Abstract
Objectives
Diabetes mellitus (DM) is an important public health problem all over the world, considering its complications and increasing prevalence. Oleanolic acid (OA) has anti-diabetic property via modulating glucose metabolism and acting as 5′–adenosine monophosphate (AMP)–activated protein kinase (AMPK) / Sirtuin–1 (SIRT–1) activator and Interleukin 6 (IL–6) / Nuclear factor kappa B (NF–κB) inhibitor. This research questioned if the OA treatment amliorates the hepatic inflammatory profile in the diabetic rats.
Methods
Twenty–eight male Sprague Dawley rats were first subjected to either no diabetes induction (healthy) or diabetes induction by i.p. injection of 50 mg/kg streptozotocin. Then rats in both groups were treated with either tap water or OA (5 mg/kg) within 1 ml tap water by oral gavage for 21 days.
Results
The diabetic rats had higher hepatic MDA (2.88x) and serum AST (2.01x), ALP (2.22x), and ALT (4.27x) levels and 50% lower hepatic SOD level than the healthy rats. The OA treatment significantly reversed these antioxidant parameters in the diabetic rats. The diabetic rats had lower AMPK (85%) and hepatic SIRT–1 (47%) levels and higher hepatic NF–κB (53%) and IL–6 (34%) levels than the healthy rats. Comparing with the health rats, the OA treatment increased hepatic SIRT–1 level, but tended to increase hepatic AMPK level and decrease hepatic NF–κB and IL–6 levels in the diabetic rats. It was also partially effective to ameliorate degenerative changes and necrosis in the diabetic rats.
Conclusion
The OA treatment can be considered to alleviate oxidative stress and reduce severity of inflammation in hepatocytes in the diabetic subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease characterized by hyperglycemia, causing metabolic degenerative complications, and developing as a result of absolute or relative insufficiency of insulin secretion in the pancreas or insulin ineffectiveness or structural defects in the insulin molecule [1]. The number of diabetic patients in the world is 463 millions according to the 2019 data of the International Diabetes Federation (IDF), and it is anticipated to reach 693 million adults in 2045 [2]. Its typical clinical symptoms include polydipsia, polyuria, polyphagia, and weight loss. In some instances it can be mortal due to its macro- and microvascular complications such as cardiovascular diseases, retinopathy, neuropathy, and nephropathy [3].
In recent years, the subject of “Hepatogenous Diabetes”, a description made in 1906 to define the high rate of DM in cirrhotic patients, has drawn new and considerable interest. Clinical researches support the fact that a decline in life expectancy of type 2 DM patients is not only associated with vascular problems and renal disease, but also with cirrhosis and hepatocellular cancer [4]. It is essential to know various metabolic pathways that are significant in the development of diabetes in order to recognize the disease itself and its effects on the liver, and to establish an early diagnosis as well as determine the appropriate treatment programs.
A serine/threonine protein complex, namely 5′-adenosine monophosphate (AMP)-activated protein kinase (AMPK), plays a major role in maintaining cell energy balance [5]. AMPK, regulating lipid, cholesterol and glucose metabolism in liver, muscle and adipose tissue, manages the cell energy requirement at the maximum level by coordinating metabolic pathways. It activates catabolic pathways in the liver, while inhibiting anabolic pathways. Synthetic drugs and phytochemical agents used in the treatment of diabetes affect the level of AMPK, improving glucose transporter-4 (GLUT4) translocation insulin, and enabling blood glucose uptake into cells [6, 7].
Sirtuin-1 (SIRT-1), predominantly localized in the nucleus but also found in the cytoplasm, is expressed in many tissues. Histones, including transcription factors, DNA repair factors and signaling proteins, regulate activities through deacetylation of various substrates [8]. SIRT-1, is effective in apoptosis inhibition, mitochondrial biogenesis, inhibition of inflammation, regulation of glucose and lipid metabolism, circadian rhythm, and cellular stress adaptation [9]. The relationship between SIRT-1 and glucose hemostasis and insulin secretion reveal that these proteins may be effective in the progression of insulin resistance (IR) and DM [10]. AMPK and SIRT-1 are present in all eukaryotic cells. Even though both molecules have been researched mainly, the similarities in their regulations and effects on such various processes as mitochondrial function and inflammation have only recently become understandable [11, 12].
Interleukin-6 (IL-6) is a pleiotropic cytokine with proinflammatory and endocrine functions, mainly secreted by vascular endothelial cells, mononuclear phagocytes, fibroblasts, and activated T lymphocytes. IL-6 plays a role in the improvement of IR and β-cell dysfunction [13]. Nuclear factor kappa B (NF-κB) is a transcription factor regulating the expression of diverse genes responsible for events including apoptosis, cell proliferation, cell differentiation and inflammation [14]. NF-κB activation is important in diabetes, in both oxidative stress and inflammatory signaling pathways. NF-κB plays a major role in the transcription of cytokines, adhesion molecules, and other mediators in the pathogenesis of many inflammatory diseases. The NF-κB family has also a significant role in the arrangement of the genes involved in inflammatory and some other signaling pathways in the cell [15, 16].
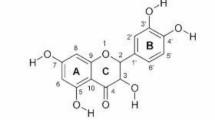
Plants containing antioxidant compounds have a extensive variety of pharmacological features, such as scavenging reactive oxygen species (ROS) and repairing or preventing the tissue damages caused by internal and external factors [17]. Oleanolic acid (OA) is an active pentacyclic triterpenoid ingredient isolated from more than 120 plant types, including many edible and medical aromatic plants. OA draws considerable attention due to its antioxidant, antimicrobial, antidiabetic, antiinflammatory and hepatoprotective effects [18,19,20]. Hyperglycemia triggers tissue damage [21, 22]. It was hypothesized that OA would ameliorate hepatic damage in diabetic subjects through loweing glucose and modulating inflammatory pathways. The objective of this experiment was to evaluate the hepatic inflammatory profile in diabetic rats.
Materials and methods
Experimental animals
Atatürk University Local Ethics Committee for Animal Experiments approved this experimental protocol number at 236/09 (date: 27/10/2021). Twenty-eight male Wistar rats weighing 250-300 g and 6–8 weeks old, were obtained from Atatürk University Experimental Animals Research Center. The rats were housed in transparent polyethylene cages and fed ad libitum consumption of a pelleted chow diet. The room was fusnished with artificially controlled temperature (23 ± 2 ºC), illumination (12:12 h photoperiod), and humidity (55 ± 5%) 1 week prior to experimentation for adaptation. In half of the rats, diabetes was induced via a single intraperitoneal (i.p.) injection of 50 mg/kg streptozotocin (STZ) in 0.4 ml (0.1 M) sodium citrate buffer, pH 4.5, (Sigma Chemical Co., St. Louis, MO) (diabetic rats). After 72 h, blood was taken from the tail vein and glucose concentration was measured using a glucometer. Fasting blood glucose > 200 mg/dl was considered diabetic [23, 24]. The other half of rats were not injected with STZ (healthy rats). Both healthy and diabetic rats were then divided randomly into two subgroups to be administered with either OA (5 mg/kg, Sigma Chemical Co.) within 1 ml tap water by oral gavage or 1 ml tap water in the same route for 21 days [25].
At the end of the experiment, intracardiac blood samples were taken from the rats. Prior to blood sampling rats were administered ketamine (80 mg/kg; Ketalar®, 50 mg/ml, Eczacıbaşı, Istanbul, Turkey) and xylazine (10 mg/kg; Rompun®, 2%, Bayer, Istanbul, Turkey), and then they were sacrificed. Blood and liver samples were collected for biochemical analyses and histopathologic evaluations.
Biochemical analyses
Blood samples were centrifuged at 4,000 g for 15 min and the sera were stored at − 80 °C for aspartate amino transferase (AST), alkaline phosphatase (ALP), and alanine amino transferase (ALT) measurements in autoanalyzer ((RX Monaco; Randox Laboratories Ltd., County Antrim, UK).
Liver tissue malondialdehyde (MDA) levels were measured to asses lipid peroxidation level [26], based on reaction with thiobarbituric acid at 90–95 °C to yield a pink colored chromogen. After 15 min, the absorbance values of the rapidly cooled samples were read spectrophotometrically at 532 nm. The MDA level was expressed as nmol/g tissue protein.
Liver tissue superoxide dismutase (SOD) level was determined based on the reduction of nitroblue tetrazolium by the xanthine–xanthineoxidase system [27]. The SOD activity was expressed as U/g tissue protein.
Liver tissue AMPK (Biocompare, South San Francisco, CA 94080 USA), SIRT–1 (Elabscience Biotechnology Inc, Houston, Texas, USA), IL–6 (Elabscience Biotechnology Inc, Houston, Texas, USA), and NF–κB (Elabscience Biotechnology Inc, Houston, Texas, USA) levels were measured by the ELISA method, is based on a specific antigen and antibody reaction. An enzyme is used as a marker in the preparation of the labeled conjugate. After completion of the reaction, separation is achieved by adding a substrate to the medium and enzyme activity is measured spectrophotometrically.
Histopathological examination
The liver samples were fixed in 10% buffered formalin and all the time processed for histological analysis by embedding in paraffin wax. Tissue sections were cut 4 μm in thickness and painted by the Haematoxylin-Eosin (H&E) for examination under a light microscope [28].
Image analysis
Liver samples were evaluated by high-power light microscopic examination using an Olympus Bx51 with a DP72 camera system. Each specimen was analyzed in 10 randomly choosed areas of approximately an X40 objective. The scores were reproduced semi-quantitatively using light microscopy on the preparations from each rat and were reported as follows: Grade 0 = – (negative); Grade 1 = + 1 (mild); Grade 2 = + 2 (moderate); Grade 3 = + 3 (severe); Grade 4 = + 4 (most severe) [29].
Statistical analysis
The normality of data was evaluated using a Kolmogorov–Smirnov test. Continuous data (biochemistry) were analyzed by 2–way ANOVA to test the main effects of health status (HS; healthy vs. diabetic) and treatment (TRT; not OA administered vs. OA administered) as well as their interaction using the General Linear Model Procedure (Yijk = HSi x Trtj + eijk) (MedCalc, Version 13.2.2; MedCalc, Ostend, Belgium). Group mean differences were attained by the LSD option. Discrete data (histopathology) were subjected to the Mann Whitney U test. Stastistical significance are declared p ≤ 0.05.
Results
Biochemistry
Alterations in serum AST, ALP, and ALT as well as hepatic MDA and SOD levels in respose to the OA treatment in the diabetic rats were summarized in Table 1. Induction of diabetes caused increases in hepatic MDA (2.88x) and serum AST (2.01x), ALP (2.22x), and ALT (4.27x) levels and a decrease in hepatic SOD level by half (p < 0.0001 for all; Table 1). Health Status by OA Treatment interaction revealed that decreases in hepatic MDA (p = 0.0073) and serum AST (p < 0.0001), ALP (p = 0.0066), and ALT (p = 0.0020) levels and a increase in hepatic SOD level (p < 0.0001) were more notable in the diabetic rats than the healthy rats in response the OA treatment (Table 1).
The effect of the OA treatment on hepatic inflammatory markers in the diabetic rats is shown in Table 2. The diabetic rats had lower AMPK (85%, p < 0.0001) and hepatic SIRT–1 (47%, p = 0.0050) levels and higher hepatic NF–κB (53%, p < 0.0001) and IL–6 (34%, p = 0.0255) levels than the healthy rats. The OA treatment increased hepatic SIRT–1 level at a greater extent and in the diabetic rats than the healthy rats (p = 0.0463; Table 2). However, an increase in hepatic AMPK level (p = 0.0851) and decreases in hepatic NF–κB (p = 0.0621) and IL–6 (p = 0.0743) levels in the diabetic rats were similar to those in the healthy rats in response to the OA treatment (Table 2).
Histopathology
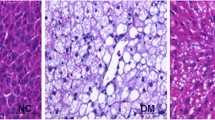
No histopathological change was observed in the liver tissues in the healthy rats not administered with OA and administered with OA (Fig. 1A, B). Dissociation in the remark cords, mild adiposity and degenerative changes in hepatocytes, as well as localized necrosis foci were noted in the liver tissue of the diabetic rats (Fig. 1C). In addition, bile duct proliferation and mononuclear cell infiltration, mostly lymphocytes, were observed. Hepatic tissue damages in the diabetic rats and their restoration by the OA treatment are shown in Table 3. Degeneration (p < 0.0001), necrosis (p < 0.0007), and inflammatory cell infiltration (p < 0.0001) were notable in hepatocytes in the diabetic rats. In terms of histopatholy scores of the hepatocytes, the OA treatment reduced necrosis (p = 0.0208; Table 3), but failed to alleviate degeneration and inflammatory cell infiltration (Fig. 1C, D).
Hepatic tissue damages in the diabetic rats and their restoration by the oleanolic acid treatment. H&E, Bar: 40 μm. A-B No histopathological lesions in the healthy rats not administered with OA and administered with OA. C Degenerative changes and necrosis in the hepatocytes, inflammation cells in the diabetic rats not administered with OA. D Moderate degenerative changes and necrosis in the hepatocytes, inflammation cells in the diabetic rats administered with OA (DM + OA)
Discussion
Diabetes, as it is a lifelong disease and requires an appropriate treatment in order to postpone or prevent complications and to eliminate its symptoms. Herbal therapy has become popular in recent years, for various reasons including medical and economic problems due to serious side effects of synthetic drugs, ecological instabilities becoming harder as a result of environmental pollution especially in industrialized countries, threats posed by various chronic diseases related to sedentary lifestyle [30, 31].
Different kinds of plants have been used for the treatment of diabetes by traditional methods, in many parts of the world. Some of these methods are considered by scientists, and supported by the World Health Organization (WHO). Up to 30% of diabetic patients are treated with medicinal plants and nutritional supplements, as well as complementary and alternative medicine [32, 33]. This study aimed at examining potential hepato-protective effects of OA in an experimental diabetes model, where inflammation and oxidative stress are involved in the pathogenesis.
Insulin resistance, recognized as the main cause of hyperglycemia and compensatory hyperinsulinemia, is one of the prevailing causes of oxidative stress and liver damage in diabetic patients [21]. Oxidative stress is a major ingredient in the mechanism of DM and hepatotoxicity. Thus, inhibition of reactive oxygen species (ROS) and/or antioxidant feature plays a very important role in protecting hepatotoxicity [34], associated with elevations in hepatic MDA [35] and liver enzymes including AST, ALT and ALP in the blood stream [22]. In diabetes, SOD enzyme causes dismutation of free oxygen radicals, leading to tissue damages [36]. The results of the present study showed that STZ–induced DM led to significant increase in MDA, AST, ALT, and ALP levels and a decrease in SOD level (Table 1), which were reversed by the OA treatment, suggesting the tissue protective effect of the OA though its antioxidant property. The MDA levels increased as a result of increased ROS in various tissues of the diabetic rats, but decreased significantly upon the administration of plant extracts or antidiabetic drugs [37, 38]. Decreased SOD activities were shown in various studies with experimental diabetes models [39, 40].
Active of AMPK plays fundamental role in protection against pathological stress such as under DM and metabolic syndrome. High AMPK levels supports energy production via different mechanism including quicken glucose uptake and enhancement fatty acid uptake and oxidation as well as glycolysis [41]. OA has strong antioxidant and anti–inflammatory properties and its hepatoprotective effect is related to potecy for loweing blood glucose, consequently inhibiting insulin resistance and hepatic gluconeogenesis [42]. Hasanvand et al. [43], and Ban et al. [44] reported that AMPK activity was low in rats experimentally induced diabetes. There are more than a hundred natural components that can activate AMPK, and despite their structural diversity, most of them are polyphenols. AMPK activation may be beneficial in treatment. In this study, the OA treatement tended to restore decreased hepatic AMPK due to DM (Table 2). This partially supports that activation of AMPK and decreased oxidation play an important role in the STZ–induced liver damage, and OA elicits hepatoprotection and the beneficial effects of AMPK activation is attributed to its anti–inflammatory effects [45], and modulation of nutrient metabolism [42, 46].
AMPK can generate an anti–inflammatory impact and restrict oxidative stress through the activation of the SIRT–1 connected pathway. AMPK and SIRT–1 share many extensive goal molecules and both arrange each other. The activation of AMPK signaling prevents the expression of the NF–κB by increasing the levels of SIRT–1, in this way contributing to the preservation against inflammation as well as DM [47, 48]. The SIRT–1/NF–κB pathway is a potential target for the development of novel treatment options in decreasing the frequency and related complications of metabolic diseases, where inflammation is involved in the pathogenesis [49,50,51]. Similar to AMPK level and as oppsed to NF–κB level, decreased SIRT–1 level increased significantly in the diabetic rats in response to the OA treatment (Table 2).
Oxidative stress and inflammation act together as important and harmful mechanisms in the pathogenesis of early and late complications of diabetes. Proinflammatory cytokines can induce IR in adipose tissue, skeletal muscle, and liver through inhibiting insulin signal transduction. Thus, inflammation–targeted therapies may be a novel treatment option for the follow–up of DM and related complications, considering that hepatic NF–κB and IL–6 activated due to diabetes can be inactived by the OA treatment [52]. NF–κB can be activated by the secretion of proinflammatory molecules such as IL–6 and TNF–α. At the same time, other non–inflammatory conditions and mediators such as oxidative stress, hyperglycemia, and obesity can also activate NF–κB levels [53]. The activation of NF–κB in DM may start a sequence of harmful cases via further elevation of proinflammatory cytokines. Additionally, NF–κB may also directly and indirectly support the generation of ROS and reactive nitrogen species, thereby increasing liver tissue damage [54].
IL–6 is a cytokine with pro– and anti–inflammatory effects. Pro–inflammatory cytokines may cause IR by inhibiting insulin signaling in skeletal muscle, liver, and adipose tissues [55]. Increased plasma concentration of IL–6 were associated with type 2 diabetes [56]. Foss–Freitas et al. [57] stated that IL–6 measurement was significant in monitoring pro–inflammatory immune responses developing in diabetic patients. OA can protect the liver not only against toxic effects of chemicals exposed, but also against diseases including fatty liver and cirrhosis [58]. However, the metabolic pathways involved in hepatoprotective effects of OA are not clear yet. Laszczyk et al. [59] have shown the anti–inflammatory and anticancer potency of OA, presumably by targeting NF–κB, but its definite mode of action remains to be explored. The studies have confirmed that OA inhibits inflammation through the suppression of NF-κB signaling, the inhibition of cytokines, including IL-6 and, the increased production of antioxidants. Anti-inflammatory features of OA were mediated by the down-regulation of the levels of NF–κB [60, 61]. In this experiment the OA treatment tended to decrease increased hepatic NF–κB and IL–6 levels in the diabetic rats (Table 2).
Liver has a crucial function in regulating glucose levels in physiological and pathological conditions such as diabetes. Necrosis, inflammatory cell infiltration, lipidosis, sinusoidal dilatation and disorders in portal spaces in hepatocytes of the diabetic rats are typical histopathological findings [62]. The OA treatment was partially effective to ameliorate degenerative changes and necrosis in the diabetic rats.
A limitation of our study is that following the model for a wider time and investigating biochemical parameters at different time points could provide more information about the model. In addition, OA results could compared to diabetic drugs.
Conclusion
In conclusion, the induction of diabetes achieved acute liver injury. The OA treatment succesfuly ameliorated antioxidant status and partially improved inflmmation through acting AMPK/SIRT–1 activator and IL–6/ NF–κB inhibitor. Therefore, OA can be considered in the protection and treatment of DM and DM–related complications.
Abbreviations
- ALT:
-
Alanine amino transferase
- AST:
-
Aspartate amino transferase
- ALP:
-
Alkaline phosphatase
- AMPK:
-
5′-adenosine monophosphate (AMP)-activated protein kinase
- DM:
-
Diabetes mellitus
- H&E:
-
Haematoxylin-Eosin
- IDF:
-
International Diabetes Federation
- IL-6:
-
Interleukin-6
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear factor kappa B
- OA:
-
Oleanolic acid
- ROS:
-
Reactive oxygen species
- SIRT-1:
-
Sirtuin-1
- STZ:
-
Streptozotocin
- SOD:
-
Superoxide dismutase
References
Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep. 2019;21(4):21. https://doi.org/10.1007/s11886-019-1107-y.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. https://doi.org/10.1016/j.diabres.2019.107843.
Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–90. https://doi.org/10.1038/s41581-020-0278-5.
Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43(1):51–64. https://doi.org/10.1111/j.1872-034X.2012.01031.x.
Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251. https://doi.org/10.1038/nrm3311.
Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48(4):e224. https://doi.org/10.1038/emm.2016.16.
Kişmiroğlu C, Cengiz S, Yaman M. Biochemistry of AMPK: mechanisms of action and importance in the treatment of diabetes. Eur J Sci Techn. 2020;18:162–70. https://doi.org/10.31590/ejosat.676335.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–8. https://doi.org/10.1038/nature03354.
Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280(21):20589–95. https://doi.org/10.1074/jbc.M412357200.
Kitada M, Koya D. SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metab J. 2013;37(5):315–25. https://doi.org/10.4093/dmj.2013.37.5.315.
Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298(4):E751-60. https://doi.org/10.1152/ajpendo.00745.2009.
Jiang X, Chen J, Zhang C, Zhang Z, Tan Y, Feng W, et al. The protective effect of FGF21 on diabetes-induced male germ cell apoptosis is associated with up-regulated testicular AKT and AMPK/Sirt1/PGC-1α signaling. Endocrinology. 2015;156(3):1156–70. https://doi.org/10.1210/en.2014-1619.
Akbari M, Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. 2018;26(3):685–98. https://doi.org/10.1007/s10787-018-0458-0.
Iskender H, Yenice G, Dokumacioglu E, Hayirli A, Sevim C, Dokumacioglu A, et al. Astaxanthin alleviates renal damage of rats on high fructose diet through modulating NFκB/SIRT1 pathway and mitigating oxidative stress. Arch Physiol Biochem. 2020;126(1):89–93. https://doi.org/10.1080/13813455.2018.1493609.
Mitchell JP, Carmody RJ. NF-κB and the Transcriptional control of inflammation. Int Rev Cell Mol Biol. 2018;335:41–84. https://doi.org/10.1016/bs.ircmb.2017.07.007.
Kunnumakkara AB, Shabnam B, Girisa S, Harsha C, Banik K, Devi TB, et al. Inflammation, NF-kappaB, and chronic diseases: how are they linked? Crit Rev Immunol. 2020;40(1):1–39. https://doi.org/10.1615/CritRevImmunol.2020033210.
Huang G, Mei X, Hu J. The antioxidant activities of natural polysaccharides. Curr Drug Targets. 2017;18(11):1296–300. https://doi.org/10.2174/1389450118666170123145357.
Yu Z, Sun W, Peng W, Yu R, Li E, Jiang T, et al. Pharmacokinetics in vitro and in vivo of two novel prodrugs of oleanolic acid in rats and its hepatoprotective effects against liver injury induced by CCl4. Mol Pharmacol. 2016;13(5):1699–710. https://doi.org/10.1021/acs.molpharmaceut.6b00129.
Ayeleso TB, Matumba MG, Mukwevho E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules. 2017;22(11):1915. https://doi.org/10.3390/molecules22111915.
Sen A. Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases. World J Clin Cases. 2020;8(10):1767–92. https://doi.org/10.12998/wjcc.v8.i10.1767.
Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6(3):456–80. https://doi.org/10.4239/wjd.v6.i3.456.
Mohamed J, Nafizah AHN, Zariyantey AH, Budin SB. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16(2):e132-41. https://doi.org/10.18295/squmj.2016.16.02.002.
Dokumacioglu E, Iskender H, Sen TM, Ince I, Dokumacioglu A, Kanbay Y, et al. The effects of hesperidin and quercetin on serum tumor necrosis factor–alpha and interleukin–6 levels in streptozotocin–induced diabetes model. Pharmacogn Mag. 2018;14:167–173. https://doi.org/10.18295/squmj.2016.16.02.002.
Iskender H, Dokumacioglu E, Terim-Kapakin KA, Yenice G, Mohtare B, Bolat I, et al. Effects of oleanolic acid on inflammation and metabolism in diabetic rats. Biotech Histochem. 2021;15:1–8. https://doi.org/10.1080/10520295.2021.1954691.
Zhao H, Liu J, Song L, Liu Z, Han G, Yuan D, et al. Oleanolic acid rejuvenates testicular function through attenuating germ cell DNA damage and apoptosis via deactivation of NF–κB, p53 and p38 signaling pathways. J Pharm Pharmacol. 2017;69(3):295–304. https://doi.org/10.1111/jphp.12668.
Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hidroxynonenal. Methods Enzymol. 1990;186:407–21. https://doi.org/10.1016/0076-6879(90)86134-h.
Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. https://doi.org/10.1093/clinchem/34.3.497.
Terim–Kapakin KA, Gumus R, Imik H, Kapakin S, Saglam YS. Effects of ascorbic and α–lipoic acid on secretion of HSP–70 and apoptosis in liver and kidneys of broilers exposed to heat stress. Ankara Univ Vet J. 2012;59:279–87. https://doi.org/10.1501/Vetfak_0000002539.
Apaydin-Yildirim B, Kordali S, Terim-Kapakin KA, Yildirim F, Aktas-Senocak E, Altın S. Effect of Helichrysum plicatum DC. Subsp, plicatum ethanol extract on gentamicin-induced nephrotoxicity in rats. J Zhejiang Univ Sci B. 2017;18(6):501–11. https://doi.org/10.1631/jzus.B1500291.
Jugran AK, Rawat S, Devkota PH, Bhatt ID, Rawal RS. Diabetes and plant-derived natural products: from ethnopharmacological approaches to their potential for modern drug discovery and development. Phytother Res. 2021;35(1):223–45. https://doi.org/10.1002/ptr.6821.
Hedayat KM, Lapraz JC, Schuff B. Medicinal plants in clinical practice. The theory of endobiogeny 5th edition 2020;57–60
Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Physician. 2016;8(1):1832–42. https://doi.org/10.19082/1832.
Salleh NH, Zulkipli IN, Mohd YH, Ja’afar F, Ahmad N, Wan WA, et al. Systematic review of medicinal plants used for treatment of diabetes in human clinical trials: An ASEAN perspective. Evid Based Complement Alternat Med. 2021;2021:5570939. https://doi.org/10.1155/2021/5570939.
Palsamy P, Sivakumar S, Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem Biol Interact. 2010;186(2):200–10. https://doi.org/10.1016/j.cbi.2010.03.028.
Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. https://doi.org/10.1155/2014/360438.
Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of oxidative stress during diabetes mellitus. J Biomark. 2013;2013:378790. https://doi.org/10.1155/2013/378790.
Masola B, Oguntibeju OO, Oyenihi AB. Centella asiatica ameliorates diabetes-induced stress in rat tissues via influences on antioxidants and inflammatory cytokines. Biomed Pharmacother. 2018;101:447–57. https://doi.org/10.1016/j.biopha.2018.02.115.
Saha MR, Dey P, Sarkar IDe Sarker D, et al. Acacia nilotica leaf improves insulin resistance and hyperglycemia associated acute hepatic injury and nephrotoxicity by improving systemic antioxidant status in diabetic mice. J Ethnopharmacol. 2018;210:275–86. https://doi.org/10.1016/j.jep.2017.08.036.
Anwer T, Alkarbi ZA, Hassan NA, Alshahrani S, Khan G, et al. Modulatory effect of zingerone against STZ-nicotinamide induced type-2 diabetes mellitus in rats. Arch Physiol Biochem. 2021;127(4):304–10. https://doi.org/10.1080/13813455.2019.1637436.
Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang X, Cui XG, et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med. 2020;24(21):12355–67. https://doi.org/10.1111/jcmm.15725.
Wang N, Zhang J, Qin M, Yi W, Yu S, Chen Y, et al. Amelioration of streptozotocin induced pancreatic β cell damage by morin: involvement of the AMPK FOXO3 catalase signaling pathway. Int J Mol Med. 2018;41(3):1409–18. https://doi.org/10.3892/ijmm.2017.3357.
Wang X, Li YL, Wu H, Liu JZ, Hu JX, Liao N, et al. Antidiabetic effect of oleanolic acid: a promising use of a traditional pharmacological agent. Phytother Res. 2011;25(7):1031–40. https://doi.org/10.1002/ptr.3385.
Hasanvand A, Amini-Khoei H, Hadian MR, Abdollahi A, Tavangar SM, Dehpour AR, et al. Anti-inflammatory effect of AMPK signaling pathway in rat model of diabetic neuropathy. Inflammopharmacology. 2016;24(5):207–19. https://doi.org/10.1007/s10787-016-0275-2.
Ban Q, Cheng J, Sun X, Jiang Y, Guo M. Effect of feeding type 2 diabetes mellitus rats with synbiotic yogurt sweetened with monk fruit extract on serum lipid levels and hepatic AMPK (5′ adenosine monophosphate-activated protein kinase) signaling pathway. Food Funct. 2020;11(9):7696–706. https://doi.org/10.1039/d0fo01860k.
Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48(7):e245-5. https://doi.org/10.1038/emm.2016.81.
Tanyıldız S, Yıldırım H, Uğur H, Yaman M. AMPK’s natural activators and relationships with diseases. Eur J Sci Technol. 2021;21:389–401. https://doi.org/10.31590/ejosat.762959.
Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage α1 AMP-activated protein kinase (α1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285(25):19051–9. https://doi.org/10.1074/jbc.M110.123620.
Abedimanesh N, Asghari S, Mohammadnejad K, Daneshvar Z, Rahmani S, Shokoohi S, et al. The antidiabetic effects of betanin in streptozotocininduced diabetic rats through modulating AMPK/SIRT1/NFκB signaling pathway. Nutr Metab (Lond). 2021;18(1):92. https://doi.org/10.1186/s12986-021-00621-9.
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Sign. 2013;25(10):1939–48. https://doi.org/10.1016/j.cellsig.2013.06.007.
Gregorio E, Colell A, Morales A, Marí M. Relevance of SIRT1-NF-kappaB Axis as therapeutic target to ameliorate inflammation in liver disease. Int J Mol Sci. 2020;21(11):3858. https://doi.org/10.3390/ijms21113858.
Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS ONE. 2012;7(9):e46364. https://doi.org/10.1371/journal.pone.0046364.
Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020;10:1607. https://doi.org/10.3389/fphys.2019.01607.
Manna P, Das J, Ghosh J, Sil PC. Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IkappaBalpha/NF-kappaB, MAPKs, and mitochondria-dependent pathways: prophylactic role of arjunolic acid. Free Radic Biol Med. 2010;48(11):1465–84. https://doi.org/10.1016/j.freeradbiomed.2010.02.025.
Romagnoli M, Gomez-Cabrera MC, Perrelli MG, Biasi F, Pallardó FV, Sastre J, et al. Xanthine oxidase-induced oxidative stress causes activation of NF-kappaB and inflammation in the liver of type I diabetic rats. Free Radic Biol Med. 2010;49(2):n171-7. https://doi.org/10.1016/j.freeradbiomed.2010.03.024.
Svistounov D, Smedsrød B. Hepatic clearance of advanced glycation end products (AGEs): myth or truth? J Hepatol. 2004;41:1038–40. https://doi.org/10.1016/j.jhep.2004.10.004.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. https://doi.org/10.1001/jama.286.3.327.
Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. In vitro TNF-alpha and IL-6 production by adherent peripheral blood mononuclear cells obtained from type 1 and type 2 diabetic patients evaluated according to the metabolic control. Ann NY Acad Sci. 2006;1079:177–80. https://doi.org/10.1196/annals.1375.027.
Pollier J, Goossens A, Oleanolic acid. Phytochemistry. 2012;77:10–5. https://doi.org/10.1016/j.phytochem.2011.12.022.
Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75(15):1549–60. https://doi.org/10.1055/s-0029-1186102.
An Q, Hu Q, Wang B, Cui W, Wu F, Ding Y. Oleanolic acid alleviates diabetic rat carotid artery injury through the inhibition of NLRP3 inflammasome signaling pathways. Mol Med Rep. 2017;16(6):8413–9. https://doi.org/10.3892/mmr.2017.7594.
Camer D, Yu Y, Szabo A, Huang XF. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes and associated complications. Mol Nutr Food Res. 2014;58:1750–9. https://doi.org/10.1002/mnfr.201300861.
Yaman T, Uyar A, Celik I, Alkan EE, Keles OF, Yener Z. Histopathological and immunohistochemical study of antidiabetic effects of heracleum persicum extract in experimentally diabetic rats. IJPER. 2017;51(3):450–7. https://doi.org/10.5530/ijper.51.3s.66.
Funding
This work was supported by Coordinator of Scientific Research Projects [2021.M84.02.04] at Artvin Coruh University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was carried out in the Atatürk University’s Experimental Animal Laboratory of the Medical and Experimental Application and Research (ATADEM) in accordance with the Atatürk University’s Local Ethical committee decision (2021/09).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this research article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iskender, H., Dokumacioglu, E., Terim Kapakin, K.A. et al. Effect of Oleanolic acid administration on hepatic AMPK, SIRT-1, IL-6 and NF-κB levels in experimental diabetes. J Diabetes Metab Disord 22, 581–590 (2023). https://doi.org/10.1007/s40200-022-01178-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01178-x