Abstract
The search for novel alpha-glucosidase inhibitory agents is currently an important scientific endeavour to the biopharmaceutical companies due to their potentials to reduce postprandial hyperglycaemia in type 2 diabetic patients. The present research attempted to investigate the alpha-glucosidase inhibitory effects of the leaf extract of Carica papaya. Aqueous, methanol, ethyl acetate and n-hexane extracts of C. papaya leaf were subjected to in vitro alpha-glucosidase inhibitory studies, and methanol extract was the most potent (IC50 = 171.83 ± 26.89 μg/mL). Consequently, the methanol extract was further fractionated by column chromatography which yielded six pooled fractions (A–F) and fraction A (FA) showed the lowest IC50 (78.62 ± 25.23 μg/mL). Subsequently, the FA was subjected to in vivo oral maltose and sucrose tolerance tests which revealed that FA had potent inhibitory effects on maltose and sucrose tolerance in rats. The computed area under the curves (AUC(0–120)) further indicates that FA (200 mg/kg bw) have better inhibitory activity on maltase than sucrase. The GC-MS analysis of FA indicates the presence of phenolics, various fatty acids and their derivatives. This research suggests that the leaf extract of C. papaya possesses alpha-glucosidase inhibitory effects and might be exploited in retarding postprandial hyperglycaemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a common metabolic abnormality associated with marked increase in blood glucose levels (hyperglycaemia) because of inadequacies in pancreatic insulin action and/or secretion. The prevalence of type 2 diabetes (T2D), representing > 90–95% of all DM cases, is rapidly increasing throughout the world. If no urgent action is taken, people affected with the disease are estimated to shoot up to 642 million by 2040 from the recorded 415 million in 2015 (IDF 2015). The hyperglycaemia associated with DM is a known leading cause of cardiovascular diseases, neurological complications, kidney and eye defects as well as premature death (Lopez-Candales 2001). Thus, the management of this postprandial hyperglycaemia is an extremely important therapeutic strategy against DM as well as lowering the associated chronic macro- and micro-complications (Shim et al. 2003).
The common therapeutic approach applied in lowering postprandial hyperglycaemia for people affected with DM involves reducing the digestion and absorption of carbohydrates after food intake (Gholamhoseinian et al. 2008). Simple sugars, such as fructose and glucose, are the major constituents of dietary carbohydrates allowed to cross from the intestinal epithelium into the blood stream. Therefore, dietary disaccharides, oligosaccharides and polysaccharides are usually catabolised into these simple sugars before their absorption into the bloodstream. The intestinal enzymes such as alpha-glucosidase attached to the intestinal epithelium facilitate this digestion and absorption processes. Hence, the inhibition of these enzymes would lower the postprandial blood glucose excursions.
Alpha-glucosidase inhibitors (acarbose and miglitol) have been exploited as oral antidiabetic drugs for the management of T2D. This is because they prevent the digestion and absorption of dietary polysaccharides (Sindhu et al. 2013) and fall under the third class of oral hypoglycemic agents (Du et al. 2005). In fact, alpha-glucosidase inhibitors obtained from natural sources have been effective in the control of postprandial hyperglycaemia as well as clinical treatment of DM (Playford et al. 2013). However, only a few of such inhibitors are available for commercial purposes in addition to their sugar mimetic structures which makes their synthesis slow because of the complex procedures involved (Yin et al. 2014). Furthermore, these currently available alpha-glucosidase inhibitors are linked with serious side effects in the gastrointestinal tract. Thus, it is worthwhile to explore for novel and safer alpha-glucosidase inhibitory agents, especially from medicinal plants.
Carica papaya Linn (Caricaceae) commonly called pawpaw tree is generally distributed throughout the world (Khalid et al. 2013). The different parts of the tree are presently utilised to manage several ailments such as diabetes, inflammation and diarrhoea (Chavez-Quintal et al. 2011; Juarez-Rojop et al. 2012). Indeed, the aqueous extract of the leaf has been reported to exert antioxidant and hypoglycemic effects as well as mitigated the upsurge in lipid profile of diabetic rats (Maniyar and Bhixavatimath 2012). Also, the leaf extract categorically affects the probity and function of both liver and pancreas (Juarez-Rojop et al. 2012). However, a detailed investigation of the alpha-glucosidase inhibitory activity of the leaf extract, which may pave way for deciphering novel alpha-glucosidase inhibitors, was not reported in the literature.
In this study, the in vitro and in vivo studies on the alpha-glucosidase inhibitory effects of the leaf extract of C. papaya were investigated along with the identification of the possible bioactive compounds.
Materials and methods
Chemicals and reagents
Yeast (Saccharomyces cerevisiae) alpha-glucosidase, p-nitrophenyl-α-D-glucopyranoside (pNPG), acarbose and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich, Germany. Other solvents and chemicals were of analytical grade purchased from Haddis International, Samaru, Zaria, Nigeria.
Plant materials
The fresh leaf of C. papaya was collected from a local farm at Samaru village, Sabongari Local Government area, Kaduna State, Nigeria. The service of the herbarium unit of Biological Sciences Department, Ahmadu Bello University, Zaria, Nigeria, was employed in the authentication of the plant material and a sample with voucher number of 1708 was deposited.
Preparation of plant extract
The collected plant leaf was rinsed with clean water and left to dry at room temperature. Subsequently, laboratory mill was used to pulverised the dried leaf and the obtained powder was used to prepare the extracts. The dried powdered leaf of C. papaya was extracted for 48 h using four different solvents (n-hexane, ethyl-acetate, methanol and water), respectively. This was achieved by transferring 50 g of the powdered leaf in 500 mL of each of the solvents for 48 h at room temperature. Thereafter, it was filtered using muslin cloth and Whatmann filter paper No. 1 to obtain the respective crude extracts. The hexane (2.8%), ethyl-acetate (6.08%) and methanol (10.24%) extracts were concentrated using a rotary evaporator while the aqueous (14.30%) extract was concentrated to dryness at 40 °C using water bath for 72 h.
Determination of alpha-glucosidase inhibition of the extracts
A protocol described Kim et al. (2005), with slight modifications, was employed in investigating the alpha-glucosidase inhibitory activity of the prepared crude extracts. Briefly, 100 μL of alpha-glucosidase (1 U/mL) was added to tubes containing 40 μL of different concentrations (50–200 μg/mL) of each extract or acarbose. In order to initiate the enzymatic reaction, 60 μL of 5.0 mM pNPG was transferred to the tubes and mixture was incubated at 37 °C for 1 h before the addition of 2 mL of 0.1 M Na2CO3 to quench the reaction. Thereafter, the absorbance of the released p-nitrophenol was monitored at 400 nm while the enzyme inhibitory activity was computed as percentage of a control sample lacking the inhibitors. The following formula was used:
where As and Ac are the absorbance values of the reaction with and without the sample, respectively.
Bioassay-guided fractionation
The methanol extract that showed the best inhibitory activity against alpha-glucosidase was fractionated using a column chromatography (size; 3 cm diameter 50 cm long) on a silica gel column with a solvent system of hexane (100%), chloroform (100%), chloroform/methanol (9:1, 8:2, 7:3) and methanol (100%). Thin-layer chromatography (TLC) with precoated silica gel TLC plates was used in monitoring the elution of the fractions. A total of 154 fractions (20 mL each) was collected, and fractions with similar Rf values were pooled together which resulted into six pooled fractions labelled A–F. Subsequently, all the six fractions were subjected to alpha-glucosidase inhibitory activity assay as previously described.
Experimental animals
A total of 20 healthy Wistar rats with a weigh range of 150–200 g were procured and kept in a well-aerated laboratory cages at the animal house of Biochemistry Department, Ahmadu Bello University, Zaria. The animals were given a period of 2 weeks to adjust to the laboratory environment before the commencement of experiment. During the stabilisation period, they were provided with growers mash (Vital Feeds Company, Nigeria) and water ad libitum. Furthermore, the rules and regulations of experimental animal ethics committee of Ahmadu Bello University, Zaria, were duly followed in maintaining the animals.
Animal grouping
After the 2-week acclimatisation period, the animals were randomly distributed into four groups of rats each, namely, NC, rats administered with distilled water; CPF100, rats administered with 100 mg/kg bw of fraction A; CPF 200, rats administered with 200 mg/kg bw of fraction A; Acarbose, administered with 100 mg/kg bw of a standard drug, acarbose.
The effects of fraction A on the in vivo maltose and sucrose tolerance in rats
The rats in all the experimental groups were fasted overnight for 12 h and after which, the animals were orally coadministered with maltose or sucrose (2 g/kg bw) and the respective dose of the fraction A in the treatment groups whereas distilled water and acarbose were administered instead of the fraction in the NC and acarbose groups, respectively. The concentrations of blood glucose in all animals were measured from the tail vein blood with a glucometer (Glucoplus Inc., Saint-Laurent, Quebec, Canada) at 0 (prior to maltose or sucrose administration), 30, 60, 90 and 120 min after the maltose or sucrose ingestion. A 3-day washout period was given between the maltose and sucrose tolerance experiments in the animals. The following formula was used for the computation of the area under the curve (AUC) for all the experimental groups:
where Ci is the concentration of blood glucose at time ti.
Gas chromatography–mass spectrometry analysis
For the identification of the phytochemical components, the fraction A was further analysed by gas chromatography-mass spectrometry (GC-MS) using a GC-MS system (Shimadzu QP 2010, Tokyo, Japan) containing a fused silica capillary column (VF-5MS; 30-m length, 0.25-mm diameter and 0.25 -mm film thickness). Ultra-pure helium served as the carrier gas at linear velocity and flow rate of 37 cm/s and 0.7 mL/min, respectively. The temperature of the injector was 250 °C whilst the initial oven temperature was 60 °C. At the rate of 10 °C/min, this temperature was set to reach 280 °C with a hold time of 3 min. The fraction A (2 μL) was injected with a manual split ratio of 20:1 while the mass spectrometer was set to operate in the electron multiplier voltage and electron ionisation mode of 1859 V and 70 eV, respectively. Additionally, the mass spectrometer was programmed to work under the following conditions: ion source temperature (230 °C), solvent delay (4 min), quadrupole temperature (150 °C) and scan range of 50–700 amu. The compounds were identified by comparison of the retention times and the interpretation of mass spectrum was carried out in conjunction with the database of National Institute of Standard and Technology (NIST) library.
Statistical analysis
Results were presented as mean ± standard deviation (SD) of three replicate determinations for in vitro assays and five animals in the case of in vivo experiment. Data were analysed using Tukey’s-HSD multiple range post hoc test with SPSS for Windows (version 18, IBM Corporation, NY, USA). Values with P < 0.05 were considered significantly different.
Result
Methanol and ethyl acetate extracts as well as the acarbose had a dose-dependent inhibitory effect on alpha-glucosidase, and upon the computation of the IC50 values, the methanol extract with an IC50 value of 171.83 ± 26.89 μg/mL was found to be the most potent extract against the enzyme (Table 1). Thus, the methanol extract was selected for the subsequent bioassay-guided fraction using column chromatography.
A total of 154 fractions were recovered after the column chromatography which pooled based on the Rf values to yield six fractions (A–F). Table 2 presents the effect of the different fractions (A–F) on alpha-glucosidase activity. All the fractions had inhibitory activity against the enzyme but fraction A with the least IC50 value of 78.62 ± 25.23 μg/mL was the most potent. Hence, it was selected for the maltose and sucrose tolerance tests in rats as well as the GC-MS analysis.
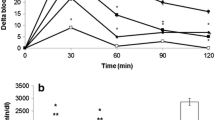
In the maltose tolerance test, the fraction A–treated groups had reduced blood glucose levels in a dose-dependent pattern in comparison to the NC group, though the difference was insignificant (P < 0.05) in some cases (Fig. 1). However, in the sucrose tolerance test, the fraction A–treated groups had a non-dose-dependent reduced blood glucose levels for most times during the 120-min experimental period (Fig. 2). It was noteworthy that the fraction A–treated groups had a profound decreased blood glucose levels from the 60 min. Moreover, the data for the computed area under the maltose tolerance curves revealed that, in comparison to the NC group, the fraction A–treated groups had lower, but insignificant (P > 0.05), AUC values (Table 3). Similarly, the AUC values for sucrose tolerance test revealed that the groups administered with fraction A also had lower but insignificant (P > 0.05) AUC values compared to the NC. However, the CPFL had lower AUC value compared to CPFH further supporting the non-dose-dependent effect of the fraction A in the sucrose tolerance test (Table 3).
The GC-MS analysis of the fraction A revealed 34 distinct peaks (Fig. 3), but only 13 different phytochemicals (Table 4) with high similarity index (> 90%) as revealed by the NIST database were considered and identified. Generally, these compounds were mainly phenolics as well as fatty acids, namely, 2,4-di-tert-butylphenol, cetene, cetyl alcohol, hexahydrofarnesyl acetone, palmitic acid, z-(13,14-epoxy)tetradec-11-en-1-ol acetate, linolelaidic acid, phytol, 1-eicosene, 5-methyl-5-(4,8,12-trimethyltridecyl)dihydrofuran-2(3H)-one, 1-heptatriacotanol, 17-pentatriacontene and squalene.
Discussion
Alpha-glucosidase inhibitors are used in the control of postprandial hyperglycaemia due to their suitability as non-surgical strategy for controlling the hyperglycaemia. In fact, the STOP NIDDM study of 2002 has clearly demonstrated the merits of these drugs in the delay of progression of impaired glucose tolerance into complete type 2 diabetic conditions in addition to mitigating the risk of macrovascular complications associated with DM (Abesundara et al. 2004). This article reported a thorough study on the potentials of C. papaya leaf as possible source of alpha-glucosidase inhibitors for subsequent application in the control of postprandial hyperglycaemia.
Report from previous research has demonstrated that the aqueous leaf extract of C. papaya possesses antioxidant and hypoglycemic effects (Juarez-Rojop et al. 2012) in addition to the improvement of the lipid profile for diabetic rats (Maniyar and Bhixavatimath 2012). However, the result of our findings revealed that the aqueous, methanol, ethylacetate and hexane extracts of the leaf could all inhibit alpha-glucosidase and consequently might reduce postprandial hyperglycemic shoot up. Among all the extracts, the methanolic extract had the best alpha-glucosidase inhibitory activity which could be as a result of the presence of phenolics, flavonoids, coumarins, alkaloids, tannins and organic acids that were reported in the methanol extract of the leaf of C. papaya (Khuzhaev and Aripova 2000; Canini et al. 2007). Subsequently, the methanol extract was subjected to column chromatography using different solvents of varying polarity to separate the compounds in the extract based on their polarity in the solvents which led to six pooled fractions (A, B, C, D, E and F) as earlier described. Fraction A had the lowest IC50 than the other fractions and even lower than the standard drug, acarbose, which makes it the most potent among the fractions and thereby, it was selected for further in vivo maltose and sucrose tolerance tests in rats.
Diabetic patients generally undergo a hyperglycaemic shoot up after consumption of dietary carbohydrates, and this takes approximately 4–5 h to revert back. The postprandial hyperglycaemic shoot up is associated with increase in the activity of disaccharidases by 1.5-fold (Tormo et al. 2002) as well as the activities of GLUT2 and SGLT1 by almost 3–4-fold (Kwon et al. 2007). In fact, in normoglycaemic individuals, the increase in the levels of blood glucose levels after dietary intake of carbohydrate begins at about 10 min and reaches maximum in ∼ 60 min (ADA 2001). Interestingly, the peak in the blood glucose level for the animals used in this study was also observed within the first 60 min of maltose but 30 min of sucrose doses. Thereafter, a decrease was observed until the blood level returns to near normal. However, the fraction A–treated groups showed reduced blood glucose levels in both maltose and sucrose tolerance tests. The observed effects of the fraction A within the initial 30 min on maltose tolerance curve and 60 min on sucrose tolerance curve might suggest a faster inhibitory activity against intestinal maltase than sucrose which are the two common disaccharidases known to be present at brush border of the intestine. Similarly, it was also evident from the computed AUC data that fraction A (200 mg/kg bw) have better inhibitory activity on maltase than sucrase which corroborates with the report of Ibrahim et al. (2016). This could possibly be due to the availability of some additional bioactive agents in the extract that specifically target maltase in addition to non-specific inhibitors of other dissaccharidases. Hence, the present in vivo sugar tolerance tests suggest that fraction A could exert an anti-diabetic effect via the mitigation of postprandial hyperglycaemia because for both in vitro and in vivo studies, the fraction showed similar inhibitory patterns with acarbose. Based on the results, it is plausible to hypothesise that fraction A mediated an anti-hyperglycaemic activity via possible inhibition of the intestinal disaccharidases.
The GC-MS analysis was conducted to identify the possible bioactive compounds in fraction A which revealed that the fraction contains phenolics which include 2,4-di-tert-butylphenol and phytol as well as fatty acids (palmitic acid) and their derivatives (squalene). Interestingly, previous studies indicated that the alpha-glucosidase inhibitory effects of Ocimum basilicum, Citrus maxima, cinnamon and tea extracts are mediated by phenolics (El-Beshbishy and Bahashwan 2012; Oboh and Ademosun 2011) which was proposed to be through hydrogen bond formation between the hydroxyl groups of the phenolics and the carboxyl acid groups of D197 and E233 at the active site of the enzyme (Piparo et al. 2008). Moreover, the identified phytol (Jananie et al. 2011), palmitic acid and squalene (Widyawati et al. 2015) have been reported to possess antidiabetic activity via the stimulation of insulin secretion and inhibition of alpha-glucosidase in diabetic rats. Thus, while not discounting the possible contribution of other components, it is plausible to connote that the phenolics along with the palmitic acid and squalene act individually or synergistically to bring about the observed inhibitory effects.
In conclusion, our data suggest that the leaf extract of C. papaya contains alpha-glucosidase inhibitors which might be exploited for the development of new agents for the control of postprandial hyperglycaemia. The findings also validate, in part, the application of the leaves of C. papaya in folk medicine for the treatment of DM.
References
Abesundara K, Mastsui T, Matsumoto K (2004) Alpha-glucosidase inhibitory activity of some Sri Lanka plant extracts, one of which, Cassia auriculata, exerts a strong antihyperglycemic effect in rats comparable to the therapeutic drug acarbose. J Agric Food Chem 52:2541–2545
American Diabetes Association (2001) Postprandial blood glucose. Diabetes Care 24:775–778
Canini A, Alesiani D, D’Arcangelo G, Tagliatesta P (2007) Gas chromatography-mass spectrometry analysis of phenolic compounds from Carica papaya L. leaf. J Food Compos Anal 20:584–590
Chavez-Quintal P, Gonzalez-Flores T, Rodriguez-Buenfil I, Gallegos-Tintore S (2011) Antifungal activity in ethanolic extracts of Carica papaya L. cv. Maradol leaves and seeds. Indian J Microbiol 51:54–60
Du WQ, Shi XF, Qui MY (2005) Progress in treatment of diabetes drugs. Chinese J Hosp Pharm 25:67–69
El-Beshbishy HA, Bahashwan SA (2012) Hypoglycemic effect of basil (Ocimum basilicum) aqueous extract is mediated through inhibition of alpha-glucosidase and alpha-amylase activities: an in vitro study. Toxicol Ind Health 28:42–50
Gholamhoseinian A, Fallah H, Sharifi-Far F, Mirtajaddini M (2008) The inhibitory effect of some Iranian plant extracts on the alpha glucosidase. Iranian J Basic Med Sci 11:1–9
Ibrahim MA, Yunus I, Kabir N, Baba SA, Yushau AM, Ibrahim SS, Bello ZI, Suleiman SH, Isah MB (2016) In vivo maltase and sucrase inhibitory activities of five underutilized Nigerian edible fruits. Mediterr J Nutr Metab 9:37–45
International Diabetes Federation.(2015) IDF Atlas. 7th edition
Jananie RK, Priya V, Vijayalakshmi K (2011) Phytoconstituents evaluation by GC-MS and anti-hyperglycemic activity of Cynodon dactylonon streptozotocin induced diabetes in rats. J Chem Pharm Res 3:460–466
Juarez-Rojop IE, Diaz-Zagoya JC, Ble-Castillo JL, Miranda-Osorio PH, Castell-Rodriguez AE, Tovilla-Zarate CA (2012) Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med 12:236
Khalid R, Meng-Ting L, Lin-Tao Z, Young-Tang Z (2013) Phytochemical screening of the polar extracts of Carica papaya Linn and the evaluation of their anti-HIV-1 activity. J App Ind Sci 1:49–53
Khuzhaev VU, Aripova SF (2000) Pseudocarpaine from Carica papaya. Chem Nat Compd 36:418–420
Kim YM, Jeong YK, Wang MH, Lee WY, Rhee HI (2005) Inhibitory effects of pine bark extract on alpha-glucosidase activity and postprandial hyperglycemia. Nutrition 21:756–761
Kwon O, Eck P, Chen S (2007) Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FEBS J 21:366–377
Lopez-Candales A (2001) Metabolic syndrome X: a comprehensive review of the pathophysiology and recommended therapy. J Med 32:283–300
Maniyar Y, Bhixavatimath P (2012) Antihyperglycemic and hypolipidemic activities of aqueous extract of Carica papaya Linn. leaves in alloxan-induced diabetic rats. J Ayur Int Med 3:70–74
Oboh G, Ademosun AO (2011) Shaddock peels (Citrus maxima) phenolic extracts inhibit alpha-amylase, alpha-glucosidase and angiotensin I-converting enzyme activities: a nutraceutical approach to diabetes management. Diabetes Metab Syndr Clin Res Rev 5:148–152
Piparo EL, Scheib H, Frei N, Williamson G, Grigorov M, Chou CJ (2008) Flavonoids for controlling starch digestion: structural requirements for inhibiting human alpha-amylase. J Med Chem 51:3555 3561
Playford RJ, Pither C, Gao R (2013) Use of the alpha glucosidase inhibitor acarbose in patients with ‘Middleton syndrome’: normal gastric anatomy but with accelerated gastric emptying causing postprandial reactive hypoglycemia and diarrhea. Can J Gastroenterol 27:403–404
Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS, Min BH (2003) Inhibitory effect of aqueous extracts from the gall of Rhus chinensis on alpha glucosidase activity and postprandial blood glucose. J Ethnopharmacol 85:283–287
Sindhu SN, Vaibhavi K, Anshu M (2013) In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur J Exp Biol 3:128–132
Tormo MA, Martinez I, Romero de Tejada A, Gil-Exojo I, Campillo J (2002) Morphological and enzymatic changes of the small intestine in an STZ diabetes rat model. Exp Clin Endocrinol Diabetes 110:119–123
Widyawati T, Yusoff N, Asmawi MZ, Ahmad M (2015) Anti-hyperglycaemic effects of methanol extract of Syzygium polyanthum (Wight.) leaf in streptozotocin-induced diabetic rats. Nutrients 7:7764–7780
Yin Z, Zhang W, Feng F, Zhang Y, Kang W (2014) Alpha glucosidase inhibitors isolated from medicinal plants. Food Sci Human Wellness 3:136–174
Acknowledgements
Authors are grateful to the authorities of Ahmadu Bello University, Zaria, for providing the facilities used in the study. The technical assistance of Mr. Aliyu Mansir is also highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
This article does not contain any studies with animal performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abubakar, M., Onyike, E. & Ibrahim, M.A. In vitro and in vivo studies on the alpha-glucosidase inhibitory effects of the leaf extract of Carica papaya Linn. Comp Clin Pathol 28, 1061–1067 (2019). https://doi.org/10.1007/s00580-019-02928-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-02928-9