. All patients who completed the initial 24 weeks of treatment were eligible to participate in the 28-week extension, regardless of whether they had required rescue treatment. The main outcome measure was change in HbA1c from baseline to week 52.
Results
In general, the outcomes achieved at week 24 were sustained to week 52. Adjusted mean change from baseline HbA1c at week 52 was greater with saxagliptin (−0.75 %) versus placebo (−0.38 %); the adjusted between-group difference was −0.37 % (95 % CI −0.55 to −0.19); between-group differences were similar in patients treated with metformin (−0.37 % [95 % CI −0.59 to −0.15]) and without metformin (−0.37 % [95 % CI −0.69 to −0.04]). At week 52, a greater proportion of patients receiving saxagliptin achieved HbA1c <7 % than those receiving placebo (21.3 vs. 8.7 %; between-group difference 12.6 % [95 % CI 6.1–19.1]). The increase from baseline in mean total daily insulin dose at week 52 was numerically smaller with saxagliptin (5.67 vs 6.67 U with placebo; difference, −1.01 U [95 % CI −3.24 to 1.22]). During the 52-week study period, the proportion of patients reporting ≥1 adverse event (AE) was 66.4 % with saxagliptin and 71.5 % with placebo, the majority being mild or moderate in intensity. The most common AEs (≥5 % with saxagliptin or placebo) were urinary tract infection, nasopharyngitis, upper respiratory tract infection, headache, influenza, and pain in extremity; the incidence of each AE was similar between treatment groups. In the saxagliptin and placebo groups, the incidence of reported hypoglycemia was 22.7 and 26.5 %, respectively; the incidence of confirmed hypoglycemia (fingerstick glucose ≤50 mg/dL [≤2.77 mmol/L] with characteristic symptoms) was 7.6 and 6.6 %, respectively. Adjusted mean change from baseline body weight was +0.8 kg with saxagliptin and +0.5 kg with placebo.
Conclusion
Saxagliptin 5 mg once daily as add-on to insulin, with or without concomitant metformin, produced a durable improvement in glycemic control and was well tolerated over 52 weeks of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease characterized by insulin deficiency and insulin resistance; management of the resulting hyperglycemia is an important treatment goal [1, 2]. Lifestyle modification (diet and exercise) and single-agent pharmacotherapy with metformin are currently recommended as initial therapy to treat patients with T2DM [3]. However, because of the progressive nature of the disease, additional agents with complementary mechanisms of action are often needed to achieve and maintain glycemic control. Complementary agents include other oral antidiabetics (e.g., sulfonylureas, thiazolidinediones, and dipeptidyl peptidase-4 [DPP-4] inhibitors), as well as injectable glucagon-like peptide-1 (GLP-1) receptor agonists or insulin. Combination therapy may reduce the occurrence of adverse effects compared with escalation of monotherapy to maximum doses.
Although current treatment guidelines recommend metformin as standard first-line therapy for patients with T2DM, insulin may also be considered as an initial therapeutic option for patients with glycated hemoglobin (HbA1c) >9 % [3–5]. Insulin has no dose-response ceiling and is a highly effective medication for controlling hyperglycemia; however, risks of hypoglycemia and weight gain may limit its use [3, 6, 7]. In addition, some insulin regimens may not provide adequate postprandial glucose control [8, 9]. Thus, adding an agent that can improve glycemic control without increasing the risk of hypoglycemia and weight gain (such as metformin or an incretin-based agent) may be preferable to increasing doses of insulin.
The DPP-4 inhibitors stimulate postprandial insulin release and suppress glucagon release and are effective in regulating postprandial glucose concentrations and fasting glucose [10]. DPP-4 inhibitors are recommended for use in combination with metformin when metformin monotherapy does not result in adequate glycemic response and for use as initial therapy when metformin is contraindicated [4]. Because their effect is glucose dependent, diminishing as glucose concentrations decline, DPP-4 inhibitors have a risk of hypoglycemia similar to that of placebo; furthermore, these agents do not induce weight gain [11]. Thus, as an add-on to insulin therapy, DPP-4 inhibitors may provide a rational alternative to escalating the dosage of insulin monotherapy.
Saxagliptin is a potent, selective DPP-4 inhibitor approved as an adjunct to diet and exercise to improve glycemic control in patients with T2DM [12]. A randomized, double-blind, placebo-controlled, 24-week trial of saxagliptin (ClinicalTrials.gov identifier: NCT00757588) was conducted in patients with T2DM with inadequate glycemic control when treated with insulin alone or in combination with metformin [13]. As add-on to continuing therapy with a stable dose of insulin with or without metformin, at 24 weeks saxagliptin 5 mg produced significantly greater reductions in HbA1c and postprandial glucose than placebo. The proportion of patients achieving HbA1c <7 % was greater in the saxagliptin group versus the placebo group; results were comparable in patients with and without metformin use. Small numerical increases in body weight from baseline were observed for patients in the saxagliptin and placebo groups at 24 weeks. In addition, the adjusted mean increase in the total daily insulin dose was numerically smaller with saxagliptin than with placebo.
To assess the long-term safety and efficacy of this approach, patients completing the initial 24-week short-term phase of that trial (NCT00757588) were eligible for inclusion in a 28-week, blinded long-term extension, totaling 52 weeks of treatment. After the 24-week short-term period, the insulin dose was flexible (able to be modified at the discretion of the physician to control the patient’s hyperglycemia), whereas the metformin dose was maintained unchanged, allowing assessment of the effects of saxagliptin add-on to an insulin regimen with or without metformin under conditions that reflect clinical practice. Results from the extension phase (which were cumulative from the preceding 24-week trial) are the subject of this report.
2 Methods
2.1 Study Design
This was a multicenter, randomized, double-blind, placebo-controlled, 2-arm, parallel-group, phase IIIb clinical trial. It comprised three phases: (1) 4-week run-in on placebo plus continued treatment with insulin alone or insulin in combination with metformin; (2) 24-week double-blind study of saxagliptin 5 mg versus placebo as add-on to continuing treatment with insulin alone or insulin in combination with metformin; and (3) 28-week site-and-patient blind extension of the 24-week trial. The study was conducted in accordance with Good Clinical Practice, as defined by the International Conference on Harmonisation and the Declaration of Helsinki. Before initiation, study approval was obtained from the institutional review board/independent ethics committee at each institution (University of Stellenbosch, South Africa; W.I.T.S. Health Consortium Ethics Committee, South Africa; Pharma-Ethics, South Africa; Leeds East Research Ethics Committee, UK; Cpp Ouest 6, France; IRB Services, Canada; University of Western Ontario Reb for Hsc, Canada; Regina Qu’Appelle Health Region, Canada; New England Institutional Review Board, USA; Cardiolink Clinical Trials S.C., Mexico; Incmnsz—Salvador Zubiran, Mexico; Unidad de Investigacion Clinica En Medicina, Mexico; Instituto Biomedico De Investigacion, A.C., Mexico; Comite De Bioetica Para, Mexico; Hosp. General De Occidente, Mexico; Hosp. Universitario De Monterrey, Mexico; Med. Research Council Ethics Committee, Hungary; Clinical Roma, Mexico; College of Physicians and Surgeons of Alberta, Canada; Committee on Ethics Under Federal Supervision Service for Public Health, Russian Federation; Komisja Bioetyczna Przy Slaskiej Izbie Lekarskiej, Poland; Centenario Hosp. Miguel Hidalgo, Mexico; Bharti Research Institute of Diabetes & Endo., India; Chowpatty Medical Center, India; Christian Medical College, India; Totall Diabetes Hormone Institute, India; Centre of Excellence for Diabetes, India; Deshmukh Clin., India; King Edward Memorial Hospital, India). All patients provided written informed consent before participation.
2.2 Patients
Men and women aged 18–78 years with T2DM, fasting C-peptide ≥0.8 ng/mL, body mass index (BMI) ≤45 kg/m2, and inadequate glycemic control (HbA1c 7.5–11.0 %) on a stable regimen of insulin (30–150 U/day, with ≤20 % variation in total daily dose for ≥8 weeks before screening) were eligible for the study. Intermediate-acting insulin, long-acting (basal) insulin, or a premixed formulation in which rapid- or short-acting insulin constituted one component was permitted. Patients could also be taking metformin if the daily dose was stable for ≥8 weeks before screening. No other antidiabetic medications were permitted. Exclusion criteria were poorly controlled diabetes (e.g., marked polyuria and polydipsia with >10 % weight loss during the 3 months before screening); history of diabetic ketoacidosis or hyperosmolar nonketotic coma; history of significant cardiovascular disease or hemoglobinopathy; contraindications to DPP-4 inhibitors, metformin, or insulin; or pregnancy, breast feeding, or not using an acceptable method of birth control.
2.3 Treatment
Patients were stratified based on metformin use at enrollment and randomized 2:1 via an interactive voice response system using a blocked randomization schedule to receive saxagliptin 5 mg (Onglyza®, Bristol-Myers Squibb Company, Princeton, NJ, USA, and AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA) or placebo once daily as add-on to baseline therapy with insulin or insulin plus metformin. To maintain blinding to patients and physicians, saxagliptin and placebo tablets were identical in appearance, and bottles were printed with a blinded label. During the initial 24-week phase, patients continued on a stable dose of their insulin regimen from baseline; patients were to be rescued by having their insulin doses increased starting at week 4 for inadequate glycemic control as defined by progressively stringent criteria (fasting plasma glucose [FPG] >240 mg/dL [13.3 mmol/L] at weeks 4 or 6; FPG >220 mg/dL [12.2 mmol/L] at week 8; FPG >200 mg/dL [11.1 mmol/L] at weeks 12, 16, or 20). Alternatively, patients were considered rescued if a >20 % increase in their mean total daily doses of insulin for two consecutive visits had occurred. After rescue during the short-term period, the insulin dosage was flexible and adjusted as deemed appropriate by the physician to control hyperglycemia without causing hypoglycemia. In patients who did not require rescue, insulin doses were kept stable until the end of the 24-week phase. All patients who completed the initial 24 weeks of treatment were eligible to participate in the 28-week extension, regardless of whether they had required rescue treatment. During the extension phase, patients continued to take the same blinded study medication assigned during the initial 24-week phase. However, the insulin dosage was flexible for all patients and adjusted as deemed appropriate by the investigator.
2.4 Assessments
Efficacy end points for the long-term extension were exploratory and included adjusted mean change from baseline HbA1c at week 52, proportion of patients achieving HbA1c <7 % at week 52, and mean change from baseline in total daily insulin dose at week 52. Safety end points included adverse events (AEs), hypoglycemia, and weight gain.
2.5 Statistical Analyses
Efficacy data were analyzed in all patients who were randomized to treatment, took ≥1 dose of double-blind treatment, and had a baseline measurement and ≥1 postbaseline measurement for the end point. Changes from baseline HbA1c, mean total daily insulin dose, body weight, and BMI at postbaseline visits were analyzed using a repeated-measures model that contained terms for treatment group, baseline measurement, metformin use at baseline, time (weeks), and time by treatment group. Repeated-measures analysis also took into account missing data. In addition, analysis of covariance (ANCOVA) was performed on the continuous efficacy end points, using last observation carried forward (LOCF), with treatment group as an effect and baseline value and metformin use at baseline as covariates. ANCOVA with observed values was also used to describe the changes from baseline in HbA1c and mean total daily insulin dose. This analysis method differs from the primary analysis used for the 24-week phase (ANCOVA, including only data before rescue). Because insulin dosage was flexible after rescue in the initial 24-week phase and throughout the 28-week extension, the current efficacy and safety analyses were conducted without regard for changes in insulin dosage. Adjusted mean change from baseline HbA1c was compared between groups; this was performed as a post hoc analysis without formal control for type I error. The proportion of patients achieving HbA1c <7 % (LOCF) at selected visits was summarized for each treatment group using counts and percentages. The 95 % confidence intervals for the difference in proportions between treatment groups were calculated based on the Mantel–Haenszel method.
Analyses carried out for each metformin use stratum included change in HbA1c from baseline to week 52, change in mean total daily dose of insulin from baseline to week 52, and proportion of patients achieving a therapeutic glycemic response of HbA1c <7 %.
The incidence of AEs, including AEs of special interest (i.e., hypoglycemia, skin disorders, lymphopenia, thrombocytopenia, localized edema, opportunistic infections, hypersensitivity, fracture, pancreatitis, and cardiovascular events) was captured for all treated patients and summarized using proportions for all randomized and treated patients regardless of insulin dose. AEs were also summarized for each metformin use stratum.
3 Results
3.1 Patients
Patient disposition is shown in Fig. 1 (enrollment and lead-in information have been reported previously [13]). Study completion rates were similar between groups (saxagliptin, n = 246 [80.9 %]; placebo, n = 125 [82.8 %]). Primary reasons for discontinuation of the extension phase included withdrawal of consent (saxagliptin, n = 3 [1.1 %]; placebo, n = 2 [1.5 %]), patient no longer meeting study criteria (saxagliptin, n = 5 [1.6 %]; placebo, n = [3.0 %]), AEs (saxagliptin, n = 4 [1.5 %]; placebo, n = 0 [0 %]), and poor compliance (saxagliptin, n = 5 [1.6 %]; placebo, n = 2 [1.5 %]). Demographic and clinical data at baseline were similar between groups (Table 1).
In the overall study population, mean age was 57 years, 59 % of patients were female, and 78 % were white. Mean baseline HbA1c was 8.7 % (saxagliptin, 8.7 %; placebo, 8.6 %). Mean body weight was 87.2 kg (saxagliptin, 87.7 kg; placebo, 86.2 kg), and mean BMI was 32.3 kg/m2 (saxagliptin, 32.6 kg/m2; placebo, 31.8 kg/m2).
3.2 Baseline Regimens
Baseline antihyperglycemic regimens are summarized in Table 1. The metformin dose was to be maintained unchanged throughout the study. The insulin regimen was premixed insulin alone in more than 50 % of patients and long- or intermediate-acting insulin alone in 35–40 % of each group. Relatively few patients used combinations of different types of insulin. The mean total daily dose of insulin at baseline was slightly lower in the saxagliptin group (53.6 U [range 19–150]) than in the placebo group (55.3 U [range 30–149]). The majority of patients (saxagliptin, 68.6 %; placebo, 69.5 %) were taking metformin with insulin. The mean total daily dose of metformin was slightly lower in the saxagliptin group (1,805 mg [range 250–3,000]) than in the placebo group (1,861 mg [range 850–3,000]). Metformin doses were ≥2,000 mg in 48 % of patients.
3.3 Study Exposure
Overall, >80 % of patients completed ≥48 weeks of blinded treatment. The mean duration of exposure to study medication was 47 weeks in both the saxagliptin and placebo groups.
3.4 Long-Term Efficacy (Week 52)
Efficacy data for the initial 24-week phase of the study have been reported previously [13]. The adjusted mean change from baseline HbA1c at week 52 was −0.75 % with saxagliptin versus −0.38 % with placebo (Fig. 2); the between-group mean difference was −0.37 % (95 % CI −0.55 to −0.19; post hoc P < 0.0001). Similar results were observed when using ANCOVA-LOCF analysis (Electronic Supplementary Material, Table S1), data before a 20 % change in insulin dose (Electronic Supplementary Material, Table S2), and observed values (Electronic Supplementary Material, Table S2). In addition, results were similar in patients with and without concomitant metformin treatment (Fig. 2). Differences between saxagliptin and placebo groups appeared fairly consistent after week 24 (Fig. 3a, b), with similar differences in the metformin subgroups.
Mean ± standard error changes from baseline in HbA1c at week 52; repeated-measures analysis. Three patients were classified as completers but had missing week-52 HbA1c values. *P < 0.0001 in a post hoc comparison with no formal controlling for type I error. HbA 1c glycated hemoglobin, INS insulin, MET metformin, PBO placebo, SAXA saxagliptin
Overall, a greater proportion of patients achieved HbA1c <7 % with saxagliptin (21.3 %) versus placebo (8.7 %; between-group mean difference, 12.6 % [95 % CI 6.1–19.1]). The proportion of patients achieving HbA1c <7 % was consistently higher in patients receiving saxagliptin versus placebo, regardless of metformin use: 23.8 versus 8.7 %, in patients receiving metformin and 16.0 versus 8.7 % in patients treated without metformin (Fig. 4).
The mean total daily dose of insulin increased from baseline in both groups at 52 weeks (Table 2); the change was 5.67 U (10.6 %) with saxagliptin and 6.67 U (12.1 %) with placebo (between-group difference, −1.01 U [95 % CI −3.24 to 1.22]). Results of the LOCF sensitivity analysis were consistent with the results of the repeated-measures analysis (Electronic Supplementary Material, Table S1). With respect to metformin use, the difference in adjusted mean change from baseline (saxagliptin vs. metformin) in the mean total daily dose of insulin at week 52 was −2.38 U (95 % CI −5.06 to 0.30) in patients with metformin use at baseline and 2.06 U (95 % CI −1.94 to 6.06) in patients with no metformin use at baseline.
3.5 Safety and Tolerability
The incidence of AEs, treatment-related AEs, serious AEs (SAEs), treatment-related SAEs, and discontinuations due to AEs were generally comparable between the saxagliptin and placebo groups (Table 3). Most AEs were mild or moderate, and the most commonly reported AEs, which had similar incidences in both treatment groups, were infections (urinary tract infection, nasopharyngitis, upper respiratory tract infection, influenza), headache, and pain in the extremities; classification of patients by metformin use revealed no unexpected differences in the pattern of AEs (Table 3). AEs occurring in ≥5 % of patients receiving saxagliptin and in a greater proportion of patients than with placebo were headache, bronchitis, and confirmed hypoglycemia. Excluding hypoglycemia, AEs of special interest (skin disorders, lymphopenia, thrombocytopenia, localized edema, opportunistic infections, hypersensitivity, fracture, pancreatitis, and cardiovascular events) were infrequent with both saxagliptin and placebo (Electronic Supplementary Material, Table S3). No patients had alanine aminotransferase or aspartate aminotransferase >3 times the upper limit of normal (ULN) or total bilirubin >2 times ULN. The incidence of reported hypoglycemic events (e.g., defined by the presence of characteristic symptoms) was 22.7 % with the addition of saxagliptin and 26.5 % with placebo, and the incidence of confirmed hypoglycemic events (fingerstick glucose <50 mg/dL [2.77 mmol/L] in a patient with characteristic symptoms) was 7.6 % with the addition of saxagliptin and 6.6 % with placebo (Table 3). The majority of the confirmed hypoglycemic events were mild or moderate in intensity. Two patients (0.7 %) in the saxagliptin group experienced a total of four severe confirmed hypoglycemic events, and three patients (2.0 %) in the placebo group experienced a total of three severe confirmed hypoglycemic events; there were no very severe confirmed hypoglycemic events. Two patients in the saxagliptin group died during the study. One patient died of a presumed myocardial infarction during the short-term phase, and one patient died owing to atherosclerotic gangrene of the small intestine in the long-term phase; both deaths were deemed unrelated to saxagliptin treatment.
Repeated measures analysis revealed a small mean increase in body weight from baseline to week 52 in both the saxagliptin (0.80 kg) and placebo (0.50 kg) groups (between-group mean difference, 0.30 kg [95 % CI −0.25 to 0.85]). This corresponded with small increases in BMI at week 52 in the saxagliptin (0.31 kg/m2) and placebo (0.18 kg/m2) groups, with a between-group mean difference of 0.13 kg/m2 (95 % CI −0.08 to 0.33).
4 Discussion
The present study evaluated the long-term safety and efficacy of saxagliptin as add-on to existing therapy in patients with inadequate glycemic control on stable doses of insulin, with or without concomitant use of metformin. Because they have a low risk of hypoglycemia and weight gain, DPP-4 inhibitors may achieve better overall outcomes as add-on therapy to insulin than escalating the dosage of insulin monotherapy. Short-term, placebo-controlled studies with sitagliptin, alogliptin, and vildagliptin have demonstrated similar results to saxagliptin [14–16]. As might be expected in patients with T2DM, the majority of patients in our study were overweight, with a mean weight >87 kg and mean BMI >32 kg/m2. The study population was diverse, representing a range of ages (18–77 years), duration of T2DM (0.2–36.8 years), and daily doses of background insulin (19–150 U).
Over 52 weeks, saxagliptin produced sustained improvements in mean HbA1c and was associated with a higher rate of therapeutic response (HbA1c <7 %) compared with placebo. Changes from baseline were only slightly smaller than those observed at week 24, indicating good durability [13]. This is notable given the flexible background dosing of insulin during the 28-week extension phase, which might be expected to reduce any between-group differences in glycemic control. Although insulin dosage increased slightly over time (approximately 5–6 U at week 52) in both treatment groups, the incidence of confirmed hypoglycemia was 7.6 % with saxagliptin (7.2 % with concomitant metformin, 8.4 % without metformin) and 6.6 % with placebo (4.8 % with concomitant metformin, 10.9 % without metformin). Moreover, the addition of saxagliptin or placebo to insulin therapy did not result in clinically relevant increases in weight. In contrast, a recent 52-week trial of several types of insulin, in which patients inadequately controlled on basal insulin received supplemental bolus pre- or postmeal insulin, showed a much higher incidence of severe hypoglycemia (17.5–20.8 %) and substantially more weight gain (adjusted mean weight change from baseline of +4.66 to +5.53 kg) [17].
The types and incidences of AEs (including infection-related AEs) [18–20] were generally comparable for saxagliptin and placebo, with or without concomitant metformin. Although metformin has been associated with gastrointestinal AEs [21], the incidence of such AEs in the present study was relatively low in patients receiving metformin plus insulin. The requirement that eligible patients be receiving metformin at stable doses for a minimum of 8 weeks before study entry may have resulted in the inclusion of patients better able to tolerate metformin in the study population.
These results extend the findings of previously published, shorter-duration studies (i.e., 24–26 weeks) that demonstrated that DPP-4 inhibitor add-on therapy to insulin improves glycemic control versus placebo add-on therapy in patients with T2DM [14–16]. To date, long-term data in this population are sparse, with 52-week findings reported only for the vildagliptin add-on therapy to insulin study, in which the 24-week placebo-controlled core phase was followed with a 28-week uncontrolled extension phase. In that study, improvements in glycemic control achieved with vildagliptin in the core phase were maintained in the extension phase, and glycemic control for patients who had received placebo in the core phase improved when patients were switched to vildagliptin in the extension phase [22]. Thus, the current study findings help establish the long-term outcomes of DPP-4 inhibitor add-on therapy to insulin compared with placebo.
4.1 Study Limitations
In this long-term extension study, flexible dosing of insulin allowed for assessment of the differences between saxagliptin and placebo under conditions that reflect ordinary clinical practice. Although flexible dosing of insulin limits the formal comparison of saxagliptin versus placebo as add-on to insulin with or without metformin, the consistency of the efficacy data from week 24 to week 52, without a disproportional increase in the mean total daily dose of insulin in the saxagliptin group versus the placebo group, implies a degree of robustness in the efficacy data. Approximately 80 % of patients in the combined treatment groups had available glycemic measurements at week 52. Statistical analyses used to compare mean changes between treatment groups assumed that unobserved glycemic measurements in patients who previously discontinued could be inferred using available pre-rescue values of these patients, along with observed values in the remaining patients. With approximately 20 % of patients having missing measurements at week 76, the estimates and P values are dependent on this assumption. In addition, P values associated with these comparisons were not prospectively specified or adjusted for multiplicity. For these reasons, the results of the long-term glycemic parameter analyses should be interpreted with a level of caution.
5 Conclusions
Over a 52-week period, saxagliptin 5 mg as add-on therapy to insulin with or without metformin improved glycemic control in patients with T2DM to an extent similar to that observed during the core phase, indicating good durability of effect. The risk of hypoglycemia with saxagliptin add-on therapy was similar to that with placebo, and both treatment groups had only small numerical increases in body weight. Incidence rates for AEs, SAEs, and discontinuations due to AEs were similar in patients receiving saxagliptin 5 mg or placebo as add-on therapy, reflecting that saxagliptin was well-tolerated as add-on therapy to insulin in this long-term study.
References
DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88(4):787–835, ix.
UK Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44(11):1249–58.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79.
Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540–59.
Pradhan AD, Everett BM, Cook NR, et al. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA. 2009;302(11):1186–94.
Barnett AH, Cradock S, Fisher M, et al. Key considerations around the risks and consequences of hypoglycaemia in people with type 2 diabetes. Int J Clin Pract. 2010;64(8):1121–9.
Charbonnel B, Cariou B. Pharmacological management of type 2 diabetes: the potential of incretin-based therapies. Diabetes Obes Metab. 2011;13(2):99–117.
Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357(17):1716–30.
Gordon J, Pockett RD, Tetlow AP, et al. A comparison of intermediate and long-acting insulins in people with type 2 diabetes starting insulin: an observational database study. Int J Clin Pract. 2010;64(12):1609–18.
Freeman JS. Managing hyperglycemia in patients with type 2 diabetes mellitus: rationale for the use of dipeptidyl peptidase-4 inhibitors in combination with other oral antidiabetic drugs. J Am Osteopath Assoc. 2010;110(9):528–37.
Freeman JS. A physiologic and pharmacological basis for implementation of incretin hormones in the treatment of type 2 diabetes mellitus. Mayo Clin Proc. 2010;85(12 suppl):S5–14.
ONGLYZA (saxagliptin). Full prescribing information. Princeton, NJ: Bristol-Myers Squibb; 2011.
Barnett AH, Charbonnel B, Donovan M, et al. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin. 2012;28(4):513–23.
Fonseca V, Schweizer A, Albrecht D, et al. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50(6):1148–55.
Rosenstock J, Rendell MS, Gross JL, et al. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11(12):1145–52.
Vilsboll T, Rosenstock J, Yki-Jarvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(2):167–77.
Ratner R, Wynne A, Nakhle S, et al. Influence of preprandial vs. postprandial insulin glulisine on weight and glycaemic control in patients initiating basal-bolus regimen for type 2 diabetes: a multicenter, randomized, parallel, open-label study (NCT00135096). Diabetes Obes Metab. 2011;13(12):1142–8.
Neumiller JJ, Campbell RK. Saxagliptin: a dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes mellitus. Am J Health Syst Pharm. 2010;67(18):1515–25.
Richter B, Bandeira-Echtler E, Bergerhoff K, et al. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4(4):753–68.
Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298(2):194–206.
Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med. 2011;123(1):15–23.
Fonseca V, Baron M, Shao Q, et al. Sustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitus. Horm Metab Res. 2008;40(6):427–30.
Acknowledgments
Drs Barnett and Charbonnel recruited and treated patients, and all authors contributed to the analysis and interpretation of study data and the preparation, review, and final approval of the manuscript. All authors confirm that this paper is an accurate representation of the study results. Bristol-Myers Squibb and AstraZeneca funded the study and participated in the study design and collection, analysis, and interpretation of the data and review of the manuscript. The decision to submit the manuscript to Clinical Drug Investigation was made independently by the authors. Medical writing support for the preparation of this manuscript was provided by Erica Wehner, RPh, and Nancy Sheridan from Complete Healthcare Communications, Inc. (Chadds Ford, PA, USA), with funding from Bristol-Myers Squibb and AstraZeneca. Karen May from Bristol-Myers Squibb contributed expertise and insights to the conduct of the study at every stage. Drs Li, Donovan, and Iqbal are employees of Bristol-Myers Squibb and have ownership of company stock. At the time of writing this manuscript, Dr Fleming was an employee of Bristol-Myers Squibb. Dr Barnett has received honoraria for lectures and advisory work as well as research funding from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi-Aventis, and Takeda. Dr Charbonnel has received fees for consultancy, speaking, travel, or accommodation from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Sanofi-Aventis, and Takeda.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov identifier: NCT00757588.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barnett, A.H., Charbonnel, B., Li, J. et al. Saxagliptin Add-on Therapy to Insulin With or Without Metformin for Type 2 Diabetes Mellitus: 52-Week Safety and Efficacy. Clin Drug Investig 33, 707–717 (2013). https://doi.org/10.1007/s40261-013-0107-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0107-8