Abstract
Background and aims
Rising levels of oxidative stress play an important role in the pathogenesis of type 2 diabetes mellitus. This study aimed to some assess salivary antioxidants in patients with type 2 diabetes, prediabetes, and healthy control. We also assessed the potential clinical relevance of salivary antioxidants with glycemic control.
Methods
This cross-sectional study included 30 prediabetes, 31 type 2 diabetes, and 39 sex-matched normoglycemic individuals. To assess the salivary oxidative status, we measured the levels of malondialdehyde (MDA), superoxide dismutase (SOD), the total antioxidant capacity (TAC), and uric acid (UA) by spectrophotometry.
Results
Salivary MDA levels were significantly higher in individuals with diabetes compared to prediabetes, and control groups (p = 0.001). MDA and SOD were significantly correlated with fasting blood sugar (FBS) and HbA1C (p < 0.001, r = 0.43, p < 0.001, r = 0.34, and p = 0.003, r = 0.29 p = 0.01, r = 0.23 respectively). Salivary TAC was also significantly correlated with FBS (p = 0.02, r = 0.23). Furthermore, salivary MDA was an independent determinant of type 2 diabetic patients compared to healthy subjects (p = 0.04). According to the cutoff point in the ROC curve, the MDA index was below 2.8 in 82.1% of the controls (specificity), and it was above 2.8 in 64.2% of the Individuals with diabetes (sensitivity).
Conclusion
The simultaneous assessment of salivary oxidative and antioxidant factors, revealed weak but a significant positive association between MDA and glycemic status in diabetes. However, further investigations are required to confirm our results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is one of the most common metabolic diseases. The incidence of type 1 and type 2 diabetes is increasing, although the incidence of type 2 diabetes is increasing more rapidly[1]. It is estimated that 10% of the population are prediabetes subjects, with only a third being aware of their disease [2]. In Iran, the prevalence of type 2 diabetes fluctuates in reports, but it is estimated to be 14.4%[3].
Poor glycemic control, hyperinsulinemia, and insulin resistance are associated with increased oxidative stress due to increased production of reactive oxygen species (ROS) by several biochemical mechanisms as well as disruption of the antioxidant defense mechanism [4, 5]. As a consequence, diabetes complications and tissue damage in the course of the disease increase. Therefore, evaluating oxidative balance factors in patients with diabetes and prediabetes can be a good criterion for evaluating the activity and severity of disease complications. Saliva is a good candidate for disease monitoring since sampling of saliva is simple and noninvasive[6]. Numerous studies have been performed on the serum and saliva of Individuals with diabetes compared with healthy individuals. However, these studies are often on a serum basis, and prediabetes patients have been less studied. Among the factors used to assess the body’s oxidative balance, Malondialdehyde (MDA) is an important indicator of changes in oxidative stress and for assessing oxygen free radicals [7, 8]. Moreover, it is the main and most useful index for assessing lipid peroxidation. MDA acts as a signaling messenger to regulate insulin secretion [7]. On the other hand, antioxidants like uric acid, superoxide dismutase (SOD), and glutathione peroxidase in high concentrations prevent the oxidation of fats, proteins, carbohydrates and DNA [9].
The total antioxidant capacity (TAC) is a measure of the body’s antioxidant status, which characterizes the antioxidant response against free radicals, and is the result of the cumulative effects of antioxidants in the body and its fluids. Determining the patient’s oxidative status sometimes requires invasive measures such as taking a blood sample. It is therefore important to examine the saliva for antioxidant markers and oxidative stress in type 2 diabetes and to find markers that accurately assess the severity of the oxidative stress and the diabetes status [9].
Some studies have identified oxidative stress as a quick way to track clinical manifestations of diabetes, which can also help determine the status of atherosclerosis and cardiovascular complications in the patients [7, 10]. Although the correlation between serum oxidative status and glycemic control status in diabetes has been investigated in some studies, few studies have examined the correlation between salivary and glycemic control factors. Shirzay et al. reported an inverse relationship between salivary MDA and glycemic control (HbA1C) [8]. In Abdolsamadi et al. study the level of salivary antioxidants such as SOD had a significantly positive association with the severity of diabetes [11]. Honarmand et al. however, showed the correlation between the total antioxidant capacity of saliva and FBS to be insignificant in diabetes [12]. In addition, in Mussaravi et al. an inverse relationship between total antioxidant activity and blood sugar level was reported [13].
Given the partially contradicting results of one or more salivary factors in previous studies, we studied salivary oxidative and antioxidant factors simultaneously. Moreover, to the best of our knowledge, by now, no study has been carried out on salivary oxidative stress factors in prediabetes subjects. In the present study, evaluation of salivary oxidative stress factors in three groups of diabetes, prediabetes, control was performed.
Furthermore, in our research, all of diabetes patients were newly-diagnosed, in contrast, to other previous studies in which patients were under anti-diabetic drug therapy and the duration of treatment was not clarified.
Antioxidants act cooperatively and employ a series of redox reactions; thus, quantitative measurement of TAC may provide more relevant biological information than measurement of individual components. Therefore, in the present study, we examined salivary factors, including TAC, SOD and MDA, (which determine oxidative and antioxidant status) in the three groups of diabetes, prediabetes, and control subjects, and their correlation with glycemic control indices.
Materials and methods
This analytical cross-sectional study was conducted between 2019 and 2021 at the Endocrinology Clinic of Imam Reza Hospital, and the Oral Medicine Department of Faculty of Dentistry in Mashhad, Iran. All newly diagnosed patients were included in the study during this period.
The study was explained to the participants and written informed consent was obtained. The study was approved by the Ethics Committee of Mashhad University of Medical Sciences (registration.
number: IR.MUMS.DENTISTRY.REC.1398.118).
Of the total of 100 participants aged 35 to 65 in the study, 30 were in the prediabetes group, 31 in the diabetes group, and 39 healthy people in the control group. FBS of 100- 125 mg/dl, two-hour sugar of 140–199 mg/dl, or HbA1C of 5.7–6.4% were considered as prediabetes, while FBS 126 ≤ mg/dl, BS2hpp of > 200 mg/dl, or HbA1C of ≥ 6.5 were considered as diabetes[1].
Inclusion criteria were newly diagnosed of diabetes who received no medication or had been untreated for 3 months. Exclusion criteria were the use of medications that affect blood sugar, and fat or cause dry mouth such as antidepressants, use of supplements, smoking, drugs and alcohol consumption, pregnancy, oral and dental problems such as periodontitis, oral lesions and salivary gland disorders, as well as known systemic diseases such as heart disease, diabetes mellitus, kidney disease (GFR < 60), liver disease, hepatitis, pulmonary tuberculosis, acute and chronic infectious diseases, rheumatic diseases and cancer [7].
Blood pressure measurement
Systolic and diastolic blood pressure were measured after 10 min in a sitting position with a calibrated digital blood pressure monitor.
Intraoral examination
The periodontal examination was performed using a Williams probe and mirror under normal dental unit light. The periodontal status was determined using the Community Periodontal Index for Treatment Needs (CPITN) [14]. Subjects were excluded from the study if they had moderate to severe pockets,Footnote 1 bleeding during probing in more than 30% of the areas taking the BOP into account, or bleeding when brushing their teeth [14]. Inclusion criteria were natural gum color (pink) and no edema. Even if there was a pathological lesion in the mouth, the person was excluded from the study.
labLaboratorysts
To collect blood samples, people had to fast for 10 to 12 h. Five ml of blood was taken from the brachial artery. After serum separation, FBS, BS2hpp, as well as fasting insulin were assessed based on available kits instruction (Man Company, Tehran, Iran), then were assessed by Mindray BS-800 M, and colorimetric method. HbA1C factors were evaluated by electrophoresis method.
Recording insulin indicators
Insulin levels, HOMA-IR and HOMA-B
In this study, insulin resistance was determined by measuring insulin level > 17 U/ml or HOMA-IR > 2.6 [15]. The HOMA-IR index was calculated using the formula: Insulin × Glucose (mg/dl) / 405. The HOMA-B index was used to determine the activity of healthy pancreatic beta cells using the following formula: Insulin × 360 / (Glucose − 63) × 100 [15].
Salivary examination
Saliva samples were taken from fasting patients between 8 a.m. and 9 a.m. Patients were advised not to drink water or fluids one hour before saliva sampling. The patient was asked to sit on a chair and collect 5 ml of unstimulated saliva in a sterile Falcon tube by spitting. The sample was immediately placed in a box containing a dry ice pack and transported to the laboratory. After saliva separation, it was stored in the freezer at − 80 °C [8].
The measurements of SOD, MDA and TAC were carried out according to standards with the available kits (Teb Pazhouhan Razi, Tehran, Iran).
In order to measure TAC, TAC Assay Kit (Teb Pazhouhan Razi, Tehran, Iran) was utilized, which is based on the oxidation of a peroxidase chromogenic substrate by ferryl myoglobin radicals and production of a water-soluble chromogen. The rate of green chromogen formation is inhibited by antioxidants that can be measured photometrically.
To measure MDA level, MDA assay Kit (Teb Pazhouhan Razi, Tehran, Iran) was used and its instruction was followed. The assay is based on the reaction between MDA and thiobarbituric acid (TBA) and generation of a MDA-TBA adduct, which can simply quantified.
In order to measure SOD activity, SOD assay kit (Teb Pazhouhan Razi, Tehran, Iran) was used. This kit measures SOD activity using tetrazolium salts. After reduction with superoxide anion, it produces water-soluble dyes. The rate of formazan formation is inhibited by the presence of SOD in the medium and can be measured by photometry.
To assess the salivary uric acid, saliva sample was examined by colorimetric method.
Statistical analysis of data
The mean and standard deviation were used to describe normally distributed quantitative data, while the median and interquartile range were used otherwise. In the case of normality, one-way analysis of variance was used for group comparisons, while the Kruskal-Wallis test was used for the non-normally distributed data. The number and percentage were used to describe qualitative data, and the Fisher and Chi-square tests were used for analysis. After data analysis, a logistic regression test was used to control confounders and variables that were heterogeneous between groups. The correlation between the measured factors was determined based on the Pearson correlation coefficient for normal data and the Spearman correlation coefficient otherwise. The data were statistically analyzed with SPSS 16, and a significance level of p = 0.05 was considered. The ROC curve was used to determine the diagnostic power of oxidative factors.
Results
In this study, we investigated the relationship between salivary oxidative stress markers and their association with HbA1C and insulin resistance in 100 individuals.
There was a significant difference between the groups in terms of age. In fact, the difference between the diabetes and control groups was significant, and the mean age in the diabetes group was higher (p = 0.006). There was no significant difference in gender between the groups (p = 0.51) (Table 1).
There was a significant difference between the groups in terms of insulin levels (p = 0.003), and the level was higher in the diabetes group. The HOMA-IR level differed significantly between the groups (p < 0.001), and was higher in the diabetes group. There was also a significant difference between the groups in terms of HOMA-B (beta cells function), and the level was higher in the control group (p < 0.001) (Table 1).
The differences between three groups according to salivary factors SOD and TAC as well as the salivary uric acid were not statistically significant (p = 0.06, p = 0.059, and p = 0.25, respectively). However, the MDA level was higher in the diabetes group than in the other two and the difference was significant (p = 0.001) (Table 1).
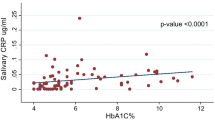
The salivary factor SOD had a significantly positive correlation with FBS (p = 0.003, rs = 0.29), and HbA1C (p = 0.01, rs = 0.23 ). The salivary factor MDA also had a significantly positive correlation with FBS (p < 0.001, rs = 0.43), HbA1C (p < 0.01, rs = 0.34) and BS2hpp (p = 0.03, rs = 0.3) in all subjects. This means that as FBS and HbA1C increase, the levels of MDA and SOD also increase. Furthermore, the salivary TAC had a significantly positive correlation with FBS and BS2hpp (both p = 0.02, both rs = 0.23). No significant correlation between salivary and glycemic factors was found in the separate analysis of the three groups. In the paired group of diabetes and prediabetes, MDA had a significant positive correlation with FBS and BS2hpp (p = 0.001, rs = 0.4 and p = 0.04, rs = 0.27 respectively) (Table 2).
The correlation test showed that SOD and MDA levels had a significantly negative correlation with HOMA-B in all subjects, which means that the function of pancreatic beta cells decreases as these salivary markers increase (p = 0.01, rs=-0.24 and p = 0.008, rs=-0.27 respectively). However, TAC had a significantly positive correlation with HOMA-IR (p = 0.02, rs = 0.23) (Table 2).
SOD showed a negative correlation with insulin in the diabetes group (p = 0.03, rs=-0.37) and also a significantly negative correlation with HOMA-IR, and insulin (both p = 0.04, rs=-0.32) in the control group. The SOD and MDA levels had a negative correlation with HOMA-B (both p = 0.04 m both rs=-0.26) in the both of diabetes, prediabetes, groups (Table 2).
Nominal regression analysis with adjusting age and sex as confounding variables, revealed that only salivary MDA level was significantly higher in the diabetes group than in the control group, so that by a per unit increase in the MDA level, it was 6.3 times more likely to have diabetes (p = 0.006, OR = 6.39(1.723–23.727)). While in the diabetes group, the factors of SOD, and TAC did not change the chance of developing diabetes according to P-value, and odds ratio (p = 0.46, p = 0.35, respectively) (Table 3).
In the prediabetes group, the amount of SOD, TAC, and MDA had no effect on the chance of developing prediabetes (p = 0.39, p = 0.33, and p = 0.40 respectively) (Table 3).
According to the cutoff point in the ROC curve, the MDA index was below 2.8 in 82.1% of subjects in the control group (specificity) and it was above 2.8 in 64.2% of subjects in the diabetes group (sensitivity) (Fig. 1).
Discussion
In this cross-sectional study, the difference was only significant for the oxidative factor MDA. So that by controlling the effect of confounders, a per unit increase in the MDA level resulted in 6.3 times higher chance of developing diabetes. MDA is a factor for oxidative stress and its level is therefore expected to increase in diabetes group compared to the control group. Consistently with the results of studies on saliva, other studies which compared serum MDA levels in diabetes group with healthy subjects came to the same conclusion [16, 17].
Similar to our study, some studies have been carried out on the salivary MDA and have shown that the MDA level has been significantly higher in diabetes group than in control group [4, 8, 9, 17]. In Abdolsamadi et al. study the salivary level of SOD in type I diabetes, and in Al-Rawi, the salivary level of uric acid was significantly higher in diabetes group compared to control group [9, 11]. In the present study, the differences between three groups based on uric acid and SOD levels were not significant.
In Madi et al. study the SOD level increased significantly in control group compared to diabetes group [18].
Although it seems that antioxidant defense capacity is reduced in individuals with diabetes, which leads to multiple complications, it is generally believed that salivary and serum TAC to be lower in individuals with diabetes than healthy subjects, according to most studies [12, 19,20,21]. However, in our study, there was no significant difference between the three groups for the level of salivary TAC. Also, Lin et al. reported a higher TAC level in diabetes group compared to control group [22]. The increased salivary antioxidants TAC, uric acid and SOD in diabetes group in the present study may be due to the increased production of antioxidants to compensate and restore the oxidative balance by increasing free radicals and oxidative stress [9]. In other words, oxidative conditions in diabetes lead to a compensatory increase in antioxidant factors. In addition, such an increase can also be due to diet and high consumption of natural and medicinal antioxidants.
There are limited studies on the relationship between salivary factors and glycemic control. Among the salivary factors in the present study, the MDA and SOD in all subjects had a significantly positive correlation with FBS and HbA1C, and the TAC with FBS. However, no significant correlation was found between salivary and glycemic factors in each group alone. Only in diabetes group the salivary SOD was reciprocally related to insulin. The salivary factors did not correlate significantly with the glucose indices in the diabetes group, presumably due to the small number of samples. However, a significantly positive correlation was observed between MDA and BS2hpp as well as FBS in the both of diabetes and prediabetes groups.
The results for MDA in Shirzaiy et al. study are in line with the present study, although they considered only two groups ,diabetes group versus healthy subjects [8]. Abdolsamadi et al. reported a significantly positive association between the SOD level and HbA1C in type 1 diabetes [11]. Honarmand et al. showed, however, that the correlation between the TAC level and FBS was not significant in the diabetes group[12]. In Bouhajja et al. the serum MDA levels correlated significantly positive with FBS and HbA1C in prediabetes and control groups [23]. Mahat et al. showed a significant correlation between serum MDA levels and FBS in prediabetes group[2]. Based on the above results, it seems that salivary factor MDA has a more established correlation with glycemic control status in most studies.
Diabetes is a multifactorial disease, which is related to various factors, including race, diet, and the environment. On the other hand, the salivary TAC can be affected by factors such as genetics, food intake, diet, intake of medicinal or natural antioxidant supplements, smoking, physical activity, hormones, and stress. In addition, hyperglycemia appears to disrupt the antioxidant system and produce more oxygen free radicals, which accelerates the peroxidation of fatty acids and nitrogen, leading to cell membrane damage and death.
Decreased antioxidants and increased oxidative stress such as MDA destroy fatty acid double bonds, further damage cells and complicate diabetes [8]. On the other hand, some studies have examined the relationship between these markers and glycemic control and concluded that oxidative stress decreases, while antioxidants increases [8, 12]. In our study, all of diabetes were new cases and therefore it appears that as fat peroxidation increases, so do antioxidants to compensate for or adopt to these conditions.
Our study showed that the salivary factors SOD and MDA had a significantly inverse correlation with HOMA-B in all subjects as well as in the paired of diabetes and prediabetes groups. Moreover, the TAC level had a significantly positive correlation with HOMA-IR in all subjects. The SOD level also showed a significantly inverse correlation with insulin levels in the diabetes group.
To the best of our knowledge, our study is the first to investigate the relationship between insulin resistance and oxidative stress markers in saliva. The only study with a similar result, albeit on a serum basis, is Bouhajja et al., in which the serum levels of MDA showed a significantly positive correlation with fasting insulin and HOMA-IR when all subjects, of prediabetes, diabetes and control groups were considered together as a group [23].
Insulin resistance and secretion deficiency are the main pathogens of diabetes. As long as pancreatic beta cells are healthy, insulin resistance is balanced out by increased insulin production. Therefore, it can take about 5 years or less from the onset of insulin resistance and beta cell damage, and the onset of diabetes. Hence, insulin resistance can be a primary deficiency that is partially compensated by increasing insulin secretion. Early detection of this stage makes a significant contribution to impede the progress of diabetes and its complications [24]. However, calculating the relative sample size is the limitation of our study. By assessment of changes in salivary markers based on the present study results, it may be possible to monitor changes in insulin resistance or production at an early stage.
Consequently, a change in MDA concentration might be used as an indicator of oxidative stress in assessment of individuals with diabetes.
Conclusion
The simultaneous assessment of salivary oxidative and antioxidant factors, revealed weak but a significant positive association between MDA and glycemic status in diabetes group. However, further investigations are required to confirm our results.
Notes
Dental pockets are classified as normal to mild (0–3 mm), moderate (4–6 mm), and severe (> 6 mm).
References
Alvin C, Kevin D, Carmella E-M. Diabetes Mellitus: Diagnosis, Classification, and Pathophysiology. In: JamesonL, Harrison TR, Kasper D, Longo D, Fauci A, Hauser S et al. Editors. Harrison’s principles of internal medicine. New York: McGraw-Hill Education; 2018. pp. 2850_2903.
Mahat RK, Singh N, Rathore V, Arora M, Yadav T. Correlates of oxidative stress and inflammation with glucose intolerance in prediabetes: a cross-sectional study. Diabetes Metab Syndr. 2019;13:616–21. https://doi.org/10.1016/j.dsx.2018.11.045.
Salehi K, Kordlu A, Rezapour-Nasrabad R. Prevalence of Type 2 Diabetes in Population Over 30 Years Old (2017–2018). Ethno Med. 2020;14:24–9. https://doi.org/10.31901/24566772.2020/14.1-2.602.
Rajeshwari SG, Choudhry AA, Guruaja A, Prabhu K. Correlation of plasma lipid profile with salivary oxidative stress markers in Type II diabetes mellitus patients. J Clin Diagn research: JCDR. 2014;8:CC08.
Desai GS, Mathews ST. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J diabetes. 2014;5:730. https://doi.org/10.4239/wjd.v5.i6730.
Amirchaghmaghi M, Delavarian Z, Ghazi A, Hashemy SI, Mozaffari Jovin S, Badzaei S, et al. Zinc Finger Protein 510 Levels in the Saliva of Patients with Oral Lichen Planus Compared with Healthy Controls. J Kerman Univ Med Sci. 2021;28:471–7. https://doi.org/10.22062/jkmu.2021.91764.
Mahat RK, Singh N, Rathore V. Association of myeloperoxidase with cardiovascular disease risk factors in prediabetic subjects. Diabetes Metab Syndr. 2019;13:396–400. https://doi.org/10.1016/j.dsx.2018.10.016.
Shirzaiy M, Dalirsani Z. The effect of glycemic control on salivary lipid peroxidation in type II diabetic patients. Diabetes Metab Syndr. 2019;13:1991–4. https://doi.org/10.1016/j.dsx.2019.04.004.
Al-Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diab Vasc Dis Res. 2011;8:22–8. https://doi.org/10.1177/1479164110390243.
Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11:45–63.
Abdolsamadi H, Mortazavi H, Goodarzi M, Ahmadi Motamayel F, Rahmani M, Moghim Beigi A. Evaluation of salivary antioxidants in type 1 diabetics. Iran J Endocrinol Metabolism. 2012;14:8.
Honarmand M, Nakhaie A, Farhadmollashahi L, Abuchenari J. Comparison of total antioxidant capacity of saliva in type2 diabetic patients and healthy persons referring to zahedan Ali Asghar hospital. Iranan South Medical. 2013;16(3):225–32.
Mussavira S, Dharmalingam M, Sukumaran BO. Salivary glucose and antioxidant defense markers in type II diabetes mellitus. Turk J Med Sci. 2015;45:141–7. https://doi.org/10.3906/sag-1306-116.
Zimmermann H, Hagenfeld D, Diercke K, El-Sayed, Fricke J, Halina Greiser K, et al. Pocket depth and bleeding on probing and their associations with dental, lifestyle, socioeconomic and blood variables: a cross-sectional, multicenter feasibility study of the German National Cohort. BMC Oral Health. 2015;15:1–9.
Gateva A, Assyov Y, Velikova T, Kamenov ZA. Increased peroxiredoxin 4 levels in patients with prediabetes compared to normal glucose tolerance subjects. Clin Endocrinol (Oxf). 2016;85:551–5. https://doi.org/10.1111/cen.13135.
Bhutia Y, Ghosh A, Sherpa ML, Sherpa ML, Pal R, Mohanta PK. Serum malondialdehyde level: Surrogate stress marker in the Sikkimese diabetics. J Nat Sci Biol Med. 2011;2:107–12. https://doi.org/10.4103/0976-9668.82309.
Smriti K, Muralidhar Pai K, Ravindranath V, Pentapati KC. Role of salivary malondialdehyde in assessment of oxidative stress among diabetics. J oral biology Craniofac Res. 2016;6:42–5.
Madi M, Babu S, Kumari S, Shetty S, Achalli S, Madiyal A, et al. Status of Serum and Salivary Levels of Superoxide Dismutase in Type 2 Diabetes Mellitus with Oral Manifestations: A Case Control Study. Ethiop J Health Sci. 2016;26:523–32. https://doi.org/10.4314/ejhs.v26i6.4.
Lotfi MH, Saadati H, Afzali M. Prevalence of diabetes in people aged ≥ 30 years: the results of screen-ing program of Yazd Province, Iran, in 2012. J Res health Sci. 2013;14:88–92.
Fathi S, Borzouei S, Goodarzi S, Poorolajal MT. Evaluation of Salivary Antioxidants and Oxidative Stress Markers in Type 2 Diabetes Mellitus: A Retrospective Cohort Study. Endocr Metab Immune Disord Drug Targets. 2020;20:584–90. https://doi.org/10.2174/1871530319666191016103222.
Ahmadi-Motamayel F, Fathi S, Goodarzi MT, Borzouei S, Poorolajal J, Barakian Y. Comparison of Salivary Antioxidants and Oxidative Stress Status in Gestational Diabetes Mellitus and Healthy Pregnant Women. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders). 2021;21:1485–1490.
Lin TK, Chen SD, Wang PW, Wei YH, Lee CF, Chen TL. Increased oxidative damage with altered antioxidative status in type 2 diabetic patients harboring the 16189 T to C variant of mitochondrial DNA. Ann N Y Acad Sci. 2005;1042:64–9. https://doi.org/10.1196/annals.1338.007.
Bouhajja H, Kacem FH, Abdelhedi R, Ncir M, Dimitrov JD, Marrakchi R, et al. Potential Predictive Role of Lipid Peroxidation Markers for Type 2 Diabetes in the Adult Tunisian Population. Can J Diabetes. 2018;42:263–71. https://doi.org/10.1016/j.jcjd.2017.06.006.
Rkhaya SA, Bulatova N, Kasabri V, Naffa R, Alquoqa R. Increased malondialdehyde vs. reduced sirtuin 1 in relation with adiposity, atherogenicity and hematological indices in metabolic syndrome patients with and without prediabetes. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2018;12:903–909.
Acknowledgements
This article was adapted from a thesis submitted to Mashhad Dental School. The authors would like to thank the Vice Chancellor for Research of Mashhad University of Medical Sciences for approving and financially supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pakfetrat, A., Delavarian, Z., Mohebbi, M. et al. Correlation of glycemic control with salivary oxidative markers in subjects with prediabetes and diabetes: A cross-sectional study. J Diabetes Metab Disord 22, 267–274 (2023). https://doi.org/10.1007/s40200-022-01127-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01127-8