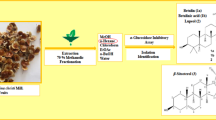
Abstract
Objectives
Type 2 diabetes is a common metabolic disease affecting millions of people worldwide. α-Glucosidase inhibitors can be used as one of the therapeutic approaches to decrease the postprandial glucose levels through the inhibition of carbohydrate hydrolysis. Medicinal plants are one of the main sources of α-glucosidase’s natural inhibitors. In this study, we report the inhibitory effects of 50 different accessions of 32 Salvia species against α-glucosidase.
Methods
To estimate the relative potency of the crude extracts, the inhibitory activities of the 80% methanol of the plants extracts were determined in three different concentrations (1000, 500 and 250 µg/ml) and compared to that of acarbose as the positive control.
Results
S. multicaulis, S. santolinifolia, S. dracocephaloides, and S. eremophila were stronger inhibitors than acarbose (p < 0.05) with IC50 values in the range of 26.23- 92.35 µg/mL. According to the LC-PDA-ESIMS and NMR analysis of crude extracts of the studied Salvia species, 8 phytochemicals including luteolin-7-O-glucoside (1) luteolin-7-O-glucuronide (2), apigenin-7-O-glucoside (3), apigenin-7-O-glucuronide (4), Hispidulin-7-O-glucuronide (5), hispidulin-7-O-glucoside (6), rosmarinic acid (7), carnosol (8) and carnosic acid (9) were identified as the most common α-glucosidase inhibitors. The above compounds constituted the major compounds in the active Salvia species in the range of 1.5–95.0%. Among them rosmarinic acid (39–95%) was detected in almost all potent α -glucosidase inhibitor species. Therefore, it can be considered as a biochemical marker in the antidiabetic Salvia species in addition to the other minor compounds.
Conclusions
Considering the high α-glucosidase inhibitory potential of the four- out of fifty Salvia species, they are suggested for further in vivo antidiabetic tests as potential medicinal plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a chronic metabolic disorder that is known as one of the major global-health threatening disease [1]. The absolute insulin deficiency and a decreased responsiveness of the tissues to the secreted insulin can induce the type 1 and type 2 diabetes, respectively [2]. Uncontrolled type 2 diabetes is more common and can cause many complications such as cardiovascular disease, retinopathy, neuropathy and renal function recession [2]. Up to now, clinicians have introduced several medicinal approaches to treat diabetes, among which suppression of the carbohydrate hydrolases is one of the main strategies via decreasing the postprandial hyperglycemia. α-Glucosidase is one of the enzymes with oligosaccharides hydrolyses function to yield glucose. On the other hand, the oligosaccharides themselves are resulted from the action of α-amylase on polysaccharides. Therefore, glucose is the end product of both enzymes catalytic actions on the carbohydrates and inhibition of α-glucosidase enzyme can decrease the carbohydrate digestion. As a result, the lower glucose uptake into the blood is achieved when the carbohydrate hydrolyzing enzymes; α-amylase and α-glucosidase lose their functions [3]. To inhibit α-glucosidase enzyme activity as a therapeutic approach, various synthetic inhibitors such as acarbose, miglitol and voglibose are effective [4]. However, to avoid their severe side effects, discovery of safer synthetic or natural alternative hydrolyzing-enzyme inhibitory medicines are developed [4].
Traditional medicinal plants contain various active constituents such as triterpenoids [5,6,7], flavonoids and phenolic acids made them an important candidate as α-glucosidase inhibitors [8]. Among the various medicinal plants that exhibit antidiabetic properties [9], Salvia species are remarkable herbs, since they are important medicinal plants of the Lamiaceae family and are very popular in the folk medicine of different countries, from Americas to Asia. Previous studies on the α-glucosidase inhibitory activities and the significant properties of the Salvia species including S. officinalis L. [10], S. nemorosa L. [11], S. atropatana Bunge [11], and S. mirzayanii Rech. F. & Esfand [12] showed that these species can be considered as important candidates for further studies in diabetes treatments. Iran is one of the hubs for Salvia diversity in the Middle East [13], some of which are used as antidiabetic medicinal herb in the Iranian traditional medicine [13]. Considering their application in the flock medicine of different countries, several researchers investigate the antidiabetic properties of various Salvia species. For instance, ethanol extracts of six Iranian Salvia species (S. hydrangea DC., S. hypoleuca Benth., S. officinalis L., S. reuterana Boiss., S. verticillata L. and S. virgata Jacq.) were examined for their α-amylase properties. Among the studied species, the extracts of S. verticillata and S. virgata inhibited the enzyme activity [14]. Petroleum ether extract of an endemic sage of Iran; S. mirzayanii inhibited α-glucosidase, significantly [12]. The methanol (MeOH) extracts of different Iranian sages; S. nemorosa L, S. atropatana Bunge, S. limbata C.A. Mey., S. syriaca L. [11] S. multicaulis Vahl. [11] and S. santolinifolia Boiss. [15], inhibited α-glucosidase with moderate to high potential.
Although the antidiabetic effects of a few Iranian Salvia species has been screened, the activities such as α-glucosidase inhibition potential of the remaining Iranian Salvia species and characterizing their active phytochemicals remain to be explored. In our previous study, we reported the antioxidant potentials, total phenol contents and total flavonoid contents of the aerial parts of different Salvia species [16]. Most of the extracts exhibited high contents of phenols and flavonoids. Previous studies revealed that flavonoids and polyphenols are among the most potent natural antidiabetic agents [17, 18]. Therefore, in the present paper we report the α-glucosidase inhibitory potential of the above mentioned 50 Iranian Salvia species, and detect their bioactive constituents with interpreting of our earlier HPLC–PDA-MS and NMR analyses [16].
Material and methods
Chemicals and instruments
HPLC-grade methanol and acetonitrile were purchased from Merck (Darmstadt, Germany). Distilled water was prepared, using a Milli-Q system (Millipore, Bedford, MA, USA).
HPLC–PDA-MS fingerprints were measured with a Shimadzu system equipped with a SPD-M20A photo diode array detector (DAD) and a heated-electrospray ionization source (ESI).
The1H and APT 13C NMR spectra of the extracts (in DMSO-D6) were run on a 300 MHz Brucker Avance III. Acarbose, ⍺-glucosidase (EC 3.2.1.20) and p-nitrophenyl-⍺-D-glucopyranoside (PNPG) were purchased from Sigma-Aldrich (Germany) and all the solvents were obtained from Merck chemical companies.
Plant material and extract preparation
The areal parts of 50 Salvia samples belonging to 32 different species, were collected, dried and extracted with 80% MeOH, as reported in our previous study [16]. The botanical characteristics; the collection dates and locations, the random identification code (1–50), and the herbarium voucher numbers were presented in Table 1 of that published earlier [16].
α-Glucosidase inhibition assay
α-Glucosidase (from Saccharomyces cerevisiae) inhibitory activity of the extracts were measured using previously described method by Park et al. [19, 20], with minor modifications. Briefly, 5 μL of 80% MeOH extracts of each of the sage extract were incubated in 96-well microplates, in three different concentrations (1000, 500 and 250 µg/ml). Then, they were diluted with 90 μL of 0.1 mM potassium phosphate buffer (pH 6.8), followed by adding 20 μL of α-glucosidase enzyme (0.25 U/mL) in phosphate buffer solution. After 10 min of incubation of the plates in the dark condition at 37 ºC, 15 μL of 2.5 mM substrate (p-nitrophenyl-α-D-glucopyranoside) in buffer solution was added into the mixture. Then the plates were incubated for further 30 min in the same condition, before quenching the reaction by addition of 80 μL of a 0.2 M Na2CO3 into each well. The absorbance (A) of each of the well’s solution was measured at λ 405 nm using a microplate reader (Bio-Rad Model 680). Acarbose was used as the standard drug and the percentage of α-glucosidase inhibitory activity was calculated with the following formula.
where Aextract is the absorbance of the sample and Acontrol is the absorbance of the control. The IC50, was expressed as the concentration (in µg/mL) of the sample that inhibited 50% of the enzyme activity, using Curve Expert software from linear regression curves.
LC–MS, 1H NMR and APT 13C NMR analysis
LC–MS and NMR spectroscopy analyses of the plant extracts were performed previously to detect the metabolites present in the plant extracts[16].
Statistical analysis
Each experiment was repeated three or four times. The results express as Mean ± standard error (SE). Statistical significance was determined using SPSS software and accepted at P < 0.05. The IC50 values were calculated with the CurveExpert software, version 1.6.5, for Windows.
Result
In vitro α-glucosidase inhibition
The in vitro α-glucosidase inhibitory activity percentage (%) of the 80% MeOH extracts of the sage samples in three different concentrations (250, 500 and 1000 µg/mL) were measured and compared to those for acarbose as the standard drug (Table 1 and Fig. 1). The highest inhibitory activity was found in S. santolinifolia (S21) with 91.68 ± 2.08 percent. While S. limbata (S33) had the lowest inhibitory activity with 2.21 ± 0.50 percent. Plant extracts including S. multicaulis (S29 and S31), S. santolinifolia (S21 and S48), S. dracocephaloides (S25), S. eremophila (S22) were stronger than that obtained for acarbose (p < 0.05). Therefore, The IC50 values of the strongest plant extracts were evaluated and shown in Table 2.
α-Glucosidase kinetic studies
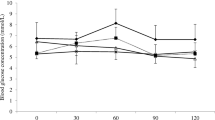
S. santolinifolia (S21), as the most effective α-glucosidase inhibitor, was selected for the study of its enzyme kinetic. Lineweaver–Burk plot of α-glucosidase inhibitory activity of the 80% MeOH extract of S. santolinifolia were examined at 0, 1 and 2 mg/mL stock solution, with different substrate concentrations of PNPG (1–5 mM) (Fig. 2) [21]. Data analysis revealed that increasing the concentration of the plants’ extract, decreases the Vmax and also Km by 2.1, 0.41 and 0.20 mM, respectively (Fig. 2).
HPLC fingerprints of the potent Salvia extracts
To characterize the bioactive chemical constituents of the most potent α-glucosidase inhibitors, S. multicaulis (S29 and S31), S. santolinifolia (S21 and S48), S. dracocephaloides (S25) and S. eremophila (S22), their HPLC fingerprints were reconsidered (Fig. 3).
Chromatographic fingerprints of 6 Salvia samples; S. santolinifolia (S21 and 48), S. eremophila (S22), S. dracocephaloides (S25), and S. multicaulis (S29 and S31). More details are visualized in the zoom plot. Peak numbers refer to components listed in Table 3. Mobile phase: water (formic acid 0.1%) and acetonitril (formic acid 0.1%). Detection is recorded at average absorbance of λ 190- 600 nm
In the previous publication we have characterized 13 phytochemicals in the plants’ extracts using LC-UV-ESIMS, 1H- and 13C NMR spectral data [16]. In the present report we have detected 8 of those compounds in the enzyme inhibitory-active extracts using reconsideration of their spectroscopic data. They include luteolin-7-O-glucoside (1) luteolin-7-O-glucuronide (2), apigenin-7-O-glucoside (3), apigenin-7-O-glucuronide (4), hispidulin-7-O-glucuronide (5), hispidulin-7-O-glucoside (6), rosmarinic acid (7), carnosol (8), and carnosic acid (9) (Figures 3 and 4). In addition, in S. multicaulis (S29) HPLC chromatogram, the peaks at Rt 23.8 and 25.4 min. presented the same pattern of UV spectrum as recorded for compound 7, but in their –ESIMS, two peaks at m/z 555, 493 and 717, 563 were compatible for the molecular formula C27H24O13 and C36H30O16 of salvianolic acid A derivative and salvianolic acid B, respectively. In addition, three peaks at Rt 48.0, 48.4, 49.3 min. were due to abiatane diterpenoids based on their UV (λmax 222; 222, 293; 222 nm, respectively), 1H NMR (Fig. 4) and MS spectral data with -ESIMS peaks at m/z 361, 343 and 345, respectively.
Discussion
In the present paper, the α-glucosidase inhibition effects of the Salvia species are measured and then their active phytochemicals are characterized.
In addition to the best Salvia species that showed better enzyme inhibition power than that of the standard drug (Table 2), some of them exhibited strong and medium enzyme inhibitory activity that can be concluded according to their inhibition %. Some of the tested extracts like S. atropatana (S8), S. russellii (S4) and S. verticillata (S42) showed 50% inhibition between 250 and 500 µg/mL concentrations, while the others like S. bracteata, (S3, 28), S. compressa (S18), S. dracocephaloides (S7) S. macrochlamys (S6), S. mirzayanii (S2) S. nemorosa (S23), S. pachystachys (S13), S. sahendica (S5) and S. sclarea (S11) displayed 50% α-glucosidase inhibition in the range of 500- 1000 µg/mL. The rest of plant extracts were suggested as less active exhibiting less than 50% inhibition with 1000 µg/mL concentration. Our results confirmed the enzyme inhibitory activities reported for some of the species in the earlier investigations [11, 12, 14, 15].
The total phenolic content (TPC), total flavonoid content (TFC) and their antioxidant activities of the under studied sages were reported in our previous paper [16]. Unlike the positive correlation between antioxidant activity, TPC and TFC, we found low positive relationship between TPC, TFC, antioxidant activity and α-glucosidase inhibition (R.2 is in the range of 0.22- 0.25, excluding non-active species). These results suggest that the enzyme inhibitory activities of the Salvia extracts depend on the quality of polyphenols and flavonoids rather than their quantities [14]. Another reason is that some of the sages like S. grossheimii consisted other types of phytochemicals like triterpenoids that inhibited the enzyme [5, 7]
Also, the kinetic study of the 80% MeOH extract of S. santolinifolia (S21) suggested the uncompetitive mechanism for the ⍺-glucosidase inhibition, but two of its major constituents, rosmarinic acid (7), carnosol (8), and carnosic acid (9) were reported to be a competitive and non-competitive inhibitors, respectively [22, 23].
HPLC–UV fingerprints of the most potent α-glucosidase inhibitor species were evaluated to characterize their bioactive chemical constituents (Fig. 3). The identified compounds are belonging to hydroxycinnamic acid derivatives, flavonoids and phenolic diterpenoids categories. Among the identified compounds, compound 7 is a key biomarker of the Salvia species and constituted as the most abundant compounds, ranging from 39.2–95.1% in the active extracts of Salvia species. Carnosol and carnosic acid are the two bioactive substances that were identified as the major constituents of S. eremophila and S. santolinifolia. Compounds 2 (11.9%, 16.7%) was also found in S. multicaulis (S29 and S31), respectively (Table 3). The remaining compounds are less distributed in the plant extracts. However, all of them are α-glucosidase inhibitors. Compound 1 and 6 were previously isolated from S. dracocephaloides [16, 24,25,26].
Most of the detected compounds in the potent enzyme inhibitor extracts have been reported with effective roles in controlling the blood glucose levels. For instance compound 1 exhibited in vitro and in vivo antidiabetic effects [27, 28]. It (1) was also isolated from aerial parts of S. chloroleuca and showed potent α-glucosidase inhibitory effect [29]. Compound 3 showed antidiabetic effects and enhanced adiponectin secretion and caused phosphorylation of insulin receptor-β, and GLUT4 translocation [30]. Compound 4 was a moderate α-glucosidase with IC50 value of 543.28 ± 11.41 μg/mL [31]. Finally, compound 7 which was the major phenolic compound in all the extracts and was reported as an important antidiabetic agents in vivo [32] and in vitro [33] tests. As a chemical marker, rosmarinic acid was isolated from S. miltiorrhiza and reported to be stronger α-glucosidase inhibitor than acarbose [34]. Hence, rosmarinic acid is likely to be the most important potent α-glucosidase inhibitor in the examined Salvia species. Carnosol (8), is another chemical marker of some Salvia species which was reported to be stronger α-glucosidase inhibitor than the standard drug, acarbose [35]. Moreover, the oral administration of 8 by normal mice reduced their postprandial blood glucose levels [35]. The last significant peak belongs to carnosic acid (9) that showed both α-amylase and α-glucosidase inhibitory activity, significantly. The mechanisms of action, in silico and in vivo studies suggested compound 9 as a potent antidiabetic diterpenoid [23]. Among the tested samples the plant extracts that were rich in carnosol, carnosic acid and rosmarinic acid showed the lowest IC50s. The presence of these compounds were deduced based on their ESIMS [16] and 1H NMR analyses (Fig. 4) [16].
Screening α-glucosidase inhibition-potential of medicinal plants is an alternative way to find new drugs with lower side effects compared to those of common medicines with the same mechanisms of action such as acarbose, voglibose and miglitol. One of these class of compounds are polyphenols that play a key role as antioxidants, which enable them to neutralize the harmful free radicals and to stop damaging the cells and reduce the risk of harmful cardiovascular, cancer and diabetes disease [36]. Via the carbohydrate hydrolyzing-enzyme inhibition, polyphenols prevent the breakdown of starch to the simple sugars, resulting in decreasing the blood glucose levels after meal consumption. In addition, they can stimulate the secretion of insulin to keep the blood sugar levels in a steady state [37]. Furthermore, polyphenol-rich diets could improve the glucose tolerance and insulin sensitivity to decrease the risk of type 2 diabetes, however they have unpleasant taste [38]. Since Salvia species are a rich source of novel bioactive polyphenolics we may consider them as an alternative medicine for treatment of diabetes after further detailed clinical tests. In addition to the crude extracts of the plant material whose chemical constituents are characterized in this research, synthetic or biotechnological approaches can afford the antidiabetic natural compounds to perform the required in vivo diabetic tests may be suggested for development of new drugs. The above mentioned approaches may be a way to overcome the limitations such as the lack of enough plant material, or their active constituents. While application of crude standardized medicinal plant preparations can reduce the cost of purified natural products as antidiabetic medicines.
Conclusion
In conclusion, the α-glucosidase inhibitory activity of 50 different accessions of 32 Salvia species were evaluated to introduce the potent medicinal herbs for diabetes treatments. Among the tested extracts, six species showed better enzyme inhibition than acarbose, while three species were comparable and ten are moderate α-glucosidase inhibitors according to the inhibition potency of the standard drug. Among them eight Salvias; S. russellii, S. bracteata, S. compressa, S. dracocephaloides, S. macrochlamys, S. pachystachys, S. sahendica and S. sclarea are reported as potential antidiabetic plants for the first time. Among the characterized bioactive compounds in the potent plants’ extracts, rosmarinic acid was both the main and the most common constituent, therefore it may be considered for further clinical tests. Finally, we suggest the Salvia species as appropriate antidiabetic medicinal plants, and their bioactive substances such as carnosol, the flavonoid derivatives of apigenin and luteoline for further in vivo and clinical tests. The presence of salvianolic acids and abietane diterpenoids in the extract of S. multicaulis (S29) together with its high α-glucosidase activity suggest these derivatives of rosmarinic acids and diterpene phenols may play the key role in the antidiabetic activity of the sage plants.
References
WHO: WHOFS. Diabetes [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed 5 April 2023.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract [Internet]. 2018;138:271–81. Available from: https://doi.org/10.1016/j.diabres.2018.02.023.
Derosa G, Maffioli P. Mini-Special Issue paper Management of diabetic patients with hypoglycemic agents α-Glucosidase inhibitors and their use in clinical practice [Internet]. Arch Med Sci. 2012;5:899–906. https://doi.org/10.5114/aoms.2012.31621.
Sugihara H, Nagao M, Harada T, Nakajima Y, Tanimura-Inagaki K, Okajima F, et al. Comparison of three α-glucosidase inhibitors for glycemic control and bodyweight reduction in Japanese patients with obese type 2 diabetes. J Diabetes Investig [Internet]. 2013/10/25. Wiley-Blackwell; 2014;5:206–12. Available from: https://doi.org/10.1111/jdi.12135.
Zare S, Pirhadi S, El Seedi HR, Jassbi AR. Anti-COVID-19 and antidiabetic activities of new oleanane and ursane-type triterpenoids from Salvia grossheimii : an in-silico approach. J Recept Signal Transduct [Internet]. 2022;1–9. Available from: https://doi.org/10.1080/10799893.2022.2072891.
Doorandishan M, Gholami M, Mirkhani H, Ebrahimi P, Jassbi A. α- glucosidase inhibitory and antioxidant activities of moluccella aucheri (Boiss.) Scheen Extracts. J Adv Biomed Sci [Internet]. 2022;12:3839–47. Available from: https://doi.org/10.18502/jabs.v11i2.8776.
Zare S, Mirkhani H, Firuzi O, Moheimanian N, Asadollahi M, Pirhadi S, et al. Antidiabetic and cytotoxic polyhydroxylated oleanane and ursane type triterpenoids from Salvia grossheimii. Bioorg Chem [Internet]. 2020;104:104297. https://doi.org/10.1016/j.bioorg.2020.104297.
Yin Z, Zhang W, Feng F, Zhang Y, Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci Hum Wellness [Internet]. 2014;3:136–74. Available from: https://doi.org/10.1016/j.fshw.2014.11.003.
Alam F, Shafique Z, Amjad ST, Bin Asad MHH. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phyther Res [Internet]. 2019;33:41–54. https://doi.org/10.1002/ptr.6211.
Mahdi S, Azzi R, Lahfa FB. Evaluation of in vitro α-amylase and α-glucosidase inhibitory potential and hemolytic effect of phenolic enriched fractions of the aerial part of Salvia officinalis L. Diabetes Metab Syndr Clin Res Rev [Internet]. 2020;14:689–94. Available from: https://doi.org/10.1016/j.dsx.2020.05.002.
Eskandani M, Babak Bahadori M, Zengin G, Dinparast L, Bahadori S. Novel natural agents from lamiaceae family: an evaluation on toxicity and enzyme inhibitory potential linked to diabetes mellitus. Curr Bioact Compd [Internet]. 2016;12:34–8. Available from: https://doi.org/10.2174/1573407212666151231183118.
Rouzbehan S, Moein S, Homaei A, Moein MR. Kinetics of α-glucosidase inhibition by different fractions of three species of Labiatae extracts: a new diabetes treatment model. Pharm Biol [Internet]. 2017;55:1483–8. https://doi.org/10.1080/13880209.2017.1306569.
Jamzad Z. Flora of Iran: Lamiaceae. Tehran: Research Institute of Forests and Rangelands; 2012.
Nickavar B, Abolhasani L, Izadpanah H. α-Amylase inhibitory activities of six Salvia species. Iran J Pharm Res [Internet]. 2008;7:297–303. Available from: https://doi.org/10.22037/ijpr.2010.779.
Babak Bahadori M, Valizadeh H, Asghari B, Dinparast L, Bahadori S, Moridi Farimani M. Biological activities of Salvia santolinifolia Boiss. A multifunctional medicinal plant. Curr Bioact Compd [Internet]. 2016;12:297–305. Available from: https://doi.org/10.2174/1573407212666160426161112.
Shojaeifard Z, Hemmateenejad B, Jassbi AR. Chemometrics-based LC-UV-ESIMS analyses of 50 Salvia species for detecting their antioxidant constituents. J Pharm Biomed Anal [Internet]. 2021;193:113745. Available from: https://doi.org/10.1016/j.jpba.2020.113745.
Mai TT, Thu NN, Tien PG, Van Chuyen N. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J Nutr Sci Vitaminol (Tokyo) [Internet]. 2007;53:267–76. Available from: https://doi.org/10.3177/jnsv.53.267.
Andrade-Cetto A, Becerra-Jiménez J, Cárdenas-Vázquez R. Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol. 2008;116:27–32.
Park SR, Kim JH, Jang HD, Yang SY, Kim YH. Inhibitory activity of minor phlorotannins from Ecklonia cava on α-glucosidase. Food Chem [Internet]. 2018;257:128–34. https://doi.org/10.1016/j.foodchem.2018.03.013.
Moheimanian N, Mirkhani H, Sohrabipour J, Jassbi AR. Inhibitory potential of six brown algae from the persian gulf on α-glucosidase and in vivo antidiabetic effect of sirophysalis trinodis. Iran J Med Sci. 2022;47(5):484–493. Available from: https://doi.org/10.30476/IJMS.2021.91258.2245.
Lineweaver H, Burk D. The Determination of Enzyme Dissociation Constants. J Am Chem Soc [Internet]. 1934;56:658–66. https://doi.org/10.1021/ja01318a036.
Lin L, Dong Y, Zhao H, Wen L, Yang B, Zhao M. Comparative evaluation of rosmarinic acid, methyl rosmarinate and pedalitin isolated from Rabdosia serra (MAXIM.) HARA as inhibitors of tyrosinase and α-glucosidase. Food Chem [Internet]. Elsevier Ltd; 2011;129:884–9. Available from: https://doi.org/10.1016/j.foodchem.2011.05.039.
Wang H, Wang J, Liu Y, Ji Y, Guo Y, Zhao J. Interaction mechanism of carnosic acid against glycosidase (α-amylase and α-glucosidase). Int J Biol Macromol [Internet]. 2019;138:846–53. Available from: https://doi.org/10.1016/j.ijbiomac.2019.07.179.
Ghoran SH, Firuzi O, Jassbi AR. Phytoconstituents from the aerial parts of Salvia dracocephaloides Boiss. and their Biological Activities. J Environ Treat Tech [Internet]. 2020;8:1274–8. Available from: https://doi.org/10.47277/JETT/8(4)1278.
Lee S-H, Kim H-W, Lee M-K, Kim YJ, Asamenew G, Cha Y-S, et al. Phenolic profiling and quantitative determination of common sage (Salvia plebeia R. Br.) by UPLC-DAD-QTOF/MS. Eur Food Res Technol [Internet]. Springer Berlin Heidelberg; 2018;244:1637–46. Available from: https://doi.org/10.1007/s00217-018-3076-6.
Hossain MB, Rai DK, Brunton NP, Martin-Diana AB, Barry-Ryan C. Characterization of Phenolic Composition in Lamiaceae Spices by LC-ESI-MS/MS. J Agric Food Chem [Internet]. 2010;58:10576–81. https://doi.org/10.1021/jf102042g.
KIM J-S, KWON C-S, SON KH. Inhibition of Alpha-glucosidase and Amylase by Luteolin, a Flavonoid. Biosci Biotechnol Biochem [Internet]. 2000;64:2458–61. Available from: https://doi.org/10.1271/bbb.64.2458.
Zang Y, Igarashi K, Li Y. Anti-diabetic effects of luteolin and luteolin-7- O -glucoside on KK- A y mice. Biosci Biotechnol Biochem [Internet]. 2016;80:1580–6. Available from: https://doi.org/10.1080/09168451.2015.1116928.
Asghari B, Salehi P, Sonboli A, Nejad Ebrahimi S. Flavonoids from Salvia chloroleuca with α-Amylsae and α-Glucosidase Inhibitory Effect. Iran J Pharm Res IJPR [Internet]. 2015;14:609–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25901170.
Rao YK, Lee M-J, Chen K, Lee Y-C, Wu W-S, Tzeng Y-M. Insulin-Mimetic action of rhoifolin and cosmosiin isolated from citrus grandis (L.) Osbeck leaves: enhanced adiponectin secretion and insulin receptor phosphorylation in 3t3-l1 cells. Evidence-Based Complement Altern Med [Internet]. 2011;2011:1–9. Available from: https://doi.org/10.1093/ecam/nep204.
Li K, Yao F, Xue Q, Fan H, Yang L, Li X, et al. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chem Cent J [Internet]. 2018;12:82. Available from: https://doi.org/10.1186/s13065-018-0445-y.
Inui A, Cheng K-C, Asakawa A, Amitani H, Amitani M, Morinaga A, et al. Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des Devel Ther [Internet]. 2016;10:2193–202. Available from: https://doi.org/10.2147/DDDT.S108539.
Kubínová R, Pořízková R, Navrátilová A, Farsa O, Hanáková Z, Bačinská A, et al. Antimicrobial and enzyme inhibitory activities of the constituents of Plectranthus madagascariensis (Pers.) Benth. J Enzyme Inhib Med Chem [Internet]. 2014;29:749–52. Available from: https://doi.org/10.3109/14756366.2013.848204.
Ma H-Y, Gao H-Y, Sun L, Huang J, Xu X-M, Wu L-J. Constituents with α-glucosidase and advanced glycation end-product formation inhibitory activities from Salvia miltiorrhiza Bge. J Nat Med [Internet]. 2011;65:37–42. Available from: https://doi.org/10.1007/s11418-010-0453-2.
Ma Y-Y, Zhao D-G, Zhang R, He X, Li BQ, Zhang X-Z, et al. Identification of bioactive compounds that contribute to the α-glucosidase inhibitory activity of rosemary. Food Funct [Internet]. 2020;11:1692–701. Available from: https://doi.org/10.1039/c9fo02448d.
Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96.
Kim Y, Keogh J, Clifton P. Polyphenols and glycemic control. Nutrients [Internet]. 2016;8:17. Available from: https://doi.org/10.3390/nu8010017.
Azzini E, Giacometti J, Russo GL. Antiobesity effects of anthocyanins in preclinical and clinical studies. Oxid Med Cell Longev [Internet]. 2017;2017:1–11. Available from: https://doi.org/10.1155/2017/2740364.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared that they have no conflict of interest. The financial support of the present research was provided by the research council of Shiraz University of Medical Sciences, Shiraz, Iran (grant no. 17131). Amir Reza Jassbi is the mentor and founder of a startup company “Aryana Phytochemistry of Tirazis”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shojaeifard, Z., Moheimanian, N. & Jassbi, A.R. Comparison of inhibitory activities of 50 Salvia species against α-Glucosidase. J Diabetes Metab Disord 22, 1685–1693 (2023). https://doi.org/10.1007/s40200-023-01301-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01301-6