Abstract
Purpose
Doogh is a famous Iranian drink based on fermented milk. Laminated film is one of the most common packaging for this beverage in Iran. So, chemical substances of the packaging may migrate to the Doogh and endanger human health.
Method
In this research, High-Performance Liquid Chromatography (HPLC) was used to determine the migration of Irganox 1010 and Irganox 1076 from the contact layer and inductively coupled plasma for Titanium dioxide (TiO2) from the second layer of three-layer laminate films into Doogh and acetic acid 3% (w/v). The influence of different storage temperatures and times was investigated by evaluating the samples stored in various conditions. The morphological, thermal and mechanical properties of the film, before and after contact with food simulant were further studied.
Result
The highest amount of Irganox 1010 concentration of the tested samples were 0.8 ± 0.04 mg/l in acetic acid 3% (w/v), and 0.62 ± 0.04 mg/l in Doogh. The highest amount of TiO2 concentration were 0.25 ± 0.04 mg/l in acetic acid 3% (w/v), and 0.12 ± 0.02 mg/l in Doogh. The migration of Irganox 1076 was determined, but it was not detected. The results indicated that the food simulant had no significant effect on the microstructure and thermal properties of the polymer, but it reduced the mechanical properties.
Conclusion
The results indicate the possible migrating of Irganox 1010 and TiO2 through laminate packaging into Doogh in some storage conditions. Since the migration value was low, the mentioned film was proven safe for Doogh packaging, imposing no hazards on human health.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food packaging is a rapidly evolving field applied to different kinds of products. Packaging offers many advantages such as food protection from external environment, increased shelf life, reduced costs, and the possibility of modern marketing. On the other hand, packaging affects the final products as their contaminants migrate into foods, some of which are harmful even at low doses [1, 2]. Laminated films are generated by a combination of two or more films. They are utilized to enhance different film properties such as heat resistance along with moisture and oxygen barrier properties; they further optimize packaging for food preservation while significantly increasing the shelf life of different types of packaged foods. Polyolefins are widely used in this industry. These materials have the advantages of processability, non-penetration of moisture, plus desirable thermal and mechanical properties [3].
Environmental factors can adversely impact polymers by making them inefficient. To prevent these changes in polymer properties, some additives should be added to the virgin polymer resin. Free radicals formed through the subjection of plastics to heat, oxygen, or irradiation can cause oxidative degradation and make many changes in the properties. In this regard, antioxidants are added as additives to inhibit or reduce degradation. Irganox 1010 and Irganox 1076 are two kinds of phenolic antioxidants widely used in polyethylene polymer [4].
These antioxidants might migrate into food matrices through direct contact, where chemicals with a molecular weight below 1000 Da can be absorbed through the gastrointestinal tract and pose a risk to the consumer’s safety [5, 6]. TiO2 is a white chemical substance that has been applied in different industries for many years. The most common use of this chemical substance is in the form of a white pigment, but it has applications including UV resistance as well as antimicrobial and brightness properties. In the food packaging industry, TiO2 is widely used as a coloring and antibacterial agent. TiO2 can migrate from packaging to food, and since there is no convincing evidence that it is safe for consumer health, concerns about the toxicity of TiO2 have increased. Hence, the migration of this chemical substance from food packaging should be investigated further [7].
The migration of chemical substances from packaging into food is affected by many parameters such as the initial concentration of the migrants in the packaging and the food, the food properties, temperature, and storage time [8].
The presence of various plastic chemical substances in food could be the consequence of the migration of substances from plastic packaging, whereby food becomes the main way of human exposure to a wide range of chemicals with potential damaging health effects, including hormone disruption, and metabolic syndromes. Thus, concerns about consumer health were raised and strict national and international standards were created specifying permitted chemicals along with allowable limits [4].
As stated in the European Union (EU) regulations, the quality of food packaging materials is mainly determined by the overall migration limit at the maximum level of 10 mg/dm2 and the specific migration limit whose level is mentioned in the regulation (EU) No.10/2011 [9]. Because the migration study on food products is not simple, food simulants can be used as substitutes. These simulants can be made of acetic acid 3% (w/v), olive oil, ethanol, and ethanol/water for liquid products, and Tenax for solid products [10]. However, the use of simulants should not underestimate the importance of complex food matrix as they may, under certain conditions, lead to the major migration of contaminants from the packaging [11].
The migration of additives from packaging materials into food or food simulants has been the subject of many studies. Using gas chromatography, Chang et al. (2016) studied the specific migration of toluene through plastic-laminated films into isooctane, ethanol 50% (v/v), acetic acid 3% (w/v), and ethanol 10% (v/v) [3]. Rastkari et al. (2012) investigated the effect of sunlight on the migration of phthalates through polyethylene terephthalate (PET) and High-density polyethylene (HDPE) packaging into acidic content. This study reported that the migration of these substances was lower than the authorized limit [12]. Goulas (2001) employed distilled water and acetic acid 3% (w/v) to measure the overall migration from commercial plastic cups and five-layer films used for dairy products such as ice cream and yogurt. He reported that the overall migration into food simulants was far lower than maximum authorized limit [13]. The migration of TiO2 into different foods and food simulants was determined elsewhere [7, 14,15,16]. Other studies have also investigated migration of chemical substances into milk and food simulants [17, 18].
Doogh is a famous Iranian drink based on fermented milk with a pH of less than 4.2; currently, it is produced in considerable amounts in the country, and its industrial production is growing. Laminated films are one of the most common and important packaging materials for Doogh drink [19, 20]. The shelf life of Doogh in this packaging is about 2 months so it may be consumed during its shelf life. Nevertheless, after a long time of its production, it thus leads to prolonged contact of food with the packaging. This beverage may be stored under various conditions including refrigerator, room temperature, and under sunlight, so different temperatures may affect that. Farhoodi et al. (2008) conducted a study on the impact of environmental factors on the migration of phthalates from PET bottles to Doogh. The obtained result showed that storage temperature and time have a significant effect on the phthalate migration process [21].
In consideration of great consumption of Doogh in the Iranian diet, it is necessary to study the migration of chemical substances from laminated films to it, because best of our knowledge, no research has been performed on the migration of contaminants from laminated films to Doogh in Iran.
Accordingly, the present research aimed to investigate the migration of specific additives including Irganox 1010 and Irganox 1076 from the contact layer, and TiO2 from the second layer, of three-layer laminate films. The effects of Doogh and acetic acid 3% (w/v) as a food simulant at different storage temperatures and times was determined in order to assess the safety of the studied packaging additives for human consumption. The effect of food simulant on the structural, thermal, and mechanical properties of the packaging materials affecting migration was further investigated.
Materials and methods
Materials
The three-layer laminate film was obtained from a local film manufacturer. Methanol (HPLC grade), acetonitrile (HPLC grade), acetic acid, nitric acid (HNO3), hydrogen peroxide (H2O2), sodium chloride, and sodium hydroxide were purchased from Merck (Darmstadt, Germany). Pure water was provided by a Mili-RO system, (Millipore, Bedford MA, USA). Standard of Irganox 1010, Irganox 1076, and TiO2 were obtained from Sigma Aldrich (Steinheim, Germany). According to data from the laminate film production company (local film manufacturer), rutile TiO2 particles were supplied by Kronos GmbH. The average diameter of the particles (according to the company) was 300 nm, and their purity was ≥92.5%.
Methods
Sample pouch preparation
The three-layer low density polyethylene (LDPE) laminate film was heat-sealed in 10 cm × 10 cm pouches. The initial percentages of Irganox 1010 and Irganox 1076 were 1.76% and 0.66%, respectively, present in the inner layer of film (in direct contact with food). The initial content of TiO2 was 5%, which was in the second layer. The thickness of each layer was 30 μm.
Overall migration measurement
The overall migration test was assessed according to BS EN 1186–7. In this test, the pouches were filled with acetic acid 3%(w/v), sealed, and kept in an oven at 40 °C for 10 days. Following exposure to simulants, the pouches were withdrawn from the oven and emptied, whereby the simulants were evaporated to dryness using a hotplate. After evaporation, the residue was kept in the oven at 105 ± 1.0 °C for 30 min and then in a desiccator for 15 min, followed by weighing. For weighing, the weight difference obtained by the analytical balance had to be less than 0.5 mg [22]. The measurement was performed in triplicate.
The overall migration (mg/dm2) was calculated as follows:
where, M is the overall migration into the simulant (mg/dm2), ma denotes the mass of the residue from the test specimen after the evaporation of the simulant filling the test specimen, (g), mb represents the mass of residue from the blank simulant equal to the volume filling the test specimen, (g), and S reflects the test specimen surface area in contact with the simulant during the exposure, (dm2) [23].
Specific migration measurement
Specific migration test was performed by filling the pouches with 100 mL Doogh and food simulant. The Doogh used in this study had a pH of 3.7; thus, as reported by regulation (EU) No.10/2011, for foods with pH < 4.5, acetic acid 3% (w/v) must be used as a food simulant. Acetic acid 3% (w/v) was prepared by a dilution of 30 g acetic acid with ultrapure water to 100 mL in a volumetric flask. The surface of the film in contact with 100 mL of food simulant in pouches was 2 dm2 (surface to volume ratio) [24]. Since Doogh in Iran is usually stored at room temperature or refrigerator, so to determine the effect of storage temperature, three temperatures of 4 °C, indicate the temperature of the refrigerator, 25 °C for the room temperature, and 40 °C, which represents the temperature of tropical regions of Iran, was considered. Therefore, the pouches were placed at 4, 25, and 40 °C for 60 days. The migration of Irganox 1010, Irganox 1076, and TiO2 were studied over a period of 60 days with time intervals of 1, 3, 7, 15, 30, 45, and 60 days. Irganox 1010 and Irganox 1076 concentrations were measured using HPLC-UV and TiO2 concentration were determined by Inductively coupled plasma - optical emission spectrometry (ICP-OES).
Migrant extraction method
To determine the amount of Irganox 1010 and Irganox 1076 migrating to the acetic acid 3% (w/v), the sample solution was injected directly into HPLC-UV. The Doogh sample needed to be prepared before injection, which was done by the solvent extraction method. Via this method, proteins and carbohydrates were separated from the samples. Briefly, 5 ml of acetonitrile and 50 μl of 0.02 mol/L sodium hydroxide were added to 1 g of the Doogh sample and then vortexed for 1 min. Following ultrasonication for 10 mins, 0.25 g of sodium chloride was then added for phase separation. Next, the sample was centrifuged to remove the particles from the solution at 2000 rpm for 10 mins. The supernatant was then evaporated. Thereafter, to redissolve the dry residue, 1 ml of methanol was added. Finally, to eliminate fats from the sample, it was placed in −20 °C freezer for 12 h. It was then centrifuged again at 2500 rpm for 2 mins. Finally, the supernatant was transferred to another vial for HPLC injection.
To measure the content of TiO2 migrating to the acetic acid 3% (w/v), the sample solution was injected directly into ICP-OES. In order to prepare the Doogh sample for measuring TiO2, 3 ml of Doogh, 3 ml of HNO3, and 1 ml of hydrofluoric acid were added. It was then digested by a microwave system (multiwave 3000) for 20 mins at 210 °C. The digested sample was injected into the ICP-OES to determine the amount of titanium.
Apparatus condition
HPLC-UV
The migration of the two antioxidants was specified by HPLC-UV model 1260 (Agilent, California, USA). The mobile phase was methanol/water (95:5) for Irganox 1010 and methanol (100:0) for Irganox 1076, both with a flow rate of 1 mL/min. The analytes were separated through a 250 × 4.6 mm column NC100-5C18–3610. The absorbance was recorded by a UV detector at 278 nm for Irganox 1010 and 280 nm for Irganox 1076.
In order to quantify the data obtained from food simulant and Doogh, the calibration curve was plotted using external standard solutions which were prepared in 6 concentrations within the range of 0.5 to 100 mg/l.
ICP-OES
The migration of TiO2 was measured by ICP-OES (Arcos EOP, Spectro, Germany). The optimal conditions of the instrument were as follows: the torch, detector, and nebulizer type were quartz-axial, 32 linear CCD, and modified lichte, respectively. The coolant, auxiliary, and nebulizer flow were 13, 0.7, and 0.7 (L/min), respectively, and RF power was 1.4 (kW). Total time and Instrument stabilization delay were 28 and 5 (s), respectively. The migration amount of titanium into acetic acid 3% (w/v) as a food simulant and Doogh as a real food were characterized by plotting the calibration curve of standard solutions. Standard solutions were prepared at different concentrations within the range of 0.5 to 100 μg/l with the calibration curve plotted based on peak area and the concentration. The limit of detection and quantification were specified at 3 and 10 times the signal-to-noise values, respectively. The analytical approach accuracy was confirmed by calculating the peak areas of six replicates of standard solution.
Field emission scanning Electron microscopy (FE-SEM)
The microscopic morphology of the surface and cross-section of the samples was analyzed after metallization by gold via FE-SEM (MIRA3, TESCAN) at the voltage of 15 kV. The samples were fractured in liquid nitrogen and maintained in acetone for 24 h.
Differential scanning calorimetry (DSC)
The thermal properties of the samples were determined by DSC (Mettler Toledo) over a temperature range of 25 °C to 200 °C, at a heating rate of 20 °C/min, and with a flow rate of 50 mL/min. Specifically, 4–5 mg of the samples were sealed in aluminum pans. The second run data of the heating and cooling processes were used for DSC curves analysis. Measurements were taken on three replicates, and the mean values were reported.
Mechanical properties
The mechanical properties were determined using Santam Machine Controller. The procedure was based on the 882 American Society for Testing and Materials (ASTM) standard. Samples (1 cm × 10 cm) were tested with a crosshead speed of 50 mm/min at room temperature. Measurements were taken on five replicate samples, and the average values were reported.
Statistical analysis
To assess the influence of different times and temperatures on the migration of Irganox 1010, Irganox 1076, and TiO2 into food and food simulants as well as thermal and mechanical properties of the laminate film, statistical analyses were performed using SPSS 22.0. All chemical measurements were accomplished in triplicate, and analysis of variance was analyzed through one-way analysis of variance (ANOVA), and Duncan’s multiple range test. P < 0.05 was considered statistically significant at a confidence level of 95%.
Results and discussion
Overall migration test
Overall migration refers to the entire amount of chemical substances able to migrate from packaging into the food simulant. The overall migration test was conducted to assess the compliance of the film with the overall migration limit and its suitability for use in food packaging. The overall migration, determined in acetic acid 3% (w/v), was 3.75 mg/dm2, which is below the 10 mg/dm2 limit for food contact surface under test conditions of 10 days at 40 °C as mentioned in regulation of plastic packaging in contact with food. This test indicates the inertness of plastic and determines whether or not the food is adulterated [25]. Bardley et al. (2008) stated that substances with a specific migration limit of 5 mg/kg or higher based on stability and volatility, could be specified by the overall migration test. Further, no other tests are required for specific migration, especially for plastics with low basic overall migration; thus, overall migration test is more economical and time-saving [26]. As a conclusion of this section, it should be noted that two phenomena cause interactions between food and packaging, which leads to the migration of chemicals. The first phenomenon is the solubility of migrants into food simulants. When a migrating substance has a high solubility in food simulants, it will tend to migrate to the simulant more than stay in the polymer. The second parameter is the absorption of liquid food simulant by the polymer film that can cause to rise the diffusivity of chemical substances in the polymer film. In the present research, the overall migration experiments showed that this laminated film was sufficiently inert and stable in contact with high polar solution [27]. A similar impact was further observed for LDPE plastic packaging, indicating a migration of lower than 10 mg/dm2 [23].
Method validation
The merit figures of the analysis methods including linearity, repeatability, the limit of detection (LOD), and limit of quantification (LOQ) were determined.
Regarding the acetic acid 3% (w/v), the quantification of selected substances was carried out via a calibration curve. Standard solutions of Irganox 1010, Irganox 1076 were provided in methanol, and TiO2 was prepared in HNO3 to investigate the linearity approach. The results of the correlation coefficient (r2) of the selected substances were greater than 0.99 for all migrants thus showing good linearity. The precision of the experiment was evaluated from relative standard deviation (RSD) which was within the 4–6% range for selected substances.
Concerning Doogh, the quantification of selected chemical substances was accomplished by a matrix-matched calibration curve. Further, the RSD of the method was in the range of 4–7%, thus showing the replicability of the experiment.
LOD and LOQ determination were performed using signal-to-noise ratio, where the amounts of LODs were 0.09, 0.18, and 0.015 mg/L, while LOQs of the substances were 0.27, 0.61, and 0.064 for Irganox 1010, Irganox 1076, and TiO2, respectively.
Migration of selected chemical substances into the food simulant
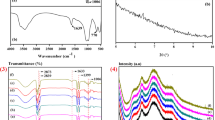
The migration of Irganox 1010, Irganox 1076, and TiO2 into the food simulant of the acetic acid 3% (w/v) through the three-layer laminate film was quantified at different times and under temperature conditions. Considering the condition of Doogh storage, three temperature points (4, 25, and 40 °C) and contact period of 60 days were selected, with the results displayed in Fig. 1 which will be explained below.
Migration of Irganox 1010 and Irganox 1076 into acetic acid 3% (w/v)
Irganox 1010
The results of the specific migration test of Irganox 1010 in Fig. 1(a) indicate that no migration was detected until 7th day; from 7th to 15th day, the migration occurred with a steep slope. Thereafter, the migration increased with a mild slope until the 60th day and reached equilibrium at temperatures of 4 and 25 °C, but at 40 °C, migration increased without reaching equilibrium.
The maximum migration was 0.8 ± 0.04 mg/L, which was observed at 40 °C on the 60th day. Similar results were obtained by Farhoodi et al. (2008) who reported that upon approaching the end of the storage time, the amount of migration increased [27].
Irganox 1076
The findings showed that the migration of Irganox 1076 was not detectable at any of the specified temperatures and times.
Migration of TiO2 into acetic acid 3% (w/v)
Figure 1(b) displays the migration results of TiO2 into acetic acid 3% (w/v). According to the results, the migration level was directly related to the time and temperature of the migration experiment. The release behavior showed that over time, the degree of migration increased. Within the first few days, the migration amount was low; a sharp increase was observed on day 7th and continued to grow until the 60th day. The results of Fig. 1(b) showed that the migration of TiO2 into the acetic acid 3% (w/v) did not reach equilibrium even after 60 days. With increasing contact temperature, the rate of migration also increased significantly and the highest migration was observed at 40 °C. Based on the findings, it was observed that temperature had a more significant effect on the migration of TiO2 than contact time.
In conclusion of this section, the results of the migration of Irganox 1010 and TiO2 into the food simulant of acetic acid 3% (w/v) showed that temperature played an important role. Similarly, Galotto et al. (2011) reported that the Irganox 1076 content of the food simulant, originating from the LDPE packaging, was significantly higher at 60 °C compared with lower temperatures. At high temperatures, the mobility and vibration of polymer chains increased, resulting in the migration of additives through the amorphous zone of the polymer into the simulant [28]. Chang et al. (2016) also found that toluene migration from laminated films significantly increased at higher temperatures [3], which can be explained by the theory of free volume. According to this theory, sufficient molecular energy as well as hollow space in the polymer cause the migration of additives from the packaging. In addition, increased temperature on one hand, leads to more free volume by weakening the forces of polymer chains and on the other hand, elevates the energy of the migrating molecule to overcome the attraction force of the surrounding molecules [29]. Contact time also had significant effects on the rate of migration, which could be due to the increase in swelling of the film via the solvent, which reduces the diffusion resistance with rising the free volume [30].
Finally, in the current research, it was concluded that the highest amount of migrated Irganox 1010, Irganox 1076, and TiO2 was less than the specific migration limit specified in regulation (EU) No. 10/2011 and its latest amendments.
Migration of selected chemical substances into the food matrix
The amounts of migrated Irganox 1010 and TiO2 from the laminated three-layer film into Doogh are summarized in Fig. 2. The migration level of targeted chemical substances was obtained at 4, 25, and 40 °C within 60 days. Further, the initial amounts of Irganox 1010, Irganox 1076, and TiO2 were determined in Doogh at zero time of storage which was not detectable.
Migration of Irganox 1010 and Irganox 1076 into Doogh
Figure 2 indicates the migration of Irganox 1010 into Doogh. The amount of Irganox 1010 into Doogh packed with the laminated film was not detectable until the 15th day. The migration of this additive increased fast within the 15th to 30th days, after which the migration level rose gently. The greatest amount of Irganox 1010 occurred as 0.62 ± 0.03 mg/kg on the 60th day. In this study, temperature played an important role as the highest migration was observed at 40 °C.
Migration of TiO2 into Doogh
Figure 2 depicts TiO2 migration into Doogh at different temperatures. Analysis of findings showed that until the 3rd day, no migration was detected, after which the migration gradually increased by the end of the storage. The highest migration was found on the 60th day at 40 °C. According to Fig. 2, as we approached the end of the product storage time, migration increased. The temperature also played an important role in migration.
Comparison of migration into food and food simulant
In this study, Table 1 and Fig. 3 present the comparison between the migration of Irganox 1010 and TiO2, respectively, into the food simulant and food matrix through the three-layer laminate film. For both substances of Irganox 1010 and TiO2, the migration amount in the acetic acid 3% (w/v) was greater than in Doogh. For Irganox 1010, the variations in the findings of the food simulant and Doogh were very small. The differences at lower temperatures and until 15th day were insignificant, but after that, by the end of the storage, the migration level to acetic acid 3% (w/v) was significantly higher than to Doogh. For TiO2, the differences in the results which can be seen from Fig. 3 were insignificant until day 15 and after which migration into the simulant grew more significantly than the migration level into Doogh. It is evident from Fig. 3 that TiO2 had a small amount of migration into both food and food simulant, which can be due to the settlement of this substance in the second layer where the first layer acts as a barrier to penetrations of acetic acid 3% (w/v) into the polymer. As a result, TiO2 particles migrate slowly from the polymer or remain in the polymer, even after an extended period of contact with the food simulant or the food matrix.
In general, acetic acid 3% (w/v) dissolved more Irganox 1010 and TiO2 than the Doogh did; the findings in this work showed that acetic acid 3% (w/v) is a suitable food simulant to investigate the migration of selected chemical substances into Doogh. This is because the migration levels into acetic acid 3% (w/v) were significantly higher than the obtained values into Doogh.
Microstructure properties
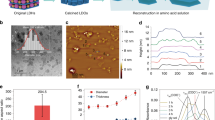
The cross-sectional and surface morphologies of the laminated films were studied by FE-SEM analysis (Fig. 4). Fig. 4(a,b) demonstrates the cross-sectional image of the laminated film, showing the three layers of the film and their thicknesses (80.8 μm) along with the layer in contact with the food.
FE-SEM micrographs of cross-sectional and surface morphologies of the laminated films. (a) and (b) FE-SEM micrographs of cross section of laminated film, the three layers of the film and their thicknesses; (c) and (d) TiO2 particles in the second layer of three-layer laminate film; (e) and (f) FE-SEM observations of the surface of laminated film before and after contact with food simulant at 40°C for 60 days. (e) Before contact with food simulant (f) after contact with food simulant
Figure 4(c) reveals the cross-section of the second layer of the laminated film, TiO2 spherical particles were 150-250 nm in size and cross-linked with polymeric chains (Fig. 4(d)). Although TiO2 particles could make aggregates, according to Fig. 4(c), no clear aggregation of these particles was seen, showing the homogenous dispersion of TiO2 in the second layer of the laminated film matrix. These TiO2 particles were uniformly dispersed, whereby the film would function better, and the particles would not separate easily from the film. This could explain the low migration of TiO2 from the laminated film.
Figure 4 (e, f) indicates the surface images of the layer in contact with the food simulant; Fig. 4(e) is a witness sample of the multilayer, and Fig. 4(f) is the sample in contact with food simulant at 40 °C for approximately 60 days. No obvious differences were noticed after contact with acetic acid 3% (w/v). Baloul et al. (2018) explored the migration of Irganox 1010 and Irgafos 168 from polypropylene packaging in contact with fatty foods at 20 and 40 °C for 10 days. In the SEM images of the plastics, they observed certain dark areas and holes, indicating the migration of the additives. Also, the surface of the treated plastics was rougher than that of the control plastics [31].
Differential scanning calorimetry (DSC)
Differential scanning calorimetry was used to examine the changes in the thermal properties of the laminated films before and after treatment. Table 2 presents the values of the thermal properties obtained from the DSC curves. As seen, the interaction between film and acetic acid 3% (w/v) did not influence the thermal properties of the polymer. This affirms the excellent resistance of the laminated films in contact with a very polar solution of acetic acid 3% (w/v).
The results of the present study indicated that the greater amount of migration at higher temperatures was not associated with changes in the laminated film structure in contact with the simulant. Therefore, there is no clear relationship between migration and thermal properties. This conclusion is consistent with the results obtained from the surface image of the polymer. Similarly, Farhoodi et al. (2008) found that the crystallinity percentage of PET bottles, in contact with acetic acid 3% (w/v) at 25 and 45 °C was constant; there anywhere no significant changes observed except for the increase in Tg at 45 °C. The significantly higher amount of migration at higher temperatures is related to the higher solubility of the migrant compounds and the diffusion rate [21]. Widén et al. (2013) found similar results for the DSC test of PET bottles in contact with acetic acid 3% (w/v). However, they observed a decrease in the Tg of the polymer that was in contact with the ethanol. As a food simulant, ethanol functions as a plasticizer and promotes the mobility of polymer chains, thereby augmenting the migration of additives [32].
Mechanical properties tests
Figure 5 (a, b) summarizes the test results of the mechanical properties of the laminated film.
As observed, the tensile strength and elongation were significantly reduced after the contact of the polymer with the simulant; the highest reduction was found in the sample at higher temperatures.
The resistance and strength of the polymer are affected by several factors, including temperature variations, solvents, and humidity. These conditions weaken the mechanical properties and make the polymer fragile [33]. Additives such as fillers are added to polymers to modify their mechanical properties and to improve their tensile strength [34].
The mechanical strength was reduced probably due to the increased migration of fillers and other additives from the polymer and causing the structure to change. This migration created certain porosities and micro-crazes in the polymer, leading to reduced mechanical strength of the polymer. The greatest reduction in the mechanical strength was observed at 40 °C, where the most migration occurred as the polymer had high micro-craze values [35, 36].
The results of this study are compiles with the findings of Urmia et al., Which states that increasing the temperature causes more changes in the mechanical properties of the polymer. These changes are due to the increased rate of migration at higher temperatures [37]. Similarly, Elgozali et al. (2008) reported the effect of additives (plasticizers and fillers) on the mechanical properties of polyvinyl chloride (PVC) polymer. Their results showed that adding fillers to PVC polymer partly increased the tensile strength, elongation at break, and shore hardness but reduced the modulus of elasticity [38].
Conclusion
In the current study, the migration of Irganox 1010, Irganox 1076, and TiO2 from three-layer LDPE laminate film as a food packaging into Doogh and acetic acid 3% (w/v) were determined.
Based on findings of migration measurement into acetic acid 3% (w/v), contact times and temperatures had a significant positive effect on the release of Irganox 1010 and TiO2. Temperature showed a more effective role than contact time on the migration level of these additives. On the other hand, no migration was detected for Irganox 1076 at different times or temperatures.
The findings of comparing the migration level of selected substances to acetic acid 3% (w/v) and Doogh suggested that acetic acid 3% (w/v) is a suitable simulant to be used instead of Doogh since migration of target substances into simulant were significantly higher than migration into real food. Nevertheless, the highest migration amount of selected chemical substances was far lower than the regulation (EU) migration limit ((EU) No.10/2011) thus confirming the safety of these laminated films for consumer health to be used for Doogh packaging.
DSC results and FE-SEM analysis revealed that the exposure of laminated packaging material to acidic food simulant did not considerably change the structure and thermal properties of packaging. On the other hand, the release of additives from the packaging material and exposure to food simulant reduced the mechanical properties of the polymer; the maximum reduction was observed at 40 °C at the end of the storage time.
In this paper, an attempt was made to provide significant results regarding chemical compounds migration and polymer structural changes during the contact of Doogh and its corresponding food simulant with the laminate film in different temperatures conditions and assess its health for the consumer. However, this study is not complete and comprehensive, and more studies are essential in this field. Further studies should be performed on laminate films and the migration of other chemical substances from different layers. Additionally, the health of other packaging such as HDPE, PS, and PET should investigate in contact with carbonated and non-carbonated Doogh.
Aknowledgments
This work was supported by the Department of Food Science and Technology, National Nutrition and Food Technology Research Institute (Shahid Beheshti University of Medical Sciences).
References
Porta M, Ballester F, Ribas-Fitó N, Puigdomènech E, Selva J, Llop S. Food packaging and migration of food contact materials: will epidemiologists rise to the neotoxic challenge? Gac Sanit. 2006;20(3):233–8.
Rohm H. Food packaging science and technology. Int J Dairy Technol. 2010;63(1):143–5.
Chang N, Zhang CH, Zheng FE, Huang YL, Zhu JY, Zhou Q, et al. Migration of toluene through different plastic laminated films into food simulants. Food Control. 2015;59:164–71.
Tian L. Targeted and non-targeted analysis of plastic-related Chemicals in Food. McGill University (Canada); 2020.
Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179–99.
Murphy J, editor. Additives for plastics handbook: Elsevier; 2001.
Chen J, Dong X, Zhang Q, Ding S. Migration of titanium dioxide from PET/TiO2 composite film for polymer-laminated steel. Food Addit Contam - Part A. 2019;36(3):483–91.
Barnes K, Sinclair R, Watson D.H. Chemical migration and food contact materials. Elsevier Science. 2007.1–464.
Commission regulation (EU) no 10/2011 on plastic materials and articles intended to come into contact with food. Off J Eur Union 2011;12(1).
Council directive 82/711/EEC of the basic rules necessary for testing migration of the constituents of plastic materials and articles intended to come into contact with foodstuffs. Off J Eur Communities. 1982; 297:26–30.
Cirillo T, Fasano E, Esposito F, De Prete EL, Cocchieri RA. Study on the influence of temperature, storage time and packaging type on di-n-butylphthalate and di(2-ethylhexyl) phthalate release into packed meals. Food Addit Contam - Part A. 2013;30(2):403–11.
Rastkari N, Zare Jeddi M, Yunesian M, Ahmadkhaniha R. Effect of sunlight exposure on phthalates migration from plastic containers to packaged juices. J Environ Health Sci Eng. 2008;16(1):27–33.
Goulas AE. Overall migration from commercial coextruded food packaging multilayer films and plastics containers into official EU food simulants. Eur Food Res Technol. 2001;212(5):597–602.
Lim JH, Bae D, Fong A. Titanium dioxide in food products: quantitative analysis using ICP-MS and Raman spectroscopy. J Agric Food Chem. 2018;66(51):13533–40.
Lin QB, Li H, Zhong HN, Zhao Q, Xiao DH, Wang ZW. Migration of Ti from nano-TiO2-polyethylene composite packaging into food simulants. Food Addit Contam - Part A. 2014;31(7):1284–90.
Golja V, Dražić G, Lorenzetti M, Vidmar J, Ščančar J, Zalaznik M, et al. Characterisation of food contact non-stick coatings containing TiO2 nanoparticles and study of their possible release into food. Food Addit Contam - Part A. 2017;34(3):421–33.
Bodai Z, Szabó BS, Novák M, Hámori S, Nyiri Z, Rikker T, et al. Analysis of potential migrants from plastic materials in milk by liquid chromatography-mass spectrometry with liquid-liquid extraction and low-temperature purification. J Agric Food Chem. 2014;62(41):10028–37.
Bodai Z, Kirchkeszner C, Novák M, Nyiri Z, Kovács J, Magyar N, et al. Migration of Tinuvin P and Irganox 3114 into milk and the corresponding authorised food simulant. Food Addit Contam - Part A. 2015;32(8):1358–66.
Codex Alimentarius commission JF. Project document for a regional standard Doogh. Codex Alimentarius Commission2009.
Tamang JP, editor. Ethnic fermented foods and alcoholic beverages of Asia. Delhi, India: Springer Sci. Rev.; 2016;1–137.
Farhoodi M, Emam-Djomeh Z, Ehsani MR, Oromiehie A. Effect of environmental conditions on the migration of di(2-ethylhexyl) phthalate from pet bottles into yogurt drinks: influence of time, temperature, and food simulant. Arab J Sci Eng 2008;33(2 B):279–87.
BS EN 1186–1. Materials and articles in contact with foodstuffs-Plastics-Part 1: Guide to Selection of conditions and test methods for overall Migration. European Committee for standardization, Brussels, Belgium.2002.
Traistaru E, Rivis A, Moldovan RC, Menelaou A. Modelling migration from plastic packaging materials used in food industry. J Agroaliment Process Technol. 2013;19(2):180–4.
CEN. Materials and Articles in contact with foodstuffs-plastics substances subjet to limitation. Guide to test methods for the specific migration of substances from plastics to foods and food simulants and the determination of substances. Cen/Ts 13130–23.2005;1.
Castle L, Damant AP, Honeybone CA, Johns SM, Jickells SM, Sharman M, et al. Migration studies from paper and board food packaging materials. Part 2. Survey for residues of dialkylamino benzophenone UV-cure ink photoinitiators. Food Addit Contam. 1997;14(1):45–52.
Bradley EL, Castle L, Jickells SM, Mountfort KA, Read WA. Use of overall migration methodology to test for food-contact substances with specific migration limits. Food Addit Contam - Part A. 2009;26(4):574–82.
Chea V, Angellier-Coussy H, Peyron S, Kemmer D, Gontard N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging: physical-chemical and structural stability under food contact conditions. J Appl Polym Sci 2016;133(2).
Galotto MJ, Torres A, Guarda A, Moraga N, Romero J. Experimental and theoretical study of LDPE: evaluation of different food simulants and temperatures. Food Res Int. 2011;44(9):3072–8.
Zabihzadeh Khajavi M, Ahmadi S, Abedi AS, Mohammadi R, Farhoodi M. A model study on the migration of Irganox 1010 from low density polyethylene into a fatty food simulant as a function of incorporated spherical and plate-like nanoparticles. Food Packag shelf Life. 2019;22.
Alin J, Hakkarainen M. Type of polypropylene material significantly influences the migration of antioxidants from polymer packaging to food simulants during microwave heating. J Appl Polym Sci. 2010;118(2):1084–93.
Baloul H, Belhaneche-Bensemra N, De Quirós ARB, Sendon R. Analysis and quantitative estimation of phenolic antioxidants in polypropylene packaging for fat products. J Polym Eng. 2018;38(9):899–904.
Widén H, Leufvén A, Nielsen T. Migration of model contaminants from PET bottles: influence of temperature, food simulant and functional barrier. Food Addit Contam. 2004;21(10):993–1006.
Ambrogi V, Carfagna C, Cerruti P, Marturano V. 4-additives in polymers. In: Modification of polymer properties. Carlos F. Jasso-Gastinel, José M. K, editors, William Andrew Publishing.2017;87–108.
Stevens MP. Polymer additives: part I. Mechanical property modifiers J Chem Educ. 1993;70:444–8.
Imel AE. Polymer Additives Effects on Structure and Dynamics 2015.
Perkins WG. Polymer toughness and impact resistance. Polym Eng Sci. 1999;39:2445–60.
Ouroumiehei AA, Ebrahimzadeh Mousavi SMA, Rabiei A, Biglari M. Study on the variations in the Physico-mechanical properties of PET bottles during shelf-life of carbonated beverages and its effect on permeation of CO2. Iran Polym J. 2004;17(6):343–39.
Joseph N, Murthy AK, Joseph KT, Santappa M. Effect of additives on the mechanical properties of polyacrylonitrile fibres. J Leather Sci Eng. 1981;28:177–80.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Forooghi, E., Ahmadi, S., Farhoodi, M. et al. Migration of Irganox 1010, Irganox 1076, and Titanium dioxide into Doogh and corresponding food simulant from laminated packaging. J Environ Health Sci Engineer 20, 363–373 (2022). https://doi.org/10.1007/s40201-021-00782-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-021-00782-y