Abstract
The aim of this study was to investigate the treatment and reuse of laundry wastewater with couple of nanofiltration (NF) and reverse osmosis (RO). In the NF process, optimal values of pH, temperature, transmembrane pressure (TMP) and cross-flow rate were determined using the Taguchi L16 (44) experimental design method. The smaller-the-better signal-to-noise (S/N) ratio was used to analyze the results of experiments. Flux decline caused by fouling was selected as response parameter. A pH of 8.5, temperature of 30 °C, TMP of 12 bar and cross-flow rate of 2 L/min were determined as optimum operating conditions in the NF process. According to analysis of variance (ANOVA), pH was the most effective factor while TMP and cross-flow rate had low effects on the fouling. Membrane fouling was also evaluated with scanning electron microscope (SEM), energy dispersive X-ray spectroscopy (EDX), zeta potential and optical profilometer measurements. In the RO process, the quality of NF permeate obtained from optimum operating conditions was improved by an RO membrane. Although a NF membrane was not effective in reducing chemical oxygen demand (COD), Orto-P and NH4+-N, these parameters were almost removed in the NF + RO system. These results showed that, the Taguchi method was successfully applied to determine the optimum operating conditions for the treatment of laundry wastewater with an NF process. Water treated with integrated membrane processes (NF + RO) is sufficient for use as laundry washing water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Domestic, industrial and hospital laundry wastewaters are important gray water sources. Almost 10% of municipal sewer discharge has a laundry wastewater origin. A laundry facility consumes 15 L of water for 1 kg of work and discharges an average of 400 m3 of wastewater daily [1]. A range of suspended solids, salts, oil-grease, surfactants, nutrients, heavy metals and pathogenic microorganisms is found in laundry wastewater [2, 3]. It is important to treat laundry wastewater to remove contaminants before its discharge to sewage or the environment. Laundry wastewater treatment is difficult since it has high surfactant content [1].
In recent years, there have been insufficient freshwater resources to meet water demand. Wastewater recovery and reuse are required due to rising industrial and public water demands. Commercial laundries have a variety of opportunities to recycle/reuse water at their facilities. Coagulation, electrocoagulation, flotation, adsorption, chemical oxidation, membrane filtration and biological treatment processes are applied to the treatment of laundry wastewater [4]. Treated laundry wastewater can be reused mainly in washing machines, for rinsing, in toilets and for irrigation. The treatment and reuse of laundry wastewater using various treatment processes has been reported in the literature. Šostar-Turk et al. (2005) studied the treatment of laundry wastewater with coagulation, adsorption and membrane filtration (Ultrafiltration-UF and Reverse Osmosis-RO). The results showed that the single coagulation process was not effective for removing surfactant, chemical oxygen demand (COD) and biochemical oxygen demand (BOD). Pollutant removal efficiency was improved by applying the adsorption process after coagulation. Additionally, a higher quality treated wastewater for reuse in the laundry industry was obtained with a combined UF and RO process [5]. Hoinkis and Panten (2008) applied a membrane bioreactor system (MBR) for the reuse of laundry wastewater in their work. The results showed that the permeate could be used as washing water. In addition, the application of the RO process improved MBR permeate quality to meet the demands of the rinsing processes [6]. Misra and Sivongxay (2009) investigated the reuse of laundry wastewater using clay soil. The results showed that treated wastewater was a valuable water resource for reuse in urban areas [7]. Ciabatti et al. (2009) tested a treatment process consisting of physico-chemical pretreatment (coagulation, flocculation and dissolved air flotation), sand filtration, ozonation, adsorption and UF a process. The results from the combined treatment processes (physico-chemical pretreatment, sand filtration, ozonation and adsorption) met the legal limits for discharge to surface water resources. In addition, a post treatment with UF reduced the residual pollutants, allowing the reuse of treated water in some washing processes [1]. Guilbaud et al. (2010) investigated laundry wastewater recycling with a nanofiltration (NF) process. The NF process produced a permeate free of microorganisms and suspended solids and with a COD of 48 mg/L [8]. Manouchehri and Kargari (2017) investigated water recovery from laundry wastewater using a microfiltration (MF) process. The results showed that MF is a reliable process for the reuse of laundry wastewater for washing machines, toilets and irrigation [9]. Bering et al. (2018) investigated the treatment of commercial laundry wastewater with a two-stage moving bed bioreactor. They reported that the quality of treated wastewater met legal requirements regarding the standards for discharge [10]. Choobar et al. (2019) studied the treatment of laundry wastewater using an MF process. The effects of feed pressure and flow rate on the flux decline were investigated. In addition, membrane fouling was evaluated using single and multistage Hermia models. They indicated that cake formation was the predominant fouling mechanism in the single Hermia model. The multistage Hermia model showed that the membrane tended to follow the cake formation rather than pore blocking as pressure increased. The opposite behavior was shown as flow rate increased [11]. Faria et al. (2019) investigated removal of anionic surfactant from commercial laundry wastewater and domestic sewage with expanded granular sludge bed reactor. The results showed that the anionic surfactant removal rate decreased when laundry wastewater was diluted with domestic sewage [12].
pH, temperature, transmembrane pressure (TMP) and cross-flow rate are important operating conditions in the membrane process since they affect flux decline and membrane fouling. The Taguchi optimization method for design of experiments (DOE) is used to find the optimum operating conditions in the treatment process [13,14,15]. There is no known notable study on determining operating conditions for laundry wastewater treatment using an NF membrane. In addition, the research on the treatment and reuse of laundry wastewater using a two-step membrane process including NF and RO has not been published. In the first stage of this study, the experimental design method was used to determine optimum values of pH, temperature, TMP and cross-flow rate to minimize flux decline. The signal-to-noise ratio (S/N) was used to determine optimum operating conditions, and analysis of variance (ANOVA) was used to determine the effect of the factors. An experiment was performed under optimum operating conditions. Scanning electron microscope (SEM), energy dispersive X-ray spectroscopy (EDX), zeta potential and optical profilometer measurements were performed to evaluate membrane fouling. At the second stage, the composite permeate quality obtained from the NF membrane was enhanced by an RO membrane. Wastewater treated with the integrated membrane processes (NF + RO) met the process water quality requirements for washing purposes.
Materials and methods
Characterization of laundry wastewater
Laundry wastewater used in this study was obtained from a laundry located in Avcılar, Turkey. Wastewater used in the experiments were directly collected from sewage discharge point. The wastewater was stored at 4 °C. The characteristics of the laundry wastewater are given in Table 1.
Membranes and experimental system
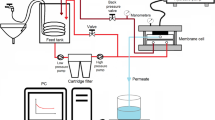
The two flat sheet membranes, Desal 5 DL (NF membrane, GE- Osmonics) and BW 30 (RO membrane, Dow-Film-Tech) were used in this study. The properties of the NF membrane were as follows: a polyamide material, a pH range of 2–11, a maximum TMP of 41 bar, a maximum temperature of 45 °C and the MWCO (molecular weight cut-off) of 150–300 Da. RO membrane is polyamide based and able to resistance a pH range of 2–11, a maximum TMP of 41 bar, a maximum temperature of 40 °C.
The membrane filtration experiments were performed in a lab-scale plant equipped with a plate and frame module (Osmo, Germany). Details of the membrane system are given in our previous study [14]. The system was operated in the concentration mode of filtration (CMF). Permeate was collected in a separate container and concentrate was circulated back to the feed tank. In the CMF tests, the volume reduction factor (VRF) was calculated using Eq. (1):
where Vf and Vc are the initial volume of feed (4 L) and the concentrate volume at the end of experiment, respectively. The VRF value was selected as 4 in the NF process. RO experiments were carried out under TMPs of 30 bar for 140 min.
Analytical methods and membrane characterization
The pH and conductivity were measured using a pH meter (Thermo Orion 3-Star) and a conductivity device (WTW Level 3), respectively. COD, sulphite, total hardness, chloride, Orto-P and NH4+-N were measured according to Standard Methods [16]. Color was measured at wavelength of 525 nm as DFZ (DurchsichtsFarbZahl = Indexes of Transparency) [17]. UV254 value was determined at a wavelength of 254 nm [18]. Jenway 6105 UV–Vis spectrophotometer was used for color and UV254 measurements. Anionic, cationic and nonionic surfactant were measured using Merck test kits (1.02552.0001, 1.01764 and 1.01787.0001, respectively).
Surface morphological features of membranes were analyzed with an SEM equipped with an EDX spectrometer (FEI Quanta FEG 200 SEM, USA). Membranes were coated with palladium-gold (3-4 nm thick) with Quorum SC7620 ion sputtering equipment. EDX analyses were conducted to detect ions that are accumulated on membrane surface. Zeta potential measurements on membranes were performed using an electrokinetic measurement instrument (Anton Paar Surpass, Austria). The value was determined as mV. 3 M KCl was used as the electrolyte. Surface roughness of membranes was determined with an optical profilometer (Zygo Brand USA). The roughness of mean (Ra), root mean square (Rrms) and maximum (Rmax) was determined as μm.
Flux and rejection
The fluxes (J) were measured as pure water flux (J0), wastewater flux (Js) and pure water flux of fouled membrane (Jf). Total flux decline, flux decline caused by concentration polarization and flux decline caused by fouling were calculated using values of J0, Js and Jf. The calculations were given in detail our previous [14]. The rejection performance of the membranes is calculated as:
where R is the rejection, Cp is the composite permeate concentration and Cf is the feed concentration.
Taguchi method
According to L16 (44) OA for the Taguchi experimental design method, four different factors with four levels were selected based on a literature survey (A = pH; B = temperature; C = TMP; D = cross-flow rate). The selected experimental design for the L16 array is given in Table 2. The flux decline caused by fouling was chosen as the response parameter to evaluate NF membrane fouling.
To analyze the experimental results of Taguchi method, three S/N ratios were used: the larger-the-better, the smaller-the-better and the nominal-the-better. In this study, the smaller-the-better ratio was used to evaluate flux decline (Eq. 3) [19]:
where n is the number of repetition applied for an experimental design, yi is the performance result for the ith experiment.
The performance value for optimum working conditions was predicted using Eq. 4 [20]:
where N is the total number of experiments, T is the sum of all experimental results, and \( \overline{{\mathrm{A}}_{\mathrm{i}}} \) and \( \overline{{\mathrm{B}}_{\mathrm{j}}} \) are the average of responses at levels i and j, respectively.
The confidence interval (CI) at the selected error level was estimated using Eq. (5) since Eq. (4) is a point estimation. Additionally, Ω transformation of percentage values was calculated when the results were given as a %. Ω transformation were calculated using Eq. (6) [21]. Detailed information about the calculations was given in our earlier study [14].
where P is the percentage of the product determined experimentally.
The effects of factors on the flux decline was determined with ANOVA. The impact of the effects was determined with an F-test of significance (F). The sum of squares (SS), degrees of freedom (DOF), mean of squares (MS) and associated F values were computed with ANOVA. The calculations were given in our earlier study [14].
Results and discussion
Taguchi and ANOVA results
The flux and flux decline results for the NF membrane are shown in Fig. 1. According to Fig. 1, the highest flux declines caused by fouling were 41.7% and 40%, occurring in exp. 14 and exp.15, respectively. The lowest flux decline caused by fouling (14.1%) occurred in exp. 9.
The mean values of the S/N ratios of the factors are shown in Fig. 2. The mean S/N ratios were used to evaluate fouling of membrane. The results were evaluated in detail as follows:
The effect of pH on the flux decline caused by fouling is shown in Fig. 2(a). The highest S/N value (−24.84) was observed at pH 8.5, whereas the lowest S/N ratio (−30.67) was found at pH 12. The S/N values for pH 6 and pH 10 were − 25.61 and − 25.52, respectively. The isoelectric point of Desal 5DL membrane based on polyamide is 3.2 [14]. Therefore, the NF membrane is negatively charged at all studied pH values. Additionally, it is known that polyamide membranes show hydrophobic properties at high pH values [22]. The higher S/N value obtained at pH 12 may be attributed to the membrane becoming more hydrophobic. In addition, metal ions can precipitate on the membrane surface at higher pH values. This situation clarified in Section 3.3.1. In addition, the negatively charged components such as anionic surfactant and sulphate ions can be effectively removed at higher pH values because repulsion forces increase between the membrane surface and these components.
As seen in Fig. 2(b), the highest S/N value (−24.72) was observed at 30 °C. S/N values at 35, 40 and 45 °C were − 26.88, −27.41 and − 27.60, respectively. At higher temperatures, the rejection of pollutants decreases since the transport of them through the membrane increases. This outcome causes the aggregation of pollutants on the membrane surface and pores [11]. Thus, flux decline and fouling increased with increasing temperatures.
According to Fig. 2 (c), the S/N ratio was −25.94 at 12 bar while S/N values were almost the same at 16, 20 and 24 bar (−26.80, −26.92 and − 26.92). It is known that concentration polarization increase with an increase in applied TMP. Therefore, more pollutants can accumulate on the membrane surface, resulting in gel layer formation. Membrane fouling increases due to cake layer formation [23].
The effect of cross-flow rate on flux decline is shown in Fig. 2 (d). S/N ratios at 2, 3 and 4 L/min were − 26.33, −24.41 and − 26.52, respectively. The lowest S/N (−27.07) was observed at 1 L/min. It is known that cross-flow rate influences concentration polarization and mass transfer on the membrane surface. A cake layer on the membrane surface can be formed faster at a lower cross-flow rate due to lower shear force [24].
According to S/N ratios of flux decline, the best results for pH, temperature, TMP and cross-flow rate were found as 8.5, 30 °C, 12 bar and 2 L/min, respectively.
The ANOVA approach was applied to determine the effects of factors on the response parameter. The evaluation of ANOVA analysis is carried out in consideration of the F ratio and p (%) values. The F ratio is compared with the Fcritic value, which is determined for a certain confidence interval from statistical charts. If the calculated F-ratio is larger than the Fcritic value, the statistical test is significant for performance at the selected confidence level. Degrees of freedom of each factor and error is 3 (Table 3). In this case, the Fcritic value is specified as 9.28 for the 95% confidence interval (F0,05; 3,3) [25]. When F ratios are compared with the Fcritic value, it can be said that the pH is a considerable factor at the 95% confidence level. While the F ratio is used in qualitative evaluation of the effects of factors, p% values are used for quantitative evaluation. As seen in Table 3, the relative effect sorting of the factors on membrane fouling is as follows: pH> > temperature > cross-flow rate > TMP. The results show that flux decline caused by fouling was mainly affected by the pH value.
Experimental results for optimum operating conditions
The experiment was conducted under the optimum operating conditions (pH:8.5; Temperature: 30 °C; TMP: 12 bar; cross-flow rate:2 L/min) to compare flux decline caused by fouling with the predicted result. J0, Js and Jf were found to be 119, 85 and 107 L/m2.h, respectively, in under optimum operating conditions. As seen in Table 4, the observed flux decline caused by fouling was 10.1% in a ± 5% error range.
Membrane autopsy results
An autopsy study was performed with SEM-EDX, zeta potential and optical profilometer analysis to evaluate membrane fouling. The analyses were performed on fouled membranes used under the optimum operating conditions and exp. of 14. In addition, all analyses were performed on clean membrane for comparison with the fouled membranes.
SEM images and EDX spectra
Figure 3. shows SEM images of clean and fouled membranes. According to cross-section and surface images of membranes, more impurities were deposited on the surface of the fouled membrane in exp. 14 while a thinner of cake-layer was observed on the fouled membrane under the optimum operating conditions. The pores of the fouled membrane in exp.14 were more clogged with impurities. EDX spectra of fouled membranes are given in Fig. 4, which shows that, Ca, Mg, Al, Si and P ions were detected in greater amounts on the cake-layer of the fouled membrane in exp.14. As mentioned in Section 3.1, it is clear that metal ions precipitated on the membrane surface in exp. 14. However, fewer metal ions were observed on the surface of the fouled membrane under optimum operating conditions. As a result, it can be said that inorganic scaling can be more effective at the higher pH values.
Zeta potential results
The zeta potential was measured to determine the surface charge of membranes with different pH values. As seen in Fig. 5., all membranes are negatively charged at a pH range of 4-12. The negative zeta potentials may originate from carboxylic (-COOH) groups in the polyamide membranes [26]. However, both fouled membranes have a higher zeta potential than the clean membrane. The fouled membrane in exp. 14 had a lower negative charge than the fouled membrane under optimum operating conditions. We theorize that the, zeta potential decreased more on the fouled membrane in exp. 14 because electrostatic repulsion forces decreased between the negatively charged membrane surface and positively charged components.
Optical profilometer results
Optical profilometer images of membranes are shown in Fig. 6. The Rrms values of membranes used under optimum operating conditions and in exp. 14 were 0.187 and 0.609 μm, respectively, while the Rrms of the clean membrane was 0.128 μm. This outcome indicates that more cake-layer was built on the surface of the fouled membrane in exp. 14. In addition, SEM images of membranes supported that the cake-layer was thicker in the fouled membrane in exp. 14.
RO experiments
An RO experiment was performed for 140 min. With permeate (3 L) obtained from an NF membrane under experimental conditions of pH 8.5, 30 bar, 30 °C and 2 L/min. J, J0 and Js were 95, 56 and 75 L/m2.h, respectively, at the end of the RO experiment. The permeate quality of the NF membrane was improved by the RO membrane. The permeate quality of both membranes was compared with process water used in the laundry. As seen in Table 5, the composite permeate obtained from the NF membrane meets process water quality requirements except for Orto-P, COD, and NH4+-N. However, the permeate quality of the RO membrane is suitable for process water. Additionally, the permeates of both membranes showed better quality than that of process water for some parameters such as UV254, conductivity, turbidity, sulphate and total hardness.
Comparison of laundry wastewater treatment processes for reuse purposes
A number of studies carried out for laundry wastewater treatment for reusing purposes are presented in Table 6. To summarize these studies for reusing of treated laundry wastewater: Physico-chemical pre-treatment did not meet the minimum quality requirements and UF or RO membrane processes were required for reuse of the treated effluents in washing processes. Also MBR system was not found effective for reuse of laundry wastewater and RO process was required to meet quality demands of water for the washing processes. Although NF membrane without pretreatment allowed to produce water for washing machines, COD and conductivity removal was not completely achieved. The MF treated laundry wastewater could be directly used for landscape irrigation or toilet flushing without the need for further post-treatments, but could not meet the desired COD standards for direct use as washing water. According to the results of these studies, we can say that a RO membrane could be required to meet minimum quality values.
Although several membrane processes were preferred for reuse of treated wastewater, integrated NF and RO membrane process has not been investigated. In the present study, high quality treated water was obtained using integrated NF-RO process and treated water met to limit values for reuse in washing machines. In addition, membrane fouling was evaluated with membrane autopsy analysis compared with in the above studies, except for only one study.
Conclusions
The integrated membrane process including the NF and RO membrane was applied to the treatment of laundry wastewater in this study. To optimize fouling, optimum conditions for pH, temperature, TMP and cross-flow rate were determined with a Taguchi experimental design in the NF process. An RO membrane was used to improve the permeate quality of the NF membrane. The optimum operating conditions were determined according to values of pH (6, 8.5, 10 and 12), temperature (30, 35, 40 and 45 °C), TMP (12, 16, 20 and 24 bar) and cross-flow rate (1, 2, 3 and 4 L/min). The lowest fouling was observed at the second level of pH (8.5), first level of temperature (30 °C), first level of TMP (12 bar) and second level of cross-flow rate (2 L/min) according to the S/N ratios. Values of pH were identified as an important factor in membrane fouling due to p% values indicated in the ANOVA results. The p% values of the factors showed that TMP and cross-flow rate did not affect fouling. pH is an important factor that affects properties of polyamide membranes such as charge, hydrophobic state and pore structure. At pH 12, the highest flux decline caused by fouling was found due to inorganic scaling. According to the ANOVA results, temperature was a significant second factor for fouling. At temperature values above 30 °C, fouling was greater since pollutants could easily pass through membrane pores.
The experiment performed under optimum operating conditions showed that predicted flux decline (11.1%) and observed flux decline (10.1%) values were within a ± 5% error range.
According to the autopsy results, impurities accumulated on the surface and pores in the fouled membranes. Impurities were especially clearly seen in the SEM images of fouled membrane used in exp. 14 (pH 12, 35 °C, 20 bar and 1 L/min). Zeta potential results showed that more inorganic scaling occurred in the fouled membrane used in exp. 14. The autopsy results comparing the fouled membranes from exp.14 and the optimum operating conditions indicate that pH was most the important factor in the fouling of the NF membrane.
Process water for laundry washing purposes is provided from well water softened using an ion exchange process. An NF membrane was not effective in reducing COD, Orto-P and NH4+-N. In the NF + RO system, these parameters were reduced compared to the NF process alone. As a result, wastewater treated by the integrated membrane processes (NF + RO) met the process water quality requirements. Comparison of laundry wastewater treatment processes for reuse purposes showed that the studied NF + RO process could be used as a technically successful method for obtaining water for reuse purposes. At the same time, the cost and workload resulting from treatment with ion exchange for the process water production (softening) will be reduced by approximately 75%.
References
Ciabatti I, Cesaro F, Faralli L, Fatsrella E, Tognotti F. Demonstration of a treatment system for purification and reuse of laundry wastewater. Desalination. 2009;245:451–9.
Misra KR, Patel JH, Baxi VR. Reuse potential of laundry for irrigation based on growth, water and nutrient use of tomato. J Hydrol. 2010;386:95–102.
Janpoor F, Torabian A, Khatibikamal V. Treatment of laundry wastewater by electrocoagulation. J Chem Technol Biotechnol. 2011;86:1113–20.
Kim H-C, Shang X, Huang J-H, Dempsey BA. Treating laundry wastewater: cationic polymers for removal of contaminants and decreased fouling in microfiltration. J Membr Sci. 2014;456:167–74.
Šostar-Turk S, Petrinić I, Simonič M. Laundry wastewater treatment using coagulation and membrane filtration. Resour Conserv Recy. 2005;44:185–96.
Hoinkis J, Panten V. Wastewater recycling in laundries-from pilot to large-scale plant. Chem Eng Process. 2008;47:1159–64.
Misra KR, Sivongxay A. Reuse of laundry greywater as affected by its interaction with saturated soil. J Hydrol. 2009;366:55–61.
Guilbaud J, Massé Andrès Y, Combe F, Jaouen P. Laundry water recycling in ship by direct nanofiltration with tubular membranes. Resour Conserv Recy. 2010;55:148–54.
Manouchehri M, Kargari A. Water recovery from laundry wastewater by the cross-flow microfiltration process: a strategy for water recycling in residential buildings. J Clean Prod. 2017;168:227–38.
Bering S, Mazur J, Tarnowski K, Janus M, Mozia S, Morawski AW. The application of moving bed bio-reactor (MBBR) in commercial laundry wastewater treatment. Sci Total Environ. 2018;627:1638–43.
Choobar BG, Shahmirzadi MAA, Kargari A, Manouchehri M. Fouling mechanism identification and analysis in microfiltration of laundry wastewater. J Environ Chem Eng. 2019;7:103030.
de Faria CV, Delferno TP, Okada DY, Varesche MBA. Evaluation of anionic surfactant removal by anerobic degradation of commercial laundry wastewater and domestic sewage. Envr Technol. 2019;40:988–96.
Gönder ZB, Arayici S, Barlas H. Advanced treatment of pulp and paper mill wastewater by nanofiltration process: effects of operating conditions on membrane fouling. Sep Purif Technol. 2011;76:292–302.
Hepsen R, Kaya Y. Optimization of membrane fouling using experimental design:an example from dairy wastewater treatment. J Ind Eng Chem. 2012;51:16074–84.
Qusay FA, Salah SI, Fatima AH. Experimental and theoretical investigation of air gap membrane distillation process for water desalination. Chem Eng Res and Des. 2018;130:95–108.
APHA/AWWA/WEF Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington DC, USA, ISBN 0–87553–223-3. 1995.
European norm, ISO 7887 Water quality examination and determination of color, Brussels. 1994.
DIN 38404-C3, Deutsche einheitsverfahren zur wasser-, abwasserund schlammuntersuchung- physikalisch-chemische kenngrößen (gruppe C) - teil 35: bestimmung der absorption im bereich der UV-strahlung spektraler sbsorptionskoeffizient (C3); Beuth Verlag: Berlin 2003.
Aber S, Salari D, Parsa MR. Employing the Taguchi method to obtain the optimum conditions of coagulation-flocculation process in tannery wastewater treatment. Chem Eng J. 2010;162:127–34.
Safarzadeh MS, Moradkhani D, Ilkhchi MO, Golshan NH. Determination of the optimum conditions for the leaching of cd–Ni residues from electrolytic zinc plant using statistical design of experiments. Sep Purif Technol. 2008;58:367–76.
Çopur M, Özmetin C, Özmetin E, Kocakerim MM. Optimization study of the leaching of roasted zinc sulphide concentrate with sulphuric acid solutions. Chem Eng Process. 2004;43:1007–14.
Yang J, Lee S, Lee E, Lee J, Hong S. Effect of solution chemistry on the surface property of reverse osmosis membranes under seawater conditions. Desalination. 2009;247:148–61.
Li K, Ma W, Han H, Xu C, Zhu H. Selective recovery of salt from coal gasification brine by nanofiltration membranes. J Environ Manag. 2018;223:306–13.
Kenneth SYN, Malavika H, Dalton JEH, Dave ED, Gregory JO. Mechanisms of flux decline in skim milk ultrafiltration: a review. J Membr Sci. 2017;523:144–62.
Montgomery DC. Design and analysis of experiments. 5th. ed. Newyork: John Wiley; 2001.
Pulido JMO, Férez AM. Impacts of operating conditions on nanofiltration of secondary-treated two-phase olive mill wastewater. J Environ Manag. 2015;161:219–27.
Acknowledgements
This work was supported by the Research Fund of the Istanbul University-Cerrahpasa (Project number: 25868).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaya, Y., Dayanir, S. Application of nanofiltration and reverse osmosis for treatment and reuse of laundry wastewater. J Environ Health Sci Engineer 18, 699–709 (2020). https://doi.org/10.1007/s40201-020-00496-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-020-00496-7