Abstract
This study focused on the potential for pentachlorophenol removal by a biological process in secondary treated wastewater (STWW). The proposed process is a combined method of phytoremediation using a native plant, Polypogon maritimus and Lemna minor, and bioaugmentation using a fungus. The bioaugmentation process was performed by a fungal isolate capable of removing PCP, isolated from the compost. The identification of the fungus was performed by morphological, biochemical, and molecular methods. A biological treatment system by bioaugmentation and phytoremediation was set up to estimate the capacity of this process to eliminate a high concentration of PCP. physico-chemical parameters, such as pH, COD, and BOD were tested at experimentation times T0 (initial) and Tf (final). The concentration of PCP is controlled by the HPLC method. Thus, the growth of the fungus was determined by spectrophotometry and enumeration on the agar medium. The results obtained show that the isolated and selected fungus is identified by Penicillium Ilerdanum. The fungal strain used has a significant capacity for tolerance and elimination of PCP. The results of the physico-chemical parameters showed an improvement in the quality of wastewater after the treatment was carried out. The elimination of PCP came with a release of Common law- and an important decrease in the DOC value in the STWW. The results obtained show that the Polypogon treatment shows a significant elimination of PCP by a percentage of the order of 92.01% and 23.58 g. L− 1 chloride concentration. The macrophytes used showed a better ability to tolerate and eliminate PCP with an increase of chlorophyll and its longer sheets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution because of hazardous waste, organic pollutants, and heavy metals has damaged the natural ecosystem by man [1, 2]. Surface waters are vital for supporting people and ecosystems; however, freshwater availability is under increasing pressure because of a growing human population requiring access to safe water [3, 4]. The surface water pollutants that are of concern and where remediation solutions are being developed. Water pollutants can be broadly categorized as either organic, e.g., hydrocarbons, pesticides, and algal toxins, or inorganic (metals or synthetic and manure-based fertilizers containing excess amounts of N and P, or biological [pathogens and algal toxins] [5]. The current biological treatment plants in the most developed countries are quite effective in removing the main pollutants from water. The wastewater treatment process requires the use of biological processes [phytoremediation, bioaugmentation, biostimulation…] which play an important role in the removal of micro-pollutants [6]. Aquatic phytoremediation is a phytotechnology used for the removal of pollutants from surface waters and the restoration of affected water bodies [rivers, streams, lakes, ponds] [7]. In surface waters, plants can be grown to remove pollutants from water and sediment [8, 9] and can be deployed either at the point source or in water bodies where pollution is diffused [10]. Thus, the process of phytoremediation can be used both for sustainable sanitation and as a management strategy for nutrient recovery from water [5]. Emergent and floating macrophytes primarily absorb nutrients and other contaminants through their roots and stem tissue [11]. Thus, Lemna macrophytes have shown high potential for pesticide removal example: L. minor, L. gibba, and Lemna [12, 13]. Besides, this phytoremediation process depends on microbial activity such as those associated with roots, which represents an important factor in the phytodegradation of pollutants [13,14,15,16]. These include bacteria that enhance macrophyte activity through their contribution to nitrification and denitrification and biological mineralization of organic phosphorus [17]. Also, through the process of bioaugmentation, microorganisms are found with the ability to degrade or convert hazardous compounds into less toxic compounds [18]. The bioaugmentation process also allows for the identification of microorganisms capable of degrading or converting hazardous compounds into less toxic ones [18]. According to the literature, most fungi are robust organisms and more tolerant to high levels of pollutants than bacteria, which is justified and supported by their ultra-sophisticated morphological and biological structure [19]. Fungi such as Phanerochaete chrysosporium, Trametes versicolor, A. sydowii, and Pleurotus are promising agents for the remediation of PCP-contaminated sites, as reported by Ryu et al. [20] and Zhou et al. [21]. Lentinus crinitus CCIBt2611 also exhibited the ability to mineralize hexachlorobenzene and pentachlorophenol and reactive textile dyes [22,23,24,25].
This study aimed to examine the removal rates of PCP added in lab microcosm-constructed wetlands composed of fixed and floated plants in secondary wastewater and supplemented with an isolated fungus strain. The novelty is the choice of a macrophyte Polypogon maritimus and Lemna minor still used for the elimination of heavy metals but it is not used for pesticide and pollutant removal in wastewater. The first part is based on the monitoring of the capacity of the fungal bioaugmentation process in the removal of PCP. The second part is devoted to the analysis of the effect of the combined phytoremediation-bioaugmentation treatment of PCP on the physico-chemical and microbiological parameters of the wastewater.
Materials and methods
Chemicals and reagents reagent-grade
Samples of wastewater with no detectable PCP were tested at the scale Charguia I [WWTP] positioned in a residential and business area in Tunis City, northern Tunisia. This mega wastewater plant in Charguia I of Tunis City treats a wastewater flow of about 60,000 m3 /day [26]. The WWTP was drained by mixed industrial, urban, domestic, 24 hospitals, and rainwater coming from different zones of the great Tunis. The WWTPs use bio-physical-chemical processes: preliminary treatment, primary settling, secondary treatment by sewage sludge, secondary clarification, and tertiary treatment. A sampling of wastewater is made at the exit of the secondary wastewater clarification system [STWW] [Fig. 1]. The wastewater samples were stored at 4° C for determination of their main physical and chemical characteristics such as cation exchange capacity and pH, total nitrogen [27], and total carbon [28]. In addition, the bacteriological analyzes were realized by NPP standard method as reported in the [www.standardmethods.org/) for an enumeration of the: total coliforms (CT), total streptococci (St), fecal coliforms (FC), Fecal Streptococci (FS), and Escherichia coli (EC) for all tested wastewater.
Selection and identification of fungi strains skilled in degrading PCP
For the selection of fungal isolates skilled in degrading PCP, we have used soil samples beforehand irrigated for 20 years with municipal treated wastewater. Soil suspension dilution in sterile physiological water was prepared, plated in growth potato dextrose Agar (PDA) supplemented by 1000 mg L− 1 of PCP, and cultured for at least three days at 26 °C. The plate showing a predominant one only mycelium type was selected and submitted for further investigation of its main morphological, microbiological characteristics, and molecular examination. Morphological and molecular identification was performed for the selected isolate. Macroscopic characteristics were studied on malt extract agar (MEA) media, according to Samson et al. [29]. The main morphological characteristics considered were colony size, color, and texture, while microscopic observation was carried out on the 7–10-day culture on MEA media using a light microscope (Leica). Genomic DNA was extracted from 7-day-old fungal cultures grown on MEA. For molecular identification, fungal DNA was extracted using the method of Liu et al. [30]. The internal transcript space (ITS) genes used were ITS4, 5’-TCCCGCTTATTGATATGC-3’) and (ITS5, 5’-GGAAGTAAAAGTCGTAACAAGG-3’) described by White et al. [31]. The fungal isolate sequence was extracted from the NCBI Gen Bank database.
Bioaugmentation process
The PCP bioaugmentation process was performed using fungi isolate and was selected. The inoculum was prepared in an enriched liquid medium potato dextrose broth for 48 h to obtain around 108 CFU· mL –1. This inoculum was then transferred to a 250 mL flask containing 100 mL of MSM or sterilized secondary wastewater. The sterilized liquid medium was supplemented with PCP at different concentrations of 50, 100, 500, 1000, and 1500. The composition of MSM earlier reported by Ryu et al. [20] was: 3 g. L− 1 NaNO3, 0.5 g. L− 1 MgSO4, 0.01 g. L− 1 FeSO4, 0.5 g. L− 1 KCl, 1 g. L− 1 K2HPO4, and 5 g. L− 1 glucose, The MSM-PCP mixture suspension was kept under agitation on an orbital shaker at 150 rpm for 168 h. The pH solution was accustomed to 7.3 ± 0.2. PCP was mixed with the sterile medium after autoclaving.
Phytoremediation–bioaugmentation process
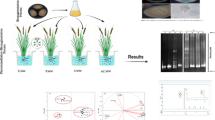
Two phytoremediation processes were realized using two specific varieties of macrophyte plants, Polypogon maritimus, and Lemna minor, known for their ability to purify PCP-contaminated secondary wastewater (Fig. 2). In the first step, some small young and healthy seedlings of Polypogon maritimus and 20 g of Lemna minor, originally collected from a local natural lagoon, were subjected to 6-week growth in the PCP-contaminated secondary wastewater to ensure an adaptation and growth change of these two macrophytes to their new environment. In the second step, the wastewater was contaminated with a known amount of 1000 mg. L− 1 of PCP as recommended by Manios et al. [32]. PCP with MW 226.34 and superior 99% purity was purchased from Sigma-Aldrich (USA) and prepared in NaOH (0.5 N). Experiments carried out in triplicates (n = 3), included two categories of control: control with planting, and control without planting, and were carried out for 20 days.
Description of treatments process of contaminated Wastewater. (T1) bioaugmentation process, (T2) phytoremediation process (Lemna), (T3) phytoremediation process (polypogon), (T4) phytoremediation process (Lemna- polypogon), (T5) phytoremediation- bioaugmentation (Lemna- polypogon- Fungi) and (T6) control SWWT.
PCP content determination
In this study, the PCP removal was controlled for different wastewater treatments of the conducted phytoremediation-bioaugmentation bioremediation process. The PCP removal measure was carried out by high-performance liquid chromatography (HPLC), and extracted by a methanol solution. An aliquot of one milliliter was taken from the different treatments on the first day of the experiment (T0), and after 20 days (TF) of experimentation, was added to 1 mL of methanol. This last suspension of methanol will be vortexed for 5 min and stored at 20 °C for 10 min. This last stored sample will be once again vortexed for 5 min and centrifuged at 8000 rpm for 5 min, and the supernatant was filtered through a 0.22 mm sterile filter. The filtrate was submitted to HPLC analysis (Perkin Elmer Series YL9100 system instrument) as reported by Karn et al. [33]. All analyzes were always carried out in triplicates.
Chloride residual continent
In different treatments of bioaugmentation and phytoremediation, the chloride analysis was released by the calorimetric method. One milliliter of K2CrO4 (5%), 3 drops of nitric acid (65%, d = 1.42), and 50 mg pure calcium carbonate were added to a 20 ml sample. The sample was then homogenized by a magnetic stirrer and titrated with 0.0282 N silver nitrate solution until the color changed from yellow to brick red. Blind tests were performed with distilled water. Chloride levels are expressed in g L − 1 [1].
Fungi growth
Indirect estimation of fungal mycelium growth Nephelometric method (OD600 determination) in liquid media. This OD at 600 nm determination of isolated and selected fungi growth is determined by a turbid culture that will increase proportionally as it multiplies. back microbial growth has largely been established in the literature Determine OD by spectrophotometry at 600 nm [28].
Fungi enumeration
The monitoring of the fungal growth of the selected strain during the treatment period of STWW supplemented with PCP was performed by the culture method on a specific medium. The capability of fungi to tolerate the organic toxicants was revealed by growing strains on solid media Potato dextrose Agar (PDA) at 25 °C for 5 to 7 days. The fungus abundance was measured using the colony-forming units (CFUs) method [36]. All analyzes were carried out in triplicates.
Plant growth
The plant growth was evaluated by the aerial and roots biomass development based on their dry weight at 80 °C between 24 and 48 h and for the different treatments [28]. All analyzes were carried out in triplicates.
Photosynthetic pigments
Fresh leaf samples, arbitrarily taken, were cut into 1–2 cm square pieces, frozen, and analyzed for chlorophyll a and b as following described: 0.2 g of prepared fresh leaf samples were submitted to 5 mL of 99.9% acetone extraction and centrifuged at 5000 g for 6 min at 4° C. Thereafter, the absorbance of the supernatant was respectively measured at 470 nm, 646.6 nm, 646.8 nm, 663.2 nm, and 720 nm as recommended by Arnon [34] and Lichtenthaler [35].
Plant photosynthetic pigments were determined using these equations:
Statistical analysis
Two-way ANOVA analysis and Principal Component Analysis (PCA) were performed on the physical and chemical parameters of different treatments of phytoremediation-bioaugmentation. Before doing PCA, data were log10 transformed. Differences between the two parameters were compared by an independent-sample t-test (two-tailed). All data were presented as mean ± SD (n¼ 3) and statistical analyzes were performed by using GraphPad Prism 5.0.
Results
The characteristics of wastewater
The first important information about the present wastewater treatment system should interest the determination of the main average bio-physical-chemical characteristics of wastewater to be treated in this study. The quality of wastewater is usually expressed in terms of its physical, chemical, and biological characteristics in Table 1. The secondary treated wastewater (STWW) is characterized by a neutral pH of about 7.06 and a high salinity of about 1827 µs. However, the STWW is characterized by an average COD of the order of 400 mg. L− 1. According to the obtained results, other parameters are high such as chloride, DOB5, SUR, and SS… The characterization of the STWW effluent shows the need for a refining treatment that can be biological such as the phytoremediation process to improve its quality.
Identification of selected fungal isolates degrading PCP
The main morphological, microbiological, and molecular investigation of the only fungal mycelium grown on a PDA medium allowed us to identify this mycelium as Penicillium ilerdanum with an accession number OP862882 Taxonomic description. The photos of colonies and fungal structures of Penicillium ilerdanum are presented in Fig. 3. The morphology of this fungal strain confirmed the characteristics of Penicillium ilerdanum reported by Alker et al. (2001). The molecular 16 S rRNA gene sequencing followed by NCBI-BLAST analysis confirmed this morphological identification.
The selected fungus gives a high capacity of resistance and tolerance of PCP at different tested concentrations of 50, 100, 500, 1000, and 1500 mgL− 1 (Fig. 4). According to the results shown in Fig. 4, maximum resistance to PCP was observed at an initially added concentration of about 1000 mg L− 1. At an initially added concentration of 1000 mg L− 1 of PCP, 57.69% PCP removal, 11.8 g L− 1 release, and growth of 18.102 CFU. mL− 1.
Bioaugmentation process by fungi
Fungi growth: The selected and identified fungal strain Penicillium ilerdanum was subjected to expression in MSM and STWW to verify its efficiency in PCP removal (Fig. 5A). Monitoring of the biomass proliferation of the selected fungus was performed by measuring the OD at 600 nm every 24 h during the incubation period in both MSM and STWW. The tracking of PCP removal is controlled by the variation of PCP content, chloride, and microbial biomass. During the incubation period, we notice three phases: adaptation, exponential and stationary. The bioaugmentation treatment performed in STWW is characterized by a short adaptation phase of 24 h with an OD at 600 nm value of 0.254 and a faster exponential phase during 48 h with a value of 0.784 OD and a decreasing lethal phase after 96 h.
According to the obtained results, the adaptation phase of the fungus Penicillium Ilerdanum OP862882 in MSM supplemented with PCP for 48 h appeared slow with a growth value about of 0.447. Thus, the exponential phase appeared higher in MSM than in STWW, in the order of 0.98 and 0.78 for 72 h, respectively.
PCP removal: Figure 3 A showed the ability of the operational fungi to tolerate and remove PCP in MSM and STWW with an average removal of about 23.32% and 5.36% of PCP, respectively, only during the first day of incubation, and this PCP removal reached 92.33% and 92.36% of PCP after seven days of incubation. According to these last results, the operational fungal isolates selected and used in this investigation showed an important capacity of tolerance and transformation/or degradation or removal of PCP in these two specific media, MSM and STWW.
Phytoremediation-bioaugmentation process
PCP content monitoring
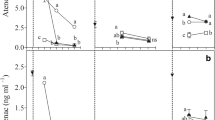
The rate of PCP removal registered in the combined phytoremediation-bioaugmentation treatments was determined after HPLC measurement. The results of PCP content are obtained in mg. L− 1 and summarized in Fig. 6. Figure 6 allowed to show that the most important percentage of PCP removal was recorded for Polypogon treatment with a 92.01% removal rate. This last result allowed showing the importance and value of the phytoremediation process regarding PCP bioremediation.
In contrast, the STWW + PCP treatment presented the lowest rate of PCP removal, with only 43.19%. All obtained results allowed us to show the intrinsic value of the microorganisms used in these PCP bioremediation processes. So, the specific fungal genus used in these bioremediation processes contributed intensively and heavily to the efficiency of these processes. The other treatments carried out in this experimentation such as L + PCP, F + L, P + L + F + PCP, L + F + PCP, and P + F + PCP usually provided low PCP removal rates.
Chloride content change in wastewater submitted to the bioaugmentation-phytoremediation process
The results of residual chloride content following the different treatments at T0 and TF were summarized in Fig. 6. These results showed that most chloride contents increase in the different treatments of phytoremediation-bioaugmentation either supplemented or not with PCP. The phytoremediation treatment with the Polypogon plant (P + PCP) presented the most important value of chloride content of 23.58 g. L− 1 when compared to the other investigated treatments such as L + PCP, F + L, P + L + F + PCP, L + F + PCP, and P + F + PCP. The lowest chloride content was recorded in the case of STWW treatment free of PCP with a value of 1.42 g. L− 1. These latter results and the PCP removal ones showed a close relationship between the chloride content in the medium and the PCP removal.
Fungal evolution during the varied bioremediation processes
The fungal enumeration in the different treatments investigated is summarized in Fig. 6. The different phytoremediation-bioaugmentation treatments showed fungal mycelium development with various consistency and intensity in STWW. The L + F + PCP treatment showed the most fungal development, with the highest number of fungi of the order of 3.3 103 CFU. mL− 1. These results allowed us to conclude the absence of antagonism between the fungus and the Lemna minor plant. In contrast, the net absence of fungal organisms at the end of the treatment of L + PCP and L + P + F + PCP was noticed. These obtained results prove the fungi Penicillium ilerdanum OP862882 viability and the PCP tolerance in STWW during the treatment process.
Change of main chemical-physical wetlands characteristics during bioremediation processes
Constructed wetlands have been effective in varying many physical and chemical parameters associated with fungi. (Table 2). Usually, vegetation has shown an improvement in PCP efficiency removal. The characteristics of the raw effluent were given in Table 1 along with the mixed wastewater effluent from the holding tanks.
The varied phytoremediation-bioaugmentation treatments were submitted to some common physico-chemical parameter analyses, like pH, EC, COD, and OC after 30 days of processing to get the treatment efficiency regarding the treated wastewater quality. The different results obtained from the physico-chemical analysis of STWW are summarized in Table 2. Thus, the STWW submitted to the different PCP bioremediation processes showed a slight pH increase from 7.06 to around 10. The EC measurement showed a slight increase for all treatments except for the control STWW without PCP. The most important values of EC were recorded in the respective treatments: P + PCP, P + L + F + PCP, and P + F + PCP, with values of 8.44, 8.97, and 8.35 µs. cm− 1. Besides, the COD results showed a significant decrease in all STWW treatments. The lowest values of COD were recorded in the treatments P + PCP and L + P + F + PCP with values of the order of 2 and 3.2 mg L− 1, respectively. The increase in COD may be related to the release of some residual compounds in wastewater likely following the transformation of PCP. As for the OC change in the PCP-STWW treatment process, the highest OC rate was registered in the F + PCP and L + PCP treatment, with respective values of around 54.35% and 53.33%.
Plant growth analysis
The effectiveness of the biological treatment process of phytoremediation is based on the viability and growth of plants used in wastewater artificially contaminated with PCP. The plant growth assessment summarized in Table 3 was tracked by counting the number of leaves and measuring their length. According to the obtained results, the Polypogon maritimus plant achieved a considerable growth yield in the treatment P + F + PCP. This plant presented a length leaf of about 35.2 cm, with an average number of about 16.
Chlorophyll determination
Figure 7 showed the chlorophyll a and b evolution during phytoremediation-bioaugmentation treatment in PCP-contaminated STWW. Chlorophylls a and b contents in Polypogon maritimus and Lemna minor plant were elevated in the STWW after 7 days of treatment. The high pigments level registered for these two macrophytes their important viability and tolerance when used for PCP phytoremediation. The Polypogon maritimus often showed a higher chlorophyll content and leaves length than Lemna minor.
Chlorophyll (leaf) g mL− 1 of the plant Lemna minor and polypogon in different wastewater treatments at time 0 (T0) and after 30 days of incubation (TF). (T2) phytoremediation process (Lemna), (T3) phytoremediation process (polypogon), (T4) phytoremediation process (Lemna- polypogon), (T5) phytoremediation- bioaugmentation (Lemna- polypogon- Fungi)
Principal component analysis (PCA)
To explore the variability observed in the phytoremediation-bioaugmentation treatments of PCP-contaminated wastewater during the incubation times, a discrimination of different treatments by the Principal Component Analysis (PCA) procedure was performed. KMO examination, generous a result of 0.717, and Bartlett sphericity test presenting p-value < 0.0001 showed the suitability of samples to apply the PCA. The procedure allowed the extraction of two principal components reported as principal components and graphically visualized in a scatterplot (Fig. 8). The two main components accounted for 77.3% of the total variance. PC expressed the variability in PCP concentration, fungi number, the organic carbon, and DOC of wastewater (Fig. 8). The separation on PC1 was principally because of the reduction, between the two-sampling time, in wastewater samples of PCP content and the increase in chlorides, bacterial, and fungi number. In T0 time, the different wastewater samples were not discriminated from each other along PC1 and PC2 (Fig. 8).
Discussion
Raw wastewater treatment processes aimed to change the wastewater quality for safe release in the natural environment, like agricultural soil irrigation, recycling in some important industries requesting large quantities and flows of water, and even why not for noble use as drinking or other suitable worthy purposes. The wastewater treatment took place in wastewater treatment plants, which should be designed and considered under well-defined circumstances and conditions [37]. So, the purpose of wastewater treatment was to remove totally or reduce some important compounds and contaminants from raw water that could cause threats to humans and the natural environment if discharged raw and without proper treatment into receiving water systems like ocean, sea, rivers, wadis, etc [38]. Bioremediation is a common biological technique often used for the removal of main residual compounds from polluted water and makes it safe for discharge in natural ecosystems or recycling usage in many domains as above cited. This technique of bioremediation is essentially based on specific microorganisms or plants for the purification of wastewater under well-defined conditions. The treated wastewater showing relatively good quality could be reused or discharged safely in natural ecosystems [39].
In this study, the bioremediation process investigated a combined phytoremediation-bioaugmentation technique of wastewater artificially contaminated with PCP. This combined process resulted in significant PCP removal rates from wastewater and confirmed the use of microorganisms and specific plants as degrading and removing agents of dyes, cosmetics, detergents, medicines, and pesticides as an effective method to reduce environmental harm and damage [40,41,42]. Thus, fungi and bacteria can transform PCP for example by incorporating one or two oxygen from the available O2 through the oxygenase process [43, 44]. This process allowed the destruction of the aromatic ring and the subsequent formation of CO2 through a slow aerobic transformation; especially for a case of highly chlorinated compounds such as PCP [45].
Microorganisms usually showed reduced growth in the presence of a toxic compound; however, the fungal strain of Trichoderma harzianum CBMAI 1677 showed an increased colony mycelium diameter at 50 mg L− 1 of PCP and it is often used in some quantitative biodegradation experiments [46]. Creswell and Curl [47] obtained similar results when T. harzianum was experienced with herbicides prometryn, norflurazon, and cyanine. Many fungi have been shown to detoxify PCP by methylation using a specific lignin-degrading system, prevailing to serve other functions such as degradation of wood components such as lignin and cellulose [48,49,50]. Using reactions catalyzed by pH oxidases such as laccases and peroxidases, fungi can make the primary PCP transformation into pentachloro anisole (PCA) [48–49, 51, 52]. The last process known for PCP removal exploits the adsorption and bio-adsorption processes into organic compounds or the microbial biomass existing in the medium as living or dead organisms. So, it has been found that some microbial cultures showed a particular affinity for binding PCP [51, 53], i.e., the adsorption took place through the charge attraction between PCP and microbial biomass [42, 51, 54–57]. Species of Lemna minor, Azolla filiculoides, Typha angustifolia L., Chrysopogon zizanioides (L.) Roberty, Eichhornia crassipes, and Cyperus isoclaudus have shown the best efficiency rate with a value > 99.9% for the removal of some tested micro-pollutants [58, 59].
The basic principles of bioremediation involved reducing the solubility of these environmental contaminants by changing pH, the redox reactions, and the adsorption of contaminants from a polluted environment [60]. Also, the batch kinetic study of Kobayashi and Kishino [61] showed that around 91% of PCP is removed from municipal wastewater spiked with around 1 mg L− 1 of PCP at an equilibrium time of 5 h. Various reports have been made on enhancing the biosorption of pentachlorophenol (PCP) by altering the pH levels in aqueous solutions. For example, the biosorption abilities of Aspergillus niger [62] and Mycobacterium chlorophenolicum [63, 64] in the removal of PCP from aqueous solutions were reported to be pH-dependent. Brandt et al. [63] also evaluated the influence of pH on the adsorption and desorption behavior of PCP by Mycobacterium chlorophenolicum and reported that pH values were an essential parameter that affected PCP adsorption, and with adsorptive capacity increasing with pH decreasing.
The physical and chemical characteristics of the reactionary environment influenced the absorption and desorption proprieties of organic as persistent organic pollutants POPs, HAP, PCP, etc [65].; so it is imperative to follow the evolution of a few indicatives and influencing parameters during each bioremediation water treatment. The macrophyte growth and development could be affected because of the well-known PCP phyto-accumulation phenomena within the organ plants as reported by Yang et al. Yang et al. [66]. Other works suggested that the pollutant bioavailability especially depended on the organic matter content of water, the pH, and other various specific properties of the pollutant. Equally severe and prolonged toxicity has shown an increase at low pH. This may be because a lower pH favors a non-ionic form of PCP, which is more easily absorbed and more toxic than ionized PCP [67]. Finally, many works released by our researchers prove that phytoremediation and bioaugmentation can eliminate PCP in wastewater (Table 4).
Conclusion
Faced with water scarcity caused by global climate change, wastewater reuse remains a promising solution in many industrial and agricultural sectors. Water treatment is the main ecosystem service in the provision of safe and clean water through biological processes such as phytoremediation and bioaugmentation that create new habitats for organisms. In this study, the phytoremediation process improved the sanitary quality of wastewater by removing this pollutant. Thus, based on the results obtained from the physico-chemical analyses of the STWW carried out, it proved to be effective in improving the quality of STWW as in the case of DOC, pH… In addition, the combined phytoremediation-bioaugmentation treatment showed the best PCP removal performance with good viability of the two plants, Polypogon maritimus and Lemna minor, and the selected fungus Penicillium ilerdanum OP862882. This biological practice and process, therefore, seems promising for the removal of PCP from PCP-contaminated sites. These processes, proposed in our study, are presented as low-cost and low-maintenance systems to treat domestic wastewater.
Data Availability
Not applicable.
References
Werheni AR, Di Rauso Simeone G, Hassen W, Smiri M, Sadfi N, Hidri Y, Hassen A. Aspergillus sydowii and Typha angustifolia as useful tools for combined bio-processes of PCP removal in wastewater. Int J Environ Sci Technol. 2022. https://doi.org/10.1007/s13762-021-03853-7
Ojuederie O, Babalola O. Microbial and Plant-Assisted bioremediation of Heavy Metal Polluted environments: a review. Int J Environ Res Public Health. 2017. https://doi.org/10.3390/ijerph1412150
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PT, …, Cooke SJ. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev, 2019.
Heathwaite AL. Multiple stressors on water availability at global to catchment scales: understanding human impact on nutrient cycles to protect water quality and water availability in the long term. Freshw Biol. 2010. https://doi.org/10.1111/j.1365-2427.2009.02368.x
Fletcher J, Willby N, Oliver DM, Quilliam RS. Phytoremediation Using Aquatic Plants R. Shmaefsky, editor, Phytoremediation, Concepts and Strategies in Plant Sciences; 2020. https://doi.org/10.1007/978-3-030-00099-8_7
Miguel RAB, Jetten MSM, Welte CU. The role of mobile genetic elements in organic micropollutant degradation during biological wastewater treatment. Water Res X. 2020. https://doi.org/10.1016/j.wroa.2020.100065
Bhat M, Shukla RN, Yunus M. Urban pond ecosystems: preservation and management through phytoremediation. In Fresh Water Pollution Dynamics and Remediation. 2020. https://doi.org/10.1007/978-981-13-8277-2_15
Newete SW, Byrne MJ. The capacity of aquatic macrophytes for phytoremediation and their disposal with specific reference to water hyacinth. Environ Sci Pollut Res. 2016. https://doi.org/10.1007/s11356-016-6329-6
Miretzky P, Saralegui A, Cirelli AF. Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere. 2004https://doi.org/10.1016/jchemosphere.2004.07.024
Lu W, Luo Y, Chang G, Sun X. Synthesis of functional SiO2-coated graphene oxide nanosheets decorated with ag nanoparticles for H2O2 and glucose detection. Biosens Bioelectron. 2011. https://doi.org/10.1016/j.bios.2011.06.008
Dhote S, Dixit S. Water quality improvement through macrophytes—a review. Environ Monit Assess. 2009. https://doi.org/10.1007/s10661-008-0303-9
Nelson-Barber S, Estrin ET. Bringing native american perspectives to mathematics and science teaching. Theory into practice. 1995;34(3):174–85.
Hafez Y, Attia K, Alamery S, Ghazy A, Al-Doss A, Ibrahim E, …, Abdelaal K. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy. 2020. https://doi.org/10.3390/agronomy10050630
Slimani NHK, Benezeth Y, Souami F. 2014. Human interaction recognition based on the co-occurence of visual words. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition Workshops: 455–460.
Stottmeister U, Wießner A, Kuschk P, Kappelmeyer U, Kästner M, Bederski O, …, Moormann H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol Adv. 2003. https://doi.org/10.1016/j.biotechadv.2003.08.010
Faulwetter JL, Gagnon V, Sundberg C, Chazarenc F, Burr MD, Brisson J, Stein OR. Microbial processes influencing the performance of treatment wetlands: a review. Ecol Eng. 2009. https://doi.org/10.1016/j.ecoleng.2008.12.030
Valipour A, Ahn YH. Constructed wetlands as sustainable ecotechnologies in decentralization practices: a review. Environ Sci Pollut Res. 2016. https://doi.org/10.1007/s11356-015-5713-y
Cevat Y. Performance and kinetics of Bioaugmentation, Biostimulation, and natural attenuation processes for bioremediation of Crude Oil-Contaminated soils. Environ Eng Coll Eng Imam Abdulrahman Bin Faisal Univ Processes. 2020;8(8):883. https://doi.org/10.3390/pr8080883
Ballaminut N, Machado KMG, Oliveira LHDS, Matheus DR. Physiological characterization of fungal inoculum for biotechnological remediation of soils. Brazilian Archives of Biology and Technology. 2014. https://doi.org/10.1590/S1516-8913201402006
Ryu YR, Zhu S, Look DC, Wrobel JM, Jeong HM, White HW. Synthesis of p-type ZnO films. J Cryst Growth, 2000.
Zhou H, Zhou L, Ma K. Microfiber from textile dyeing and printing wastewater of a typical industrial park in China: occurrence, removal, and release. Sci Total Environ. 2020. https://doi.org/10.1016/j.scitotenv.2020.140329. 739, 140329.
Matheus MC, Lourenço GR, Solano BA, Dezotti MW, Bassin JP. Assessing the impact of hydraulic conditions and absence of pretreatment on the treatability of pesticide formulation plant wastewater in a moving bed biofilm reactor. J Water Process Eng. 2020;36:101243.
Matheus CJ, Kokar MM, Baclawski K. A core ontology for situation awareness. In Proceedings of the sixth international conference on information fusion 2003; 1, 545–552).
Machado JA, Mata J. Counterfactual decomposition of changes in wage distributions using quantile regression. J Appl Econom. 2005. https://doi.org/10.1002/jae.788
Moreira-Neto JJS, Gondim JO, Raddi MSG, Pansani CA. Viability of human fibroblasts in coconut water as a storage medium. Int Endod J. 2009. https://doi.org/10.1111/j.1365-2591.2009.01591.x
Ibrahim C, Hammami S, Khelifi N, Pothier P, Hassen A. The effectiveness of activated sludge procedure and UV-C254 in Norovirus inactivation in a tunisian industrial wastewater treatment plant. Food and Environmental Virology; 2020. https://doi.org/10.1007/s12560-020-09434-0
Brookes CA, O’neill JB. & Redfern B A W. Anisotropy in the hardness of single crystals. Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences; 1971.
Werheni Ammeri R, Kraiem K, Riahi K, Eturki S, Hassen W, Mehri I, Hassen A. Removal of pentachlorophenol from contaminated wastewater using phytoremediation and bioaugmentation processes. Water Sci Technol. 2021a;84(10–11):3091–103.
Samson A. The behavioral economics guide 2014. London, UK: Behavioral Economics Group; 2014.
Liu F, Ng TB. Effect of pineal indole on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem Cell Biol. 2000. https://doi.org/10.1139/o00-018
White FJ. Comparative socio-ecology of Pan paniscus. Great ape societies; 1996.
Manios T, Stentiford EI, Millner PA. The effect of heavy metals accumulation on the chlorophyll concentration of Typha latifolia plants, growing in a substrate containing sewage sludge compost and watered with metaliferus water. Ecol Eng. 2003. https://doi.org/10.1016/S0925-8574. (03)00004 – 1.
Karn SK, Chakrabarty SK, Reddy MS. Characterization of pentachlorophenol degrading Bacillus strains from secondary pulp-and-paper-industry sludge. Int Biodeterior Biodegrad. 2010. https://doi.org/10.1016/j.ibiod.2010.05.017
Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. https://doi.org/10.1104/pp.24.1.1
Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In Methods in enzymology, 1987. https://doi.org/10.1016/0076-6879 (87)48036-1.
Kurashvili MV, Adamia GS, Amiranashvili LL, Ananiasvili TI, Varazi TG, Pruidze MV, …, Khatisashvili GA. Targeting of detoxification potential of microorganisms and plants for cleaning environment polluted by organochlorine pesticides. Annals of Agrarian Science, 2016.
Nuralhuda AJ. The design for wastewater treatment plant (WWTP) with GPS X modeling. Cogent Engineering; 2020. https://doi.org/10.1080/23311916.2020.1723782
Avijit M, Md A, Mhia MZ. Design and feasibility analysis of a low-cost water treatment plant for rural regions of Bangladesh. AIMS Agri and Food. 2018. https://doi.org/10.3934/agrfood.2018.3.181
Ayangbenro AS, Babalola OO. A new strategy for heavy metal polluted environments: a review of microbial bio-sorbents. Int J Environ Res Public Health. 2017;14:94. https://doi.org/10.3390/ijerph14010094
Harms H, Schlosser D, Wick LY. Untapped potential: exploiting fungi in bioremediation of hazardous chemicals. Nat Rev Microbiol. 2011. https://doi.org/10.1038/nrmicro2519
Tortella GR, Diez MC, Durán N. Fungal diversity and use in the decomposition of environmental pollutants. Crit Rev Microbiol. 2005. https://doi.org/10.1080/10408410500304066
Yazid NA, Joon YC. Co-precipitation synthesis of magnetic nanoparticles for efficient removal of heavy metal from synthetic wastewater. In AIP Conference Proceedings 2019; 2124, 1, 020019. AIP Publishing LLC.
De Franca Lopes LG, Junior FSG, Holanda AKM, de Carvalho IMM, Longhinotti E, Paulo TF …, Sousa EHS. Bioinorganic systems responsive to the diatomic gases O2, NO, and CO: from biological sensors to therapy. Coordination Chemistry Reviews; 2021.
Bosso L. Fungi in Pentachlorophenol Adsorption and Degradation: Novel Bioremediation and Biotechnological Tools (Doctoral dissertation, Ph.D. Thesis. University of Naples Federico II). 2014.
Reddy GVB, Gold MH. Degradation of pentachlorophenol by Phanerochaete chrysosporium: intermediates and reactions involved. Microbiology. 2000;146(2):405–13.
Vacondio B, Birolli WG, Ferreira IM, Seleghim MHR, Gonçalves S, Vasconcellos SP, Porto ALM. Biodegradation of pentachlorophenol by the marine-derived fungus Trichoderma harzianum CBMAI 1677 isolated from ascidian Didemnun ligulum. Bioc and Agri Biotech. 2015. https://doi.org/10.1016/j.bcab.2015.03.005
Creswell TC, Curl EA. Effects of some herbicides on rhizoctonia-solani and trichoderma harzianum. In Phytopathology 1982, 72, 3, 356–356. 3340 PILOT KNOB ROAD, ST PAUL, MN 55121: Amer Phytopathological Soc.
McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;0080239–1. https://doi.org/10.1016/S0896-6273
Bosso L, Cristinzio G. A comprehensive overview of bacteria and fungi used for pentachlorophenol biodegradation. Rev Environ Sci Bio/Techn. 2014. https://doi.org/10.1007/s11157-014-9342-6
Gadd GM, Gadd GM, editors. Fungi in bioremediation: 23. Cambridge University Press; 2001.
Rubilar O, Diez MC, Gianfreda L. Transformation of chlorinated phenolic compounds by white rot fungi. Crit Rev Environ Sci Technol. 2008. https://doi.org/10.1080/10643380701413351
Singh KK, Singh AK, Hasan SH. Low-cost bio-sorbent ‘wheat bran for the removal of cadmium from wastewater: kinetic and equilibrium studies. Bioresour Technol. 2006. https://doi.org/10.1016/j.biortech.2005.04.043
Ahmaruzzam M. Adsorption of phenolic compounds on low-cost adsorbent: a review. Adv Colloid Interface Sci. 2008. https://doi.org/10.1016/j.cis.2008.07.002
Crawford M J, Dy CJ, Alexander JW, Thompson M, Schroder SJ, Vega CE, ... Noble PCThe 2007 Frank Stinchfield Award: the biomechanics of the hip labrum and the stability of the hip. Clinical Orthopaedics and Related Research®, 2007. 465, 16–22.
Xun J, Reynolds J. Applying ethnography to market research: the case of the online forum. J Target Meas Anal Mark. 2010;18:17–31.
Carvalho DC, Neto DA, Brasil BS, Oliveira DA. DNA barcoding unveils a high rate of mislabeling in a commercial freshwater catfish from Brazil. Mitochondrial DNA. 2011;22(sup1):97–105.
Copley MR, Babovic S, Benz C, Knapp DJ, Beer PA, Kent DG., ... Eaves CJ. The Lin28b–let-7–Hmga2 axis determines the higher self-renewal potential of fetal hematopoietic stem cells. Nature cell biology, 2013; 15(8), 916–925.
Dosnon-Olette R, Trotel-Aziz P, Couderchet M, Eullaffroy P. Fungicides and herbicide removal in Scenedesmus cell suspensions. Chemosphere. 2010;79(2):117–23.
Gorzerino C, Quemeneur A, Hillenweck A, Baradat M, Delous G, Ollitrault M, Azam D, Caquet T, Lagadic L. Effects of diquat and fomesafen applied alone and in combination with a nonylphenol polyethoxylated adjuvant on Lemna minor in aquatic indoor microcosms. Ecotoxicol Environ Saf; 2009.
Jain S, Arnepalli D. Biomineralization as a remediation technique: A critical review. In Proceedings of the Indian Geotechnical Conference (IGC2016), Chennai, India; 2016.
Kobayashi K, Kishino T. Effect of pH on the toxicity and accumulation of pentachlorophenol in goldfish. Bull Japan Soc Sci Fish. 1980;46:167.
Mathialagan T, Viraraghavan T. Biosorption of pentachlorophenol from aqueous solutions by fungal biomass. Bioresour Technol. 2009. https://doi.org/10.1016/j.biortech.2008.06.054
Brandt S, Zeng AP, Deckwer WD. Adsorption and desorption of pentachlorophenol on cells of mycobacterium chlorophenolicum PCP-1. Biotechnol Bioeng; 1997.
Bosso L, Lacatena F, Cristinzio G, Cea M, Diez MC, Rubila O. Biosorption of pentachlorophenol by Anthracophyllum discolor in the form of life fungal pellets. New Biotechnol; 2015. https://doi.org/10.1016/j.nbt.2014.08.001
Salam JA, Das N. Lindane degradation by Candida VITJzN04, a newly isolated yeast strain from contaminated soil: kinetic study, enzyme analysis, and biodegradation pathway. World J Microbiol Biotechnol. 2014;30:1301–13.
Yang CW, Liu C, Chang BV. Biodegradation of amoxicillin, tetracyclines, and sulfonamides in wastewater sludge. Water. 2020. https://doi.org/10.3390/w12082147
Spehar RL, Nelson HP, Swanson MJ, Renoos JW. Pentachlorophenol toxicity to amphipods and fathead minnows at different test pH values. Environ Toxicol Chemistry: Int J. 1985. https://doi.org/10.1002/etc.5620040314
Werheni Ammeri R, Hassen W, Hidri Y, Simeone GDR, Hassen A. Macrophyte and indigenous bacterial co-remediation process for pentachlorophenol removal from wastewater. Int J Phytorem. 2022. https://doi.org/10.1080/15226514.2021.1933897
Werheni AR, Simeone GDR, Hassen W, Ibrahim C, Ammar RB, Hassen A. Bacterial consortium biotransformation of pentachlorophenol contaminated wastewater. Arch Microbiol. 2021.
Werheni Ammeri R, Eturki S, Di Rauso Simeone G, Ben Moussa K, Hassen W, Moussa M, Hassen A. Effectiveness of combined tools: adsorption, bioaugmentation, and phytoremediation for pesticides removal from wastewater. Int J of Phytorem.2021d; https://doi.org/10.1080/15226514.2022.2164249
Acknowledgements
This research was funded by the Water Treatment and Recycling Laboratory member lab (CERTE), Eremology and combating desertification, arid regions Institute of Medenine, Tunisia, and National Agency for the Promotion of Scientific Research (Mobidoc Promise 2019). So, this research was partly funded by the Tunisian Ministry of Higher Education and Scientific Research in the frame of the program contract 2019–2020 (CERTE). The authors would also gratefully acknowledge the Tunis International Center for Environmental Technologies for providing macrophyte species Polypogon maritimus and Lemna minor. We thank all the co-authors for their technical support and their editing of the manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no conflicts of interest in the publication of this article.
Ethics approval and consent to contribute
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ammeri, R.W., Kouki, S., Hassen, W. et al. Bioaugmentation and phytoremediation wastewater treatment process as a viable alternative for pesticides removal: case of pentachlorophenol. J Environ Health Sci Engineer 21, 373–387 (2023). https://doi.org/10.1007/s40201-023-00865-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-023-00865-y