Abstract
Lab studies have suggested that exposure to Bisphenol A (BPA) could disturb glucose homeostasis, but epidemiologic studies are limited and show inconsistent results for pregnant women. For this, 535 pregnant women were selected from a pregnant women cohort established in Tangshan City in North China between 2013 and 2014. Serum concentrations of BPA were measured in the early term of pregnancy, and fasting glucose and insulin levels were repeatedly measured in each of three terms of pregnancy (early, middle, and late). Gestational diabetes mellitus (GDM) were examined by Oral Glucose Tolerance Test (OGTT) in the middle and late terms of pregnancy. BPA was detected in 97.5% of pregnant women with a median of 6.50 ng/ml. Natural log-transformed BPA (Ln BPA) was positively associated with fasting glucose level (β (95% CI): 0.038 (0.015~0.061)), fasting insulin level (0.195 (0.069~0.321)), and homeostasis model insulin resistance index (HOMA-IR) (0.226 (0.087~0.364)) in the middle term of pregnancy by multiple linear regression model after adjusting for potential confounders. After serum BPA levels were divided into three groups (low, middle, and high), BPA showed a positive dose-response relationship with blood glucose, insulin, and HOMA-IR in the middle term of pregnancy. Increased BPA concentration tended to increase the RR of GDM although not statistically significant (risk ratio: 2.51 (95% CI: 0.68~9.30) for high vs low tertile of BPA concentrations). These findings suggested that exposure to BPA might affect glucose homeostasis and the middle term of pregnancy was a potentially sensitive period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last few decades, the incidence of gestational diabetes mellitus (GDM) is increasing (Zhu and Zhang 2016), resulting in multiple adverse health outcomes, such as excessive fetal growth, neonatal hypoglycemia, jaundice, polycythemia, and stillbirth (Buchanan et al. 2012; Pergialiotis et al. 2018). GDM often occurs when the compensatory effect of the pancreatic β-cell is insufficient (Catalano et al. 1993). Endocrine disrupting chemicals (EDCs) have been found to target several pathophysiological features of GDM, such as linked to weight gain, insulin resistance, and pancreatic β-cell (Filardi et al. 2020). Further, EDCs has been linked to the activity of peroxisome proliferator-activated receptors (PPARs), which are crucially involved in glucose metabolism and energy homeostasis (Filardi et al. 2020). Bisphenol A (BPA), as a typical EDC, is extensively used in the production of plastic products (Huang et al. 2017; Huang et al. 2012) and has been frequently found in various types of human biological samples (Włodarczyk 2014). BPA has been demonstrated to induce hepatic DNA hypomethylation in a rat experiment (Ma et al. 2013), bind to both estrogen receptor α and β (Tuduri et al. 2018), and increase the phosphorylation state of extracellular-regulated protein kinases (ERK)1/2 in developing cerebellar neurons (Zsarnovszky et al. 2005), which can be involved in the programming of metabolic disorders such as obesity and Type 2 diabetes mellitus (T2DM) (Alonso-Magdalena et al. 2015).
Seven epidemiological studies indicated that exposure to BPA might affect the glucose homeostasis in pregnant women, but the findings were inconsistent (Bellavia et al. 2018; Chiu et al. 2017; Fisher et al. 2018; Robledo et al. 2013; Shapiro et al. 2015; Wang et al. 2017; Zhang et al. 2019). These studies screened for GDM by oral glucose tolerance test (OGTT) only once during pregnancy, and six of them only explored the association between exposure to BPA and glucose levels in OGTT measured once rather than repeated measurements during pregnancy (Bellavia et al. 2018; Chiu et al. 2017; Fisher et al. 2018; Robledo et al. 2013; Wang et al. 2017; Zhang et al. 2019), while none of them explored the association with serum insulin levels. Considering that blood glucose and serum levels changes along the pregnancy, these studies had a relatively weak power to explore the effect of BPA exposure on glucose homeostasis during pregnancy. Furthermore, although physical activity and diet factor are known to play an important role in the occurrence of GDM (Silva-Zolezzi et al. 2017) and food is a main exposure source of BPA (Lorber et al. 2015), only one of these studies took these two factors into consideration (Chiu et al. 2017).
Due to different diet pattern or genetic background, Chinese pregnant women are likely to have a different susceptibility to GDM than other countries. However, only two studies were conducted in China (Wang et al. 2017; Zhang et al. 2019) and both only screened for GDM and did not collect food consumption and physical activity information. In this study, we included 535 pregnant women in Tangshan City, an industrial city adjacent to Beijing in North China between 2013 and 2014. After sociodemographic variables, detailed food consumption and physical activity data during whole pregnancy were collected; we measured serum BPA concentrations in early term of pregnancy and follow up to determine the associations between serum BPA and glucose homeostasis.
Methods
Study population
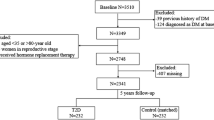
A pregnant women cohort was established in the Tangshan Maternal and Child Health Hospital of Hebei Province in North China from September 2013 to December 2014. Detailed information has been described elsewhere (Wang et al. 2018). Briefly, healthy pregnant women aged 20–40 years old in the early term of pregnancy (5–15 gestational weeks) were included and pregnant women with serious metabolic or immune diseases, including previous history of diabetes mellitus, chronic hypertension, systemic lupus erythematosus, and hypothyroidism, were excluded. A total of 924 pregnant women were enrolled in the cohort originally after signing informed consent. Among them, 838 completed a questionnaire survey and 771 provided blood specimens. For the current study, 557 women were randomly chosen from those with blood specimens to determine serum BPA concentration, and among them, 535 women were followed up to the delivery with complete data. The study was reviewed and approved by the Institutional Review Board of Fudan University.
Questionnaire surveys
The information on gestational weeks, partner smoking, disease history, reproductive history, career, and education was collected by trained interviewers using a structured questionnaire. Food consumption was obtained by using a food frequency questionnaire (FFQ), which was slightly modified from the 2010 China National Nutrition and Health Survey questionnaire (Zhao et al. 2016), in each of three terms of pregnancy. Physical activity of pregnant women was assessed through the Physical Activity Questionnaire in three terms of pregnancy. Physical activity was expressed in the form of metabolic equivalent tasks (MET), which were physical activity metabolic equivalent coefficient multiplied by daily duration (hours) (Haskell et al. 2007).
Laboratory analysis
The peripheral venous blood was collected by obstetric nurses in each of the three terms of pregnancy following a standard procedure.
Serum BPA in early term of pregnancy was determined in our lab, using an isotope-dilution method based on ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-Q/TOF MS). In short, after an aliquot (300 μL) of serum was spiked with isotope-labeled internal standard (BPA-D16), the mixture was hydrolyzed by β-glucuronidase. The hydrolyzed mixture was purified by Oasis 96-well Prime hydrophilic-lipophilic-balanced (HLB) solid phase extraction (SPE) plate and then BPA in serum was determined by UPLC-Q/TOF MS. Ninety-two serum samples, two water blank samples, and two serum samples spiked with 10 ng of BPA were prepared for each batch. Tiny background BPA interference was found and subtracted from final BPA concentration. The recoveries of BPA ranged from 87.5 to 107.8% with a limit of detection (S/N = 3) of 0.15 ng/ml.
Health outcomes
Blood biochemical tests, including fasting serum insulin levels (mIU/L) and fasting glucose levels (mmol/L), were conducted in the Tangshan Maternal and Child Health Hospital. The insulin resistance status was expressed by the homeostasis model assessment of insulin resistance (HOMA-IR). HOMA-IR = fasting blood glucose × fasting blood insulin ÷ 22.5 (Gayoso-Diz et al. 2013). OGTT was performed in the second (gestational age: 24~28 weeks) and third trimester (gestational age: 36~40 weeks) to screen for GDM. The diagnosis process was based on GDM diagnostic criteria recommended by the International Diabetes and Pregnancy Research Group (IADPSG) in 2010 (International Association of et al. 2010).
Statistical analysis
BPA concentrations were natural log-transformed (Ln BPA) and non-detected values were replaced by LOD/\( \sqrt{2} \) (Ganser and Hewett 2010) in the following statistical analysis. One-way ANOVA test was used to compare serum BPA and Chi-square test was used to compare GDM incidence among different sociodemographic character subgroups. The associations of serum BPA in the early term of pregnancy with serum glucose, insulin levels, and HOMA-IR in three terms of pregnancy were tested by four multiple linear regression (MLR) model. Model 1 only included serum BPA and model 2 adjusted for age, body mass index (BMI), gestational age, career, GDM family history, parity (nulliparous, multiparous), husband smoke, education, fetal sex, and per-capita income. Model 3 additionally adjusted for energy intake (kcal/day) and MET in the corresponding term of pregnancy based on model 2. In Model 4, carbohydrate, protein, and fat intake were used to replace total energy intake in Model 3 as covariates. When examining averaged glucose, insulin, and HOMA-IR, averaged carbohydrate, protein, fat, energy intake, and physical activity in three terms of pregnancy were used in the models.
To determine the changes of glucose, insulin levels, and HOMA-IR during pregnancy and their association with BPA levels, a repeated-measures mixed model with trimester and BPA concentration as fixed effect was used. Potential covariates for the model included all the covariates adjusted in Model 4. After serum BPA concentrations were divided into tertiles and coded into dummy variables, the associations between each tertile and glucose, insulin level, and HOMA-IR were tested to explore the dose-response relationship. Cox regression was used to calculate the risk ratio (RR) for GDM incidence associated with serum BPA.
All statistical analyses were performed using the SAS version 9.4 (SAS Institute INC, USA). A p value less than 0.05 was considered statistically significant.
Results
Thirty-three out of 535 women were diagnosed as having GDM in the middle term of pregnancy and other two in the late term of pregnancy based on the testing results of OGTT. BPA was detected in 97.5% of the participants ranging from 0.55 to 43.8 ng/ml with a median of 6.50 ng/ml. Table 1 showed the distribution of BPA concentrations and the GDM incidence by maternal characteristics. BPA concentrations were similar among the study groups. GDM incidence increased with age. In addition, pregnant women with higher BMI or giving birth to a boy tended to have higher incidence of GDM although the differences did not reach the significance level (p value = 0.065 and 0.064).
Table 2 showed that serum BPA concentrations were positively associated with fasting serum glucose and fasting insulin levels, and HOMA-IR in the middle term of pregnancy by MLR model, with β coefficient of 0.038, 0.195, and 0.226, respectively. After BPA concentrations were divided into tertiles, glucose, insulin levels, and IR in the middle term of pregnancy showed an increasing trend with BPA (Fig. 1). Insulin levels and HOMA-IR in the early term of pregnancy tended to be higher in pregnant women in the second tertile of BPA levels as compared to those in the first tertile. Table 3 presents the results of the repeated-measures mixed model, in which glucose levels showed a decreasing trend during pregnancy while insulin levels showed an increasing trend. BPA concentrations were not associated with glucose, but positively associated with insulin levels and HOMA-IR throughout pregnancy, with β coefficient of 0.092 and 0.107, respectively.
Serum BPA was not significantly associated with GDM risk before and after adjusting for sociodemographic factors (Fig. 2), but an increasing trend of RR with BPA concentrations was found. After adjusting for sociodemographic factors, average energy intake, and physical activity, RR was 1.16 (95% CI: 0.30~4.53) and 2.51 (95% CI: 0.68~9.30) in the second and third tertile of BPA concentration, respectively.
Discussion
This study investigated the associations of serum BPA in the early term of pregnancy with fasting blood glucose, fasting blood insulin, and HOMA-IR during whole pregnancy and GDM risk in Chinese pregnant women. Serum BPA was positively associated with fasting blood glucose, fasting blood insulin, and HOMA-IR in the middle term of pregnancy.
The GDM incidence was 6.5% in our cohort, which was comparable to other studies. For example, the GDM incidence varied from 5.12% (Xinjiang) to 33.3% (Henan) in China (Gao et al. 2018), and from 5.8% (Europe) to 12.9% (Middle East and North Africa) globally (Zhu and Zhang 2016). We found that higher BMI and giving birth to male infant might be potential risk factors for GDM, which were consistent with previous findings (Pons et al. 2015; Retnakaran and Shah 2015). The median of serum BPA was 6.50 ng/ml in our study, which was relatively higher than BPA in serum, plasma, or whole blood of pregnant women from other areas in China, including Tianjin (whole blood, 0.81 ng/ml) (Zhang et al. 2013), Taiwan (serum, 2.5 ng/ml) (Chou et al. 2011), and other studies conducted in other countries, such as in Korea (serum, 2.73 ng/ml) (Lee et al. 2008), the UK (serum, 1.76 ng/ml) (Fisher et al. 2018), Canada (serum, 1.36 ng/ml) (Aris 2014), and Germany (plasma, 3.1 ng/ml) (Schonfelder et al. 2002). Because Tangshan is a major industrial city producing coal, steel, and ceramics in North China, it was possible to have high BPA contamination in food and aquatic environment. The specific reason for this needs to be investigated further.
As the pregnancy progresses, the serum glucose level of pregnant women decreased, while insulin increased as found in the mixed model. This might be caused by an increased amount of insulin production to overcome the resistance levels during pregnancy (Sonagra et al. 2014). We found that serum BPA was positively associated with fasting glucose, fasting insulin, and HOMA-IR in the middle term of pregnancy by MLR model. Positive associations between BPA and glucose were also found in two previous studies (Bellavia et al. 2018; Chiu et al. 2017), while other two studies found no significant association between glucose levels and BPA exposure (Fisher et al. 2018; Robledo et al. 2013) and one study even found negative association (Wang et al. 2017). An American case-control study including 22 cases of GDM and 72 controls (Robledo et al. 2013) and another British nested case-control study including 232 pregnant women (Fisher et al. 2018) both found null association between internal BPA concentrations and plasma glucose in OGTT tests. A Chinese maternal cohort included 620 pregnant women and found that urinary BPA was associated with lower plasma glucose concentration after adjusting for covariates (Wang et al. 2017). Different study design (case-control study vs cohort study) and relatively small sample size might partly account for the discrepancy. Besides, these three studies all explored the association between BPA and 1-h or 2-h OGTT glucose rather than fasting glucose, which could also lead to differences.
The results of MLR model and mixed model showed partial discrepancies. We did not find significant associations between BPA and glucose changes at an overall level by mixed model, which might be due to that the weak correlation between BPA and glucose in the first and third trimester of pregnancy diluted the overall association. The associations between serum BPA and fasting glucose level tended to be stronger in the middle term of pregnancy, which might be related to the increased production of placental hormones and insulin resistance at this period (Handwerger and Freemark 2000). The insulin resistance is mediated by placental hormones, which could be disrupted by BPA through activating estrogen receptors and increasing inflammation and oxidative stress as proved in animal studies (Alonso-Magdalena et al. 2006; Quesada et al. 2002; Strakovsky and Schantz 2018). Screening for GDM is usually done at 24–28 weeks of gestation because insulin resistance increases during the middle term and glucose levels rise faster than other two terms when insulin is not produced enough (Rani and Begum 2016). Moreover, this finding was partly supported by some studies. For example, BPA exposure in the middle term of pregnancy was found to be associated with increased glucose levels among subfertile women in a previous American study (Chiu et al. 2017). These findings suggested that the middle term of pregnancy may be a more susceptible period than other two terms.
In this study, an increased RR of GDM with BPA was seen although the association between risk of GDM and exposure to BPA did not reach significant threshold (Fisher et al. 2018; Robledo et al. 2013; Shapiro et al. 2015; Wang et al. 2017). Some studies found that BPA analogue could increase GDM risk. A Chinese study included 1841 pregnant women and measured four bisphenols (BPA, BPS, BPF, and BPAF) in first-trimester urine samples. They found that BPAF was associated with increased odds of GDM and BPS was associated with increased fasting plasma glucose levels (Zhang et al. 2019). This indicated that BPA might also be an influencing factor of GDM but its effect needs to be further investigated.
This study provided information on the association between BPA exposure and glucose homeostasis during pregnancy with two major strengths. First, we prospectively explored the association of serum BPA measured in the early term of pregnancy with glucose homeostasis during whole pregnancy and GDM risk with repeated measurements of fasting glucose and insulin levels. Second, numerous important sociodemographic and dietary factors, such as carbohydrate, protein, fatty acid, and energy intake, and physical activity information were controlled in statistical model to enhance the accuracy of associations. However, this study is subject to two limitations. First, BPA has a short half-life; thus, one measure might not well reflect a long-term exposure and could result in misclassification bias. Second, the number of pregnant women who were diagnosed as having GDM was small, and this might increase the variation of association between serum BPA and GDM risk.
Conclusion
We prospectively explored the association of serum BPA with glucose homeostasis and GDM risk during pregnancy in 535 pregnant women. Exposure to BPA was found to be positively associated with higher levels of fasting insulin, fasting glucose, and HOMA-IR in the middle term of pregnancy, but not in other two terms. This suggested that exposure to BPA was a potential influencing factor of glucose homeostasis during pregnancy and the middle term of pregnancy may be a susceptible period. Further studies with repeated measurement of BPA are warranted to confirm such association.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A (2006) The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect 114:106–112
Alonso-Magdalena P, Quesada I, Nadal A (2015) Prenatal exposure to BPA and offspring outcomes: the diabesogenic behavior of BPA. Dose-Response 13:1559325815590395
Aris A (2014) Estimation of bisphenol A (BPA) concentrations in pregnant women, fetuses and nonpregnant women in Eastern Townships of Canada. Reprod Toxicol 45:8–13
Bellavia A, Cantonwine DE, Meeker JD, Hauser R, Seely EW, McElrath TF, James-Todd T (2018) Pregnancy urinary bisphenol-A concentrations and glucose levels across BMI categories. Environ Int 113:35–41
Buchanan TA, Xiang AH, Page KA (2012) Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 8:639–649
Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, Sims EA (1993) Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Phys 264:E60–E67
Chiu YH, Minguez-Alarcon L, Ford JB, Keller M, Seely EW, Messerlian C, Petrozza J, Williams PL, Ye X, Calafat AM, Hauser R, James-Todd T, for EST (2017) Trimester-specific urinary bisphenol A concentrations and blood glucose levels among pregnant women from a fertility clinic. J Clin Endocrinol Metab 102:1350–1357
Chou WC, Chen JL, Lin CF, Chen YC, Shih FC, Chuang CY (2011) Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environ Health 10:94
Filardi T, Panimolle F, Lenzi A, Morano S (2020) Bisphenol A and phthalates in diet: An emerging link with pregnancy complications. nutrients 12(2):525. https://doi.org/10.3390/nu12020525
Fisher BG, Frederiksen H, Andersson AM, Juul A, Thankamony A, Ong KK, Dunger DB, Hughes IA, Acerini CL (2018) Serum phthalate and triclosan levels have opposing associations with risk factors for gestational diabetes mellitus. Front Endocrinol (Lausanne) 9:99
Ganser GH, Hewett P (2010) An accurate substitution method for analyzing censored data. J Occup Environ Hyg 7:233–244
Gao C, Sun X, Lu L, Liu F, Yuan J (2018) Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig 10(1):154–162. https://doi.org/10.1111/jdi.12854
Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, Gude F, Garcia F, De Francisco A, Quintela AG (2013) Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 13:47
Handwerger S, Freemark M (2000) The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab 13:343–356
Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39:1423–1434
Huang YQ, Wong CK, Zheng JS, Bouwman H, Barra R, Wahlstrom B, Neretin L, Wong MH (2012) Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42:91–99
Huang RP, Liu ZH, Yuan SF, Yin H, Dang Z, Wu PX (2017) Worldwide human daily intakes of bisphenol A (BPA) estimated from global urinary concentration data (2000-2016) and its risk analysis. Environ Pollut 230:143–152
International Association of D, Pregnancy Study Groups Consensus P, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33:676–682
Lee YJ, Ryu HY, Kim HK, Min CS, Lee JH, Kim E, Nam BH, Park JH, Jung JY, Jang DD, Park EY, Lee KH, Ma JY, Won HS, Im MW, Leem JH, Hong YC, Yoon HS (2008) Maternal and fetal exposure to bisphenol A in Korea. Reprod Toxicol 25:413–419
Lorber M, Schecter A, Paepke O, Shropshire W, Christensen K, Birnbaum L (2015) Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ Int 77:55–62
Ma Y, Xia W, Wang DQ, Wan YJ, Xu B, Chen X, Li YY, Xu SQ (2013) Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood. Diabetologia 56:2059–2067
Pergialiotis V, Kotrogianni P, Christopoulos-Timogiannakis E, Koutaki D, Daskalakis G, Papantoniou N (2018) Bisphenol A and adverse pregnancy outcomes: a systematic review of the literature. J Matern Fetal Neonatal Med 31:3320–3327
Pons RS, Rockett FC, de Almeida RB, Oppermann MLR (2015) Bosa VL (2015) Risk factors for gestational diabetes mellitus in a sample of pregnant women diagnosed with the disease. Diabetol Metab Syndr 7:A80. https://doi.org/10.1186/1758-5996-7-S1-A80
Quesada I, Fuentes E, Viso-Leon MC, Soria B, Ripoll C, Nadal A (2002) Low doses of the endocrine disruptor bisphenol-A and the native hormone 17beta-estradiol rapidly activate transcription factor CREB. FASEB J 16:1671–1673
Rani PR, Begum J (2016). Screening and diagnosis of gestational diabetes mellitus, where do we stand. J Clin Diagn Res 10(4):QE01–4. https://doi.org/10.7860/JCDR/2016/17588.7689
Retnakaran R, Shah BR (2015) Fetal sex and the natural history of maternal risk of diabetes during and after pregnancy. J Clin Endocrinol Metab 100:2574–2580
Robledo C, Peck JD, Stoner JA, Carabin H, Cowan L, Koch HM, Goodman JR (2013) Is bisphenol-A exposure during pregnancy associated with blood glucose levels or diagnosis of gestational diabetes? J Toxicol Environ Health A 76:865–873
Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I (2002) Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect 110:A703–A707
Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Fraser W, Fisher M, Taback S, Keely E, Bouchard MF, Monnier P, Dallaire R, Morisset AS, Ettinger AS (2015) Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC study. Environ Int 83:63–71
Silva-Zolezzi I, Samuel TM, Spieldenner J (2017) Maternal nutrition: opportunities in the prevention of gestational diabetes. Nutr Rev 75:32–50
Sonagra AD, Biradar SM, Dattatreya K, Murthy DSJ (2014) Normal pregnancy- a state of insulin resistance. J Clin Diagn Res 8:CC01–CC03
Strakovsky RS, Schantz SL (2018) Using experimental models to assess effects of bisphenol A (BPA) and phthalates on the placenta: challenges and perspectives. Toxicol Sci 166:250–268
Tuduri E, Marroqui L, Dos Santos RS, Quesada I, Fuentes E, Alonso-Magdalena P (2018) Timing of exposure and bisphenol-A: implications for diabetes development. Front Endocrinol (Lausanne) 9:648
Wang X, Wang X, Chen Q, Luo ZC, Zhao S, Wang W, Zhang HJ, Zhang J, Ouyang F (2017) Urinary bisphenol A concentration and gestational diabetes mellitus in Chinese women. Epidemiology 28 Suppl 1:S41–S47
Wang H, Yang J, Du H, Xu L, Liu S, Yi J, Qian X, Chen Y, Jiang Q, He G (2018) Perfluoroalkyl substances, glucose homeostasis, and gestational diabetes mellitus in Chinese pregnant women: a repeat measurement-based prospective study. Environ Int 114:12–20
Włodarczyk E (2014) Occurrence of bisphenol a and its effects on the human body. Arch Physiother Glob Res 18:13–26. https://doi.org/10.15442/apgr.19.2.8
Zhang T, Sun H, Kannan K (2013) Blood and urinary bisphenol A concentrations in children, adults, and pregnant women from China: partitioning between blood and urine and maternal and fetal cord blood. Environ Sci Technol 47:4686–4694
Zhang W, Xia W, Liu W, Li X, Hu J, Zhang B, Xu S, Zhou Y, Li J, Cai Z, Li Y (2019) Exposure to Bisphenol a substitutes and gestational diabetes mellitus: a prospective cohort study in China. Front Endocrinol (Lausanne) 10:262
Zhao L, Ma G, Piao J, Zhang J, Yu D, He Y, Huo J, Hu X, Yang Z, Yang X (2016) Scheme of the 2010-2012 Chinese nutrition and health surveillance. Zhonghua Yu Fang Yi Xue Za Zhi 50:204–207
Zhu Y, Zhang C (2016) Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 16:7
Zsarnovszky A, Le HH, Wang HS, Belcher SM (2005) Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology 146:5388–5396
Funding
This study was supported by the Ministry of Science and Technology of the People’s Republic of China (No. 2017YFC1600500), the Project of Shanghai Municipal Commission of Health and Family Planning Foundation (No. 201740113), National Natural Science Foundation of China (No.81773413) and the key research project of National Natural Science Foundation of China (No. 21537001).
Author information
Authors and Affiliations
Contributions
Jiaqi Yang: Writing, Investigation; Hexing Wang: Writing, Methodology; Hongyi Du: Resources; Linji Xu, Shuping Liu, Jianping Yi: Investigation; Yue Chen: Writing —Review and Editing; Qingwu Jiang: Project administration; Gengsheng He: Resources, Supervision. All authors have given approval to the final version of the manuscript. †These authors contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was reviewed and approved by the Institutional Review Board of Fudan University.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Wang, H., Du, H. et al. Serum Bisphenol A, glucose homeostasis, and gestational diabetes mellitus in Chinese pregnant women: a prospective study. Environ Sci Pollut Res 28, 12546–12554 (2021). https://doi.org/10.1007/s11356-020-11263-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11263-4