Abstract
Purpose
A growing number of evidence have assessed the association between bisphenol A (BPA) as an endocrine-disrupting agent and the risk of gestational diabetes (GDM). This meta-analysis aimed to reassess the data on the association of BPA levels in women with GDM compared to the control.
Methods
A comprehensive literature search was conducted in Medline, Embase, Scopus, and Web of Science to extract relevant published studies up to May 2024. 12 articles were included in the meta-analysis. DerSimonian and Liard random-effects model was used to estimate the pooled odds ratio (OR). Sensitivity analysis was conducted to assess the robustness of the pooled results by removing each study from the pooled effect size. Subgroup analyses were performed depending on the subgroups of gestational age, GDM trimester, BMI, study design and geographical area.
Results
The results showed that there was no significant association between circulating and urinary BPA concentrations with the risk of GDM (OR: 0.79; 95% CI 0.60–1.04; P = 0.095). No significant heterogeneity was found among the studies. Using Begg’s correlation (P = 0.95) and Egger’s linear regression (P = 0.86) tests, no publication bias was observed. The sensitivity analysis shows that our findings were completely robust and stable. Meta-regression indicated a significant association between BPA levels and study design and geometric mean as an index of the risk of GDM.
Conclusion
The present meta-analysis demonstrates exposure to BPA was associated with a reduced risk of GDM. Further studies are needed for obtain the reliable results.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is recognized as one of the most common serious complications during pregnancy and is related to a higher risk of unfavorable maternal and perinatal outcomes. These adverse pregnancy outcomes include overgrowth of the fetus that results in macrosomia, or infants that are larger than average, regardless of gestational age [1, 2], obstructed labor, increase in the rate of surgical deliveries, especially by cesarean Sects. [2, 3]. Preterm delivery and hypertensive disorders are other adverse effects seen in pregnancies complicated by GDM [4]. The studies have indicated a relationship between gestational diabetes and increased risk of neonatal complications [5, 6]. These well-known complications include hypoglycemia, birth trauma, polycythemia, hypocalcemia, hyperbilirubinemia, and neonatal respiratory distress. Infants born to mothers with GDM are also at increased risk of early obesity and are more likely to suffer from cardiovascular disease (CVD) and type 2 diabetes in the future. Moreover, there is evidence that mothers with a history of GDM may indicate an underlying susceptibility to complications such as hypertension, type 2 diabetes, metabolic syndrome, and ischemic heart disease compared with mothers without prior GDM [5, 6]. Bisphenol A (BPA) is recognized as a ubiquitous endocrine disruptor that exists in polycarbonate plastics, epoxy resins and other polymer materials [7]. BPA has been reported to cause multiple harmful effects affecting various organs, such as the immune system, reproductive system, nervous system, etc. [7]. Exposure to BPA can alter placental microRNA expression levels and cause epigenetic changes such as DNA methylation and histone methylation and acetylation [8]. Animal studies have indicated that exposure to BPA can lead to metabolic disorders relevant to glucose homeostasis, which is considered a risk factor for the development of diabetes [7, 9, 10]. Additional studies also suggest a relationship between BPA exposure and increased type 2 diabetes. Several studies have also addressed the link between BPA exposure and the risk of developing GDM. Some studies have indicated that increased cases of GDM can be associated with BPA exposure. A prospective cohort study in China investigated 1,841 pregnant women and selected samples with GDM to assess the relationships between BPA substitutes and GDM. The researchers suggested that BPA substitutes (BPS and BPAF) might be potential risk factors for developing GDM [11]. A positive association was observed between BPA exposure during the second trimester and blood glucose levels [12]. However, results regarding the association between BPA and GDM are inconsistent and further research is needed. In this study, we sought to conduct a meta-analysis for further evaluation of these relationships, considering articles including human studies.
Methods
The present meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [13].
Search strategy
PubMed, Medline, ISI Web of Science, EMBASE, and Scopus systematically searched using the following keywords: (“Bisphenol A” [Mh] OR 2,2-bis(4-hydroxyphenyl)propane OR 4,4’-dihydroxy-2,2-diphenylpropane OR Diphenylolpropane OR bisphenol A, sodium salt OR bisphenol A, disodium salt) in combination with (“Diabetes, Gestational“[Mesh] OR Gestational Diabetes Mellitus OR Pregnancy-Induced Diabetes OR Gestational Diabetes). Only articles written in English up to May 2024 were included and duplicates were removed by using Endnote software version X7.8. The primary eligibility of articles was first evaluated by screening titles and abstracts. Then, full-text manuscripts were assessed for inclusion of articles in the study. Additionally, we checked the reference lists of the eligible articles to detect other relevant studies. The literature search and the study screening were performed by two independent authors (M.K. and N.AD.). An independent author (M.F.) assessed the potential eligibility of articles. Disagreements were resolved by discussion with a fourth reviewer (A.D.). The eligibility criteria comprised (i) population exposed to BPA, (ii) gestational diabetes mellitus (GDM), (iii) outcome of GDM related to BPA described, (iv) published in English. A meta-analysis on our topic was published by Taheri et al., in September 2021. To assess Taheri’s article, we designed the present meta-analysis by obtaining results from the AMSTAR checklist (data not shown).
Data extraction and quality assessment
Selected studies were reviewed by two reviewers (M.K. and N.AD.) and the following data from included studies were collected and tabulated using a standardized data extraction form. Data are listed as follows: first author name, publication year, study country, study design, sample size case who has GDM, sample size of control, gestational age, BMI, BPA exposure levels in the case and control, exposure indicator (serum/plasma or urinary) and geometric mean of BPA levels in women with GDM. On the other hand, two investigators (M.F. and N.AD.) independently assessed the quality of the included studies based on the Newcastle–Ottawa Scale (NOS) system. The studies were evaluated across 4 domains: (1) study population selection, (2) exposure, (3) comparability, and (4) outcome. The maximum score for a study was 9 points. After quality assessment, the studies were assigned a score from zero to nine. Finally, the studies were categorized into three classes based on scoring: (I) low quality (0 to 4 points), (II) moderate quality (5 to 7 points), and (III) high quality ( > = 8). The reviewers resolved any disagreements in scoring processing by discussion.
Outcome
Primary outcome includes the Bisphenol A concentrations in women with GDM and secondary outcomes were affective variables that altered the concentration of Bisphenol A in the included studies.
Statistical analysis
We used the odds ratio (OR) and 95% CI to quantitatively determine the association between BPA exposure and the risk of GDM. Heterogeneity among studies was assessed using the Q test and the Higgins I-square (P < 0.1, values < 25%, 25-50%, and > 50% were set to indicate mild, moderate, and significant heterogeneity, respectively) [14]. If I2 > 50%, the DerSimonian and Laird random-effect model were used to obtain the ORs. Sensitivity analysis was also performed to evaluate the impact of removing each study using the “leave-one-out method”. To find the variables involved in significant heterogeneity among the studies, subgroup analyses were conducted based on subgroups of gestational age ( = < 31, > 31 years), pregnancy trimester (first, second and third trimester), BMI (< 25, > 25), sample type (urine and serum), study design (cohort, case-control and cross-sectional), geographical area (countries) and geometric mean (< 1, > 1). We also applied Begg’s rank correlation test and Egger’s regression asymmetry test as statistical analysis to evaluate the potential publication bias produced by the funnel plot as a graphical analysis [15, 16]. The trim-and-fill analysis was used to adjust any significant publication bias detected. Lastly, a random-effects meta-regression analysis was conducted, using an unrestricted maximum likelihood method, to explore the association between the urinary or circulating levels of BPA with gestational age, GDM trimester, BMI, study design, sample size, geographical area and geometric mean. A P-value threshold of 0.05 was used to determine statistical significance. Meta-analysis was conducted using the Comprehensive Meta-Analysis (CMA) V3 software (Biostat, NJ).
Overall results
The flow of record selection
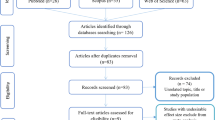
A systematic search of electronic databases was performed to find 147 articles according to the PRISMA flow chart explaining the study selection process. Of which 62 articles were removed to be duplicated. A total of 85 records were screened for title and abstract, and 59 were finally excluded due to were not original or research article or because they were not original or research article or because they were animal and in vitro models. Thus, 26 articles were selected as potentially reviewed. Fourteen of them were excluded since the measurement of the level of Bisphenol A was not relevant to gestational diabetes. Finally, 12 articles were selected based on inclusion and exclusion criteria for the meta-analysis. The PRISMA flowchart of the study selection is shown in Fig. 1.
Characteristics of the included studies
The primary characteristics of the included studies are summarized in Table 1. The publication year of all studies between 2013 and 2022. Among the selected articles, four were case-control articles, six articles were cohort studies and one was cross-sectional study. Regarding to the location of article, four studies were performed in the USA, 5 in china, 1 in the UK and 1 in Canada. In terms of sample type, eight articles were measured the level of Bisphenol A in the urine sample and three articles in the serum sample. Concerning the GDM trimester, seven studies were evaluated the level of Bisphenol A in the second trimester, four studies in the first trimester and two in the third trimester. The average age of the GDM group was 31 years. The mean BMI was 26.6 in the GDM group. The measurement unite of Bisphenol A in all studies were uniform as µg/L. A total of 1267 women participated in 12 articles as GDM group. We carried out the data quality assessment using NOS scale. The quality of the data was moderate to strong in all studies. The present meta-analysis includes 12 articles with 13 studies.
Bisphenol A and risk of GDM
13 studies within 12 articles in this meta-analysis are involved in the analysis of Bisphenol A and the risk of GDM. A random-effect model was utilized to estimate the OR. There is a negative association between Bisphenol A and the risk of GDM (OR = 0.79) (95% CI 0.60–1.04) P = 0.095 suggesting that Bisphenol A was not significantly associated with the risk of GDM. The random forest plot is presented in Fig. 2. A significant heterogeneity was observed among the included studies (I2 = 71.74%, Tau2 = 0.12, SE = 0.10, Q = 42.4, P = 0.00). In this regard, subgroup analysis was performed according to the subgroups of pregnancy trimester, sample type and study design to find the source of significant heterogeneity in the meta-analysis.
Sensitivity analysis
Sensitivity analyses were conducted using the leave-one-out method. In this meta-analysis, we omitted each study from highest sample size to lowest sample size. The results of sensitivity analysis showed the overall effect size from OR = 0.78 to OR = 0.89 indicating robust stability of the overall result in this meta-analysis. Thus, Bisphenol A concentration was inversely associated with GDM risk.
Subgroup analysis
Subgroup analyses were conducted based on subgroups of age ( = < 31, > 31 years), pregnancy trimester (first, second and third trimester), BMI (< 25, > 25), sample type (urine and serum), study design (cohort, case-control and cross-sectional), geographical area (countries) and geometric mean (< 1, > 1). In the subgroup analysis, a significant association was observed between BPA levels in the urine sample and GDM risk. When the subgroup analysis was classified by the design of study, the pooled OR from case-control studies significantly was associated with the risk of GDM. Moreover, the OR of the other subgroups did not show a significant association between Bisphenol A and the risk of GDM. Therefore, this study reported that the variables of urine samples and case-control studies are the sources of significant heterogeneity in the included studies. The results of the subgroup analysis are summarized in Table 2.
Meta-regression
Random-effects meta-regression was performed by using an unrestricted maximum likelihood method, to explore the association between the urinary or circulating levels of BPA with gestational age, GDM trimester, BMI, study design, sample size, geographical area and geometric mean (Fig. 3). The results showed no significant association between urinary or circulating levels of BPA, gestational age, GDM trimester, BMI, sample size and geographical area. However, a significant negative association between study design and geometric mean as reporting the growing rate of women with GDM was found. The outcome of meta-regression is shown in Table 3.
Publication bias
According to the Begg’s rank correlation (Kendall s Tau with continuity correction = 0.012; Z = 0.061; 2-tailed p-value = 0.95) and Egger’s linear regression (intercept = 0.15; standard error = 0.84; 95% CI = -1.70–2); t- value = 0.17; df = 11; 2-tailed p-value = 0.86) as statistical analysis, no publication bias was observed in the meta-analysis. As a graphical analysis, the funnel plot of study precision (standard error) by pooled effect size (log odds ratio) was symmetric and proposed no publication bias in studies reporting the Bisphenol A level and the risk of GDM. The observed publication bias was imputed by trim-and-fill correction. Two imputed studies were found in the meta-analysis. The pooled result did not change affected by two imputed studies (OR = 0.74) (95% CI 0.56–0.96) (Fig. 4).
Discussion
Several studies have shown contradictory results about bisphenol A exposure and the risk of GDM. Towards this end, we carried out a meta-analysis on the levels of urinary and circulating bisphenol A in women with GDM. In this regard, we obtained 12 articles that have investigated the BPA concentration of women with GDM. In the present study, we found that there is no significant association between the levels of BPA and the development risk of GDM. Bisphenol A dysregulates glucose hemostasis, insulin level and HOMA-IR in the pregnancy periods [17, 18]. As in the middle of pregnancy, circulating glucose levels in the pregnant women decreased following an increase in the insulin level [19]. While BPA in the higher concentration increases insulin resistance by placental hormones through stimulating inflammation and oxidative stress [20]. Apart from that, BPA has a similar molecular structure to 17β-estradiol (E2), therefore, BPA may bind to estrogen receptors (ERs) and act as endocrine-disrupting agents [21, 22]. Consistent with our results were reported in several previous studies. These studies found a negative association between BPA and the risk of GDM [11, 17, 23, 24]. Moreover, a study reported that maternal urine BPA exposure was associated with a reduced risk of GDM [25]. Consequently, the findings from the present study, which confirm that BPA levels are reduced in women with GDM, correspond with the findings from previous studies, and taken together, the findings implicate BPA as a potential factor in the pathogenesis of GDM.
To find the significant heterogeneity among included studies, subgroup analyses were conducted. Firstly, the results of subgroup analysis based on subgroups of pregnancy trimester showed a significant reduction in BPA levels between the third trimester of pregnancy with first of second trimesters. This might be due to increase in glucose levels and insulin resistance to BPA exposure in the middle or late pregnancy.
In addition, we analyzed BPA levels in subgroups of age and BMI. The subgroup of age based on lower or greater than 31 years age has no significant effect on urinary or circulating levels of BPA in women with GDM. Moreover, pooled results from studies stratified based on BMI < 25 and BMI > 25 indicated no significant difference in BPA levels between GDM and controls. These results were also confirmed by meta-regression analysis. Apart from that, subgroup analyses based on sample type of urine or serum, study design and geographical area were also carried out. BPA levels were significantly lower in GDM women compared to controls in subgroups of sample type of urine and case-control studies. On the other hand, BPA levels were not significantly different from control in studies stratified by geographical area. These results implicate increased BPA levels in urine samples in women with GDM compared to serum samples, suggesting more metabolism of BPA through the kidneys.
Importantly, meta-analysis results showed a strong association between urinary or circulating levels of BPA and case-control study design and geometric mean. The geometric mean is an index that is often used for showing a growth rate of GDM affected by BPA levels. In the present study, our results confirm that long-term exposure to BPA is an important factor that can affect the risk of GDM.
The present subjected to several limitations: A limitation was significant heterogeneity among included studies. Thus, we performed subgroup analysis and meta-regression to explore the source of heterogeneity. The relatively small number of pregnant women with GDM in the subgroup analysis could be another limitation of the current study that needed more studies with larger sample sizes. Moreover, BPA has a short half-life. Therefore, the duration time of exposure to BPA in studies could result in bias. On the other hand, we were not able to control the variety of potential confounding variables such as diet and plastic-containing compounds as important sources of bisphenol in the present study. All in all, the present study provided information on BPA exposure and the risk of GDM with a major strength point being the analysis of BPA levels in both urine and serum samples. Nevertheless, further experimental and epidemiologic studies are required to validate these results in the future.
Conclusion
We evaluated the BPA exposure effect on the maternal risk of GDM by using a meta-analysis. The results showed no significant association between circulating and urinary BPA concentration and the risk of GDM. Taken together, these results could be important for public health implications and clinical decisions and provide new insight for assessing the health impacts of BPA.
Availability of data and materials
The data used to support the findings of this study are included within the article. Additional information can be requested by contacting the corresponding author.
Abbreviations
- GDM:
-
Gestational diabetes
- BPA:
-
Bisphenol A
- BPS:
-
Bisphenol S
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- NOS:
-
Newcastle-Ottawa scale
- OR:
-
Odds ratio
References
Preda A, et al. Gestational diabetes and preterm birth: what do we know? Our experience and mini-review of the literature. J Clin Med. 2023;12(14):4572.
Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Annals Nutr Metabolism. 2015;66(Suppl 2):14–20.
Gorgal R, et al. Gestational diabetes mellitus: a risk factor for non-elective cesarean section. J Obstet Gynecol Res. 2012;38(1):154–9.
Innes K, Wimsatt J. Pregnancy-induced hypertension and insulin resistance, evidence for a connection. Acta Obstet Gynecol Scand. 1999;78(4):263–84.
Ye W et al. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ, 2022. 377.
Murray SR, Reynolds RM. Short-and long‐term outcomes of gestational diabetes and its treatment on fetal development. Prenat Diagn. 2020;40(9):1085–91.
Ma Y, et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ Res. 2019;176:108575.
Mitra T, et al. Endocrine disrupting chemicals: gestational diabetes and beyond. Diabetol Metab Syndr. 2024;16(1):95.
Ding S, et al. High-fat diet aggravates glucose homeostasis disorder caused by chronic exposure to bisphenol A. J Endocrinol. 2014;221(1):167–79.
Amiri-Dashatan N et al. Potential molecular mechanisms of Bisphenol A in obesity development: Bisphenol A and obesity. Int J Med Toxicol Forensic Med, 2023. 13(4).
Zhang W, et al. Exposure to bisphenol a substitutes and gestational diabetes mellitus: a prospective cohort study in China. Front Endocrinol. 2019;10:262.
Chiu Y-H, et al. Trimester-specific urinary bisphenol a concentrations and blood glucose levels among pregnant women from a fertility clinic. J Clin Endocrinol Metabolism. 2017;102(4):1350–7.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (Chinese edition). J Integr Med. 2009;7(9):889–96.
Higgins JP, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics, 1994: p. 1088–101.
Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Yang J, et al. Serum bisphenol A, glucose homeostasis, and gestational diabetes mellitus in Chinese pregnant women: a prospective study. Environ Sci Pollut Res. 2021;28:12546–54.
Fisher BG, et al. Serum phthalate and triclosan levels have opposing associations with risk factors for gestational diabetes mellitus. Front Endocrinol. 2018;9:99.
Sonagra AD, et al. Normal pregnancy-a state of insulin resistance. J Clin Diagn Research: JCDR. 2014;8(11):CC01.
Strakovsky RS, Schantz SL. Using experimental models to assess effects of bisphenol A (BPA) and phthalates on the placenta: challenges and perspectives. Toxicol Sci. 2018;166(2):250–68.
Soriano S, et al. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS ONE. 2012;7(2):e31109.
Tang P, et al. Associations of bisphenol exposure with the risk of gestational diabetes mellitus: a nested case–control study in Guangxi, China. Volume 30. Environmental Science and Pollution Research; 2023. pp. 25170–80. 10.
Shapiro G, et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC study. Environ Int. 2015;83:63–71.
Hou Y, et al. Associations of urinary phenolic environmental estrogens exposure with blood glucose levels and gestational diabetes mellitus in Chinese pregnant women. Sci Total Environ. 2021;754:142085.
Wang X, et al. Urinary bisphenol A concentration and gestational diabetes mellitus in Chinese women. Epidemiology. 2017;28:S41–7.
Acknowledgements
The authors would like to thank Yasuj University of Medical Sciences, Yasuj, Iran, for the support.
Funding
This project was financially supported by grants from the Deputy of Research, Yasuj University of Medical Sciences (Grant No: 4020157).
Author information
Authors and Affiliations
Contributions
All the authors drafted the manuscript. N A.D, MK and AD contributed to the improvement of the selection criteria, the risk of bias assessment strategy and data extraction criteria. MF, SA and RV started selecting publications and assessing the eligibility and quality. All the authors promoted the search strategy. MK and HC provided statistical expertise. All authors read, provided feedback and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study was reviewed and approved by the Institutional Ethics Committee of the Yasuj University of Medical Sciences (IR.YUMS.REC.1402.153).
Conflict of interest
The authors declare have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koushki, M., Doustimotlagh, A.H., Amiri-Dashatan, N. et al. Impact of bisphenol A exposure on the risk of gestational diabetes: a meta-analysis of observational studies. J Diabetes Metab Disord (2024). https://doi.org/10.1007/s40200-024-01485-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40200-024-01485-5