Abstract
There is an increase in the incidence and prevalence of type-2 diabetes and obesity which leads to the structural and functional changes in myocardium leading to a lethal complication called diabetic cardiomyopathy (DCM). In the present study, we investigated the preventive effect of cinnamon (3% of Cinnamomum zeylanicum bark powder in AIN-93 diet for 3 months) feeding on DCM and the concerned mechanisms in a rodent model. Experimental diabetes was induced by a single intraperitoneal injection of 40 mg/kg b.w streptozotocin (STZ), 15 min after the ip administration of 60 mg/kg b.w of nicotinamide (NA) in Wistar-NIN (WNIN) male rats. The oxidative stress parameters were investigated by assessing superoxide dismutase (SOD), glutathione-s-transferase (GST) enzyme activity, protein carbonyls and malondialdehyde (MDA) levels. The histopathology of myocardium was analyzed by H&E and Masson’s trichrome staining, and scanning electron microscopy. The changes in diabetic rat heart involved the altered left ventricular parietal pericardium, structural changes in myocardial cells, enhanced oxidative stress. Masson’s trichrome and H&E staining have shown increased fibrosis, and perinuclear vacuolization in NA-STZ induced diabetic rat myocardium. Cinnamon feeding prevented the oxidative stress and myocardial alterations in the heart of diabetic rats. Taken together, these results suggest that cinnamon can effectively prevent the metabolic and structural changes in NA-STZ induced diabetic cardiomyopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a major health challenge faced by the both developing and developed countries in the world. According to International Diabetes Federation (IDF) the global burden of diabetes reached 463 million by 2019 and it is expected to be 700 million by 2045 [1]. Among the total diabetes people, type-2 diabetes (T2D) contributes to 90—95 percent. T2D is metabolically distinct from type-1 diabetes (T1D) with the occurrence of hyperinsulinemia during its early stages. With the disease progression hyperglycemia develops due to pancreatic β-cell loss. One in five of the people who are above 65 years old have diabetes [1]. People with diabetes have an increased risk of developing a number of serious health problems including cardiovascular disease, blindness, kidney failure, and lower limb amputation [2,3,4,5]. Cardiovascular disease is the most common cause of death in people with diabetes [2]. Hyperglycemia can trigger the blood coagulation system thereby elevating the risk of blood clots [6]. Diabetes is also associated with high blood pressure and cholesterol levels, which lead to increased risk of cardiovascular complications [7].
Myocardial changes in diabetes include at the physiological, structural and molecular level. Primary changes are due to insulin resistance and later hyperglycemia cause oxidative stress which leads to the reduced ventricular elasticity, atrial fibrillation [8], cardiac autonomic neuropathy [9], altered myocardial contractile proteins, raise in interstitial fibrosis, mitochondrial dysfunction [10], altered rennin angiotensin system [11], activation of apoptotic machinery [12], activation of Endoplasmic Reticulum (ER) stress and autophagy [13, 14].
So far therapeutic treatment is not available for diabetic cardiomyopathy (DCM). The master action of cellular insulin resistance in the pathological progress of the cardiomyopathy might be the logical point to target DCM in T2D. We hypothesized that the drugs with anti-hyperglycemic and insulin sensitizing properties can effectively target the DCM. Several dietary agents have been successfully reported for the therapeutic treatment of diabetic secondary complications like diabetic retinopathy [15, 16], diabetic cataract [17, 18], diabetic nephropathy [19], and DCM [12]. Most of the dietary agents reported are targeting diabetes and diabetic complications by inhibiting the formation of non-enzymatic glycation and sorbitol, reducing oxidative stress and lowering the blood glucose levels [12, 17, 20,21,22].
Medicinal plants possess strong antioxidants and efficient anti-diabetic compounds of various classes like flavonoids, tannins, phenols, and alkaloids that improve glucose utilization by various diverse mechanisms [23, 24]. Cinnamon (bark of tree species from the genus Cinnamomum) is a well-known spice for its medicinal properties and widely used as dietary ingredient in Asian countries. Cinnamon is reported to have anti-diabetic property [21]. The recent reports have been revealing the potential of cinnamon in treating the T2D, prediabetes and also its antihypertensive, antihyperglycemic effects [25]. Consuming 500 mg/day of cinnulin (water-soluble cinnamon extract) in prediabetic men and women prevented the metabolic syndrome [25]. Intravenous administration of extract of Cinnamomum zeylanicum stem bark can reduce the mean atrial blood pressure in N-nitro-L-arginine methyl ester induced hypertensive rats [26]. Some studies also suggest that cinnamon can mimic insulin [27]. It has been determined that cinnamon reduces fasting blood glucose and regulates systolic blood pressure in the prediabetic humans [25], postprandial glucose in rats and acts as an alpha glucosidase inhibitor [27]. Previously we have evaluated a number of dietary sources and found that cinnamon has significant potential in inhibiting advanced glycation end products (AGEs) formation under in vitro conditions [28]. Based on bioassay-guided fractionation we have characterized procyanidin-B2 as the active component of cinnamon that is involved in AGEs inhibition [18]. In the present study, we have investigated the effect C. zeylanicum on diabetes-induced cardiomyopathy using nicotinamide-streptozotocin (NA-STZ) rat model.
Materials and methods
Materials
Streptozotocin (STZ), β-mercaptoethanol, SDS, TEMED, NaN3, pyrogallol, TBA, DETPA, 1-chloro-2,4-dinitro benzene (CDNB), 2,4-dinitro phenyl hydrazine (DNPH), GSH, nicotinamide, anti-actin antibody (Cat.No-A5060), horse radish peroxidase (HRP) conjugated anti-rabbit (A6154) and anti-mouse (A9044) secondary antibodies were purchased from Sigma Chemicals (St. Louis, MO, USA). Anti-VEGF antibody was purchased from Santa Cruz Biotechnology, Inc. (Dallas, Texas, USA). Nitrocellulose membrane (Pall Corporation, Pensacola, FL, USA), Tri sodium citrate, citric acid, MOPS, sodium acetate, butyl hydroxy toluene (BHT), TCA, TEP (tetra ethoxy propane), ethanol, ethyl acetate, urea, Tris, EDTA are obtained from local chemicals suppliers. Alexafluor-555 conjugated anti-mouse (A-21427) antibody was obtained from Molecular Probes, Inc. (Eugene, OR, USA).
Experimental design
Two-month-old male Wistar-NIN (WNIN) rats weighing around 180–200 g were obtained from the Animal Facility of the National Institute of Nutrition, Hyderabad, India, and maintained at a temperature of 22 ± 20C, 50% humidity, and 12-h light/dark cycle. Diabetes was induced in overnight fasted rats by a single intraperitoneal (ip) injection of 40 mg/kg b.w of STZ, 15 min after the ip administration of 60 mg/kg b.w of nicotinamide. Another set of rats, which received only vehicle (0.1 M citrate buffer, pH 4.5) served as the control. Fasting and postprandial blood glucose levels were measured 10 days after NA-STZ injection. After one month of NA + STZ injection, animals having high postprandial glucose (> 140 mg/dl) were considered as diabetic. A subgroup of diabetic animals was fed with 3% cinnamon powder (bark of C. zeylanicum) in AIN-93 diet. The dosage of C. zeylanicum is fixed based on our earlier work [16, 29]. Thus, the rats were categorized into three groups of seven each: control (C), untreated diabetic (D) and diabetic rats fed with bark of C. zeylanicum (D + Cin) groups. Experiment was continued for another three months. All the rats were fed with AIN-93 diet ad libitum. Animal care and protocols were in accordance with and approved by the Institutional Animal Ethics Committee (IAEC) of National Institute of Nutrition. Animals were sacrificed at the end of experimental period and heart tissue was collected and frozen at -80C° until further analysis.
Biochemical and physiological parameters
Fasting and postprandial plasma glucose levels were analyzed every month. The postprandial plasma glucose levels were estimated 2-h after the oral feeding of glucose as a bolus at 2 g/kg b.w to overnight fasted rats. Glucose and glycosylated hemoglobin (HbA1c) in plasma were measured by the glucose oxidase–peroxidase (GOD–POD) method [30] and ion-exchange resin, respectively, using commercially available kits (Bio systems, Barcelona, Spain) and plasma insulin by RIA kit (BRIT-DAE) Mumbai, India) method as per the manufacturer’s instructions [31].
Antioxidant enzymes
Snap-frozen heart tissues were homogenized in 50 mM sodium phosphate buffer (pH 7.4) to prepare 10% homogenate and centrifuged at 10,000 g for 15 min. Supernatant was collected to monitor the myocardial oxidative stress by measuring the specific activity of superoxide dismutase (SOD) [32], glutathione-s-transferase (GST) [33] and protein carbonyls [34] according to the previously reported methods.
Lipid peroxidation assay
Malondialdehyde (MDA), the end product of polyunsaturated fatty acid lipid peroxidation, was estimated by its reactivity with 2-thiobarbutyric acid (TBA). Total tissue homogenate was used to determine the MDA levels in myocardial tissue according to the reported method [35].
Scanning electron microscopy
At the end of the experiment, animals were sacrificed and left ventricle of heart was collected and fixed in adequate Karnowsky’s fixative (pH 7.2) for 24 h at 4 °C. Later washed three times with 0.1 M phosphate buffer (pH 7.2) for 15 min each, then dehydrated with 50%, 70%, 80%, 90% of acetone each for 30 min followed by 100% acetone with two repeats. After dehydration, samples were dried under vacuum, molded on aluminum stubs then coated with 600A° thickness of gold at 10–2 Torre pressure (E1010 sputter coating unit) and observed under scanning electron microscope (SEM) instrument (S3400N Hitachi) [36].
Whole tissue lysate preparation
Heart tissue (100–200 mg) was homogenized in TNE buffer (pH 7.5) containing 20 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM DTT and protease inhibitors. Homogenization was performed on ice using a glass homogenizer and the homogenate was centrifuged at 14,000 g at 4 °C for 20 min. The protein concentrations were measured by Lowry Method.
SDS-PAGE and immunoblotting
Equal amounts of protein were subjected to 12% SDS-PAGE and transferred to nitrocellulose membrane (0.22 µm pore size) by western blot transfer system (Bio-Rad, USA) at a voltage of 40 V for 2 h. Nonspecific binding was blocked with 5% nonfat dry milk powder in PBST (20 mM phosphate buffer; pH 7.2, 137 mM NaCl, 0.1% Tween 20) and incubated overnight at 4 °C with anti-VEGF (1:1000) and anti-actin (1:500) antibodies diluted in PBST. After washing with PBST, membranes were incubated with anti-rabbit IgG (1:3500) or anti-mouse IgG (1:3500) secondary antibodies conjugated to HRP. The immunoblots were developed using ECL detection kit (RPN2232, GE Health Care, Buckinghamshire, UK) by Image analyzer (G-Box iChemi XR, Syngene, UK) and images were analyzed and quantitated using imageJ software (available in the public domain at https://rsbweb.nih.gov/ij/).
Morphological analysis and immunohistochemistry
The harvested tissue was immediately placed in 4% paraformaldehyde in phosphate buffer (pH-7.2), fixed overnight, embedded in paraffin blocks, and cut into 4 µm sections and used for Haemotoxylin and Eosin (H&E) staining, Masson’s Trichome staining, immunostaining with specific antibodies. Sections for immunostaining were deparafinized by incubating in xylene for 5 min followed by dehydration in decreasing grades of ethanol. Deparafinized sections were boiled in 0.01 M Na-citrate pH 6.0 for 10 min at 60 °C and blocked with blocking solution (3% horse serum, 3% BSA, 0.3% TritonX) in PBS. Slides were washed 3 times with PBS and incubated with anti-VEGF antibody (1:1000) in PBS overnight at 4 °C. Sections were washed 3 times with PBS and the binding of primary antibody was visualized by Alexafluor-488 conjugated anti-rabbit (1:1000) IgG antibody for 1 h. Sections were mounted in medium containing 4, 6-diamidino-2-phenylindole (DAPI; # 1500, Vector Laboratories, Burlingame, CA, USA) and visualized using a Leica laser microscope (LMD6000, Leica microsystems, Germany).
Statistical analysis
The data is expressed as the mean ± SEM. Statistical evaluation of the data was determined by performing the one-way ANOVA followed by Mann Whitney test. The p-value less than 0.05 was considered significant.
Results
Effect of C. zeylanicum on metabolic changes in diabetic rats
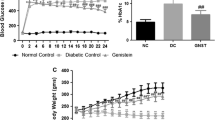
Diabetic rats have shown reduced body weight in spite of increased food intake when compared to controls. Feeding of C. zeylanicum to diabetic rats could not improve the body weight (Fig. 1a). There was a significant decrease in the heart weight of diabetic rats when compared with control rats. However, heart weight was significantly (50%) increased in C. zeylanicum fed diabetic rats compared to untreated diabetic rats (Fig. 1b). Diabetic rats have shown increased postprandial glucose levels (> 140 mg/dL) but normal fasting plasma glucose levels similar to early stages of T2D (Fig. 2b&a). Though fasting plasma glucose levels were comparable in C. zeylanicum fed diabetic rats, there was significant (28.6%) reduction in postprandial glucose levels when compared to untreated diabetic rats (Fig. 2b&a). There is slight decrease in insulin levels in diabetic rats as compared to control. Though, cinnamon feeding has not altered insulin levels at initial period of experiment, there was a gradual increase in insulin levels in C. zeylanicum fed diabetic animals which was increased significantly by third month (Fig. 2c). The HbA1c levels were significantly increased in untreated diabetic rats, however, C. zeylanicum feeding prevented the rise in HbA1C by 44.45%, though the difference is statistically insignificant (Fig. 2d).
(a) Body weight and (b) heart weights of control, diabetic and cinnamon fed diabetic rats. Body weights were measured at each month after NA + STZ administration. Heart weights were measured at the end of the experiment. Data represent mean ± SEM for control, (D) diabetes and (D + Cin) diabetes + cinnamon (n = 7). p < 0.05 considered significant
C. zeylanicum prevents the pericardial damage in diabetic heart
Scanning electron microscopic images of left ventricular region of heart showed the pericardial damage in diabetic rats but it was ameliorated by C. zeylanicum feeding (Fig. 3b). Further, to support this, we have also done H&E staining on paraffin tissue sections of the heart. The H&E staining showed the pericardial detachment in left ventricles of diabetic heart and this pericardial detachment was prevented in C. zeylanicum fed animals (Fig. 3a).
Representative morphology of rat heart analysed by H&E staining and electron microscope. Left ventricular myocardial cross sections of control (I), diabetic (II) and cinnamon fed diabetic rat (III) heart was stained with H&E (a). (Magnification = 63x); arrow ‘A’ indicates the damage of outer most pericardial layer of the diabetic heart which was successfully prevented by cinnamon feeding. (b) Representative pictures of heart left ventricular outer surface by SEM. (Magnification = 4000x), Scale bars 10 µm; Arrow ‘B’ indicates the damage of left ventricular outer surface of the diabetic heart which was ameliorated by cinnamon
C. zeylanicum prevents the perinuclear vacuolization, interstitial fibrosis and arteriolar smooth muscle fibrosis
Morphological analysis of myocardium was done by H&E, Masson’s trichrome staining. Diabetic rats have shown perinuclear vacuolization, increased interstitial, arteriolar and smooth muscle fibrosis whereas these changes were markedly prevented in C. zeylanicum fed rats (Fig. 4). Furthermore, arteriolar smooth muscle loss was also inhibited in cinnamon fed diabetic rats.
Representative histology of rat heart analyzed by H&E and Masson’s trichrome staining. Left ventricular myocardial cross sections of control (I), diabetic (II) and cinnamon treated rat (III) heart was stained with H&E (a). Arrow ‘A’ indicates the perinuclear vacuolization. (b) Left ventricular myocardial cross sections of control (I), diabetic (II) and cinnamon treated rat (III) heart stained with Masson’s trichrome. Arrow ‘A’ and ‘B’ indicates increased interstitial space as well as fibrosis stained in blue. (c) Left ventricular myocardial transverse sections of control (I), diabetic (II) and cinnamon treated rat (III) heart was stained with Masson’s trichrome. (Magnification = 63x), Scale bars 50 µm; arrow ‘A’ indicates the increased fibrosis stained in blue. Arrow ‘B’ indicates the increased interstitial space and arrow ‘C’ indicates the perinuclear vacuolization
C. zeylanicum prevents the oxidative stress in diabetes
To determine the effect of cinnamon on oxidative stress in diabetes, we investigated the specific activities of SOD, GST, lipid peroxidation (MDA levels), and protein oxidation (protein carbonyls) in the heart. The activity of SOD was decreased in the heart of untreated diabetic rats and significantly increased after the treatment with C. zeylanicum (Fig. 5a). Further, activity of GST was increased in untreated diabetic group which was significantly improved in C. zeylanicum fed rats (Fig. 5b). On the other hand, MDA and protein carbonyls were increased in untreated diabetic rats and significantly decreased in C. zeylanicum fed diabetic rats (Fig. 5c-d). Decreased activity of SOD, increased activity of GST and increased levels of MDA and protein carbonyls in diabetic heart suggest increased lipid peroxidation, oxidative stress and decreased antioxidant potential in comparison with control rats which were improved by treatment with C. zeylanicum (Fig. 5).
Oxidative stress parameters in rat heart. (a) Superoxide dismutase activity. (b) Glutathione-s-transferase activity. (c) Protein carbonyls in control, diabetic and cinnamon treated rat heart. (d) Malondialdehyde content for g heart tissue in control, diabetic and cinnamon treated rats. Data represent mean ± SEM for control (n = 4), diabetes (n = 4) and diabetes + cin (n = 4). p < 0.05 considered significant
C. zeylanicum increases the angiogenic marker VEGF in diabetic heart
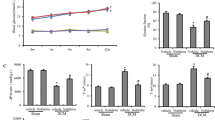
Diabetes significantly decreased the vascular endothelial growth factor (VEGF) protein expression by 70.6% when compared with control (Fig. 6a). To support the immunoblotting of VEGF, we have also shown immunostaining for VEGF on paraffin tissue sections of heart. Decreased immunostaining of VEGF was found in diabetic rat heart (Fig. 6b). Interestingly, C. zeylanicum feeding has significantly improved VEGF protein expression by 51.08% in diabetic heart (Fig. 6).
Expression of VEGF protein in rat heart. (a) Representative immunoblot and quantification bars demonstrating the levels of VEGF. Expression was normalized with actin and results are represented as percent of control. Data represent as mean ± SEM for control, diabetes and cinnamon treated. p < 0.05 considered significant. (b) Paraffin tissue cross sections of control, diabetes and cinnamon treated rat heart were labeled with DAPI (blue), anti-VEGF antibody (red), merged (blue + red). (n = 4). Magnification = 63x. Scale bars 50 µm
Discussion
Numerous studies throughout the world indicate the epidemic drift of diabetes and its expanded threat for both developing and developed countries. Cardiomyopathy is one of the major diabetic complications responsible for increasing mortality of diabetic patients. Therefore, prevention of diabetic complications plays a significant role in extending the life span of diabetic patients in a healthy way. The role of medicinal plants in the treatment of diabetes and its complications is well appreciated, however, only few studies exist on cardiomyopathy.
In the present study, C. zeylanicum (cinnamon) feeding to diabetic rats significantly reduced the postprandial glucose levels compared to untreated diabetic rats and thereby decreased the HbA1c levels. The glucose lowering activity of C. zeylanicum bark is well evident in the literature both in animals and humans [37, 38]. Chemical constituent analysis of C. zeylanicum bark by earlier workers revealed several constituents responsible for its glycemic control efficacy [39, 40]. The major constituents were found to be cinnamaldehyde (65 to 80%), eugenol (5 to 10%), cinnamyl-alcohol, cinnamic acid, procyanidins and catechins [39, 41]. Cinnamaldehyde was shown to decrease blood glucose and ameliorate T1D in rat through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction [42, 43]. The potential dual role of eugenol in lowering blood glucose and inhibiting AGEs in diabetes is evident [44]. Taher et al., isolated a proanthocyanidin, cinnamtannin B1 from the bark of C. zeylanicum, that has insulin like activity in phosphorylation of insulin receptor β-subunit on 3T3-L1 adipocytes [45]. Several previous studies showed that good glycemic control plays significant role in the delay or prevention of secondary complications of diabetes and hence, this glucose lowering activity of C. zeylanicum would certainly beneficial in preventing cardiomyopathy.
H&E, and Masson’s trichrome staining on diabetic rat heart sections in the current study has shown the perinuclear vacuolization and increased interstitial fibrosis (Fig. 3). These histological alterations in diabetic rats were in agreement with the previous findings [12]. Fibrosis in the heart tissue is a common pathophysiological alteration of many myocardial diseases, and is associated with systolic and diastolic dysfunction and arrhythmogenesis [46]. However, C. zeylanicum feeding to diabetic rats markedly decreased the interstitial fibrosis, arteriolar smooth muscle fibrosis, perinuclear vacuolization which otherwise will decrease the elasticity of cardiac muscles and alters the function of heart leading to its failure [47]. Niknezhad et al., showed that cinnamon prevented hepatic fibrosis in rats [48] but so far no one reported in cardiac fibrosis. Further, C. zeylanicum feeding prevented the pericardial damage in diabetic rats which is one of the hall marks of DCM [49]. It is well established that diabetes increases the oxidative stress and decreases the antioxidant defense network in heart. Several past studies have shown a decrease in antioxidant mechanism in diabetic heart [12]. All these changes were also reflected in NA + STZ treated rats. C. zeylanicum fed rats showed significant increase in SOD activity and it is thought to be involved in the protection of cardiac mitochondria and DCM [50]. Increased GST activity shows the metabolic activation of GSH and thought to be a defense action to inhibit the increased quenching of intracellular ROS required for cellular insulin signaling. In addition, C. zeylanicum treatment significantly decreased MDA and protein carbonyls. This data suggests that C. zeylanicum plays a pivotal role in reduction of oxidative stress and improvement in antioxidant defense network. Previous studies showed the polyphenols belonging to the oligomeric procyanidins of C. zeylanicum have strong antioxidant potential, hypoglycemic and hypolipidemic effects in diabetic patients and animals [51, 52]. Further, cinnamaldehyde was also reported to potentially attenuate oxidative stress associated with diabetes [43].
As evidenced by previous studies, the expression of angiogenic factors like VEGF will decrease in diabetic heart which may lead to decreased perfusion and imbalance between myocardial supply and demand [53]. Interestingly expression of VEGF was significantly improved by C. zeylanicum treatment in diabetic rats that would facilitate a balance of myocardial nutritional and hormonal supply and demands.
In conclusion, above results suggest that C. zeylanicum bark feeding to T2D rats at a dose of 3% in diet has postprandial blood glucose lowering activity and thereby decreased HbA1C levels. It also prevented cardiac fibrosis, pericarditis and morphological alterations induced by diabetes. It showed strong antioxidant activity against diabetes associated cardiac oxidative stress. Hence, the preventive potency of C. zeylanicum bark against T2D induced cardiomyopathy is evident in rats.
Abbreviations
- T1D:
-
Type-1 diabetes
- T2D:
-
Type-2 diabetes
- DCM:
-
Diabetic cardiomyopathy
- AGEs:
-
Advanced glycation end products
- STZ:
-
Streptozotocin
- NA:
-
Nicotinamide
- VEGF:
-
Vascular endothelial growth factor
- SOD:
-
Superoxide dismutase
- GST:
-
Glutathione-s-transferase
References
IDF (2019) International Diabetes Federation DIABETES ATLAS 9th edition; 2019.
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83.
Strain WD, Paldanius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57.
Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983;25(6):496–501.
Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arterioscler Thromb Vasc Biol. 2020;40(8):1808–17.
Stegenga ME, et al. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes. 2006;55(6):1807–12.
Dal Canto E, et al. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26(2_suppl):25–32.
Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105(3):315–8.
Patel HP, Gandhi A, Trivedi S, Patel S. Prevalence and risk factors of cardiac autonomic neuropathy in diabetes mellitus. J Assoc Phys India. 2020;68(5):54.
Pinti MV, et al. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am J Physiol Endocrinol Metab. 2019;316(2):E268–85.
Modesti A, et al. Hyperglycemia activates JAK2 signaling pathway in human failing myocytes via angiotensin II-mediated oxidative stress. Diabetes. 2005;54(2):394–401.
Yu W, et al. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PloS One. 2012;7(12):e52013.
Ceylan-Isik AF, et al. Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis. J Mol Cell Cardiol. 2010;48(2):367–78.
Zhang C, Yu H, Yang H, Liu B. Activation of PI3K/PKB/GSK-3beta signaling by sciadopitysin protects cardiomyocytes against high glucose-induced oxidative stress and apoptosis. J Biochem Mol Toxicol. 2021;e22887.
Kowluru RA, et al. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr Metab. 2014;11(1):8.
Kommula SR, et al. Cinnamon attenuated long-term IGT-induced retinal abnormalities via regulation of glucose homeostasis in neonatal streptozotocin induced rat model. Indian J Clin Biochem. 2020;35(4):442–50.
Saraswat M, et al. Antiglycating potential of Zingiber officinalis and delay of diabetic cataract in rats. Mol Vis. 2010;16:1525–37.
Muthenna P, et al. Inhibition of protein glycation by procyanidin-B2 enriched fraction of cinnamon: delay of diabetic cataract in rats. IUBMB Life. 2013;65(11):941–50.
Pradeep SR, Barman S, Srinivasan K. Attenuation of diabetic nephropathy by dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) via suppression of glucose transporters and renin-angiotensin system. Nutrition. 2019;67–68:110543.
Hasanzade F, Toliat M, Emami SA, Emamimoghaadam Z. The effect of cinnamon on glucose of type II diabetes patients. J Tradit Complement Med. 2013;3(3):171–4.
Vafa M, et al. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int J Prev Med. 2012;3(8):531–6.
Mahdavi A, Bagherniya M, Mirenayat MS, Atkin SL, Sahebkar A. Medicinal plants and phytochemicals regulating insulin resistance and glucose homeostasis in type 2 diabetic patients: a clinical review. Adv Exp Med Biol. 2021;1308:161–83.
Ardalani H, Hejazi Amiri F, Hadipanah A, Kongstad KT. Potential antidiabetic phytochemicals in plant roots: a review of in vivo studies. J Diabetes Metab Disord. 2021.
Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Phys. 2016;8(1):1832–42.
Ziegenfuss TN, Hofheins JE, Mendel RW, Landis J, Anderson RA. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J Int Soc Sports Nutr. 2006;3:45–53.
Nyadjeu P, et al. Acute and chronic antihypertensive effects of Cinnamomum zeylanicum stem bark methanol extract in L-NAME-induced hypertensive rats. BMC Complement Altern Med. 2013;13:27.
Mohamed Sham Shihabudeen H, Hansi Priscilla D, Thirumurugan K. Cinnamon extract inhibits alpha-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr Metab. 2011;8(1):46.
Saraswat M, Reddy PY, Muthenna P, Reddy GB. Prevention of non-enzymic glycation of proteins by dietary agents: prospects for alleviating diabetic complications. Br J Nutr. 2009;101(11):1714–21.
Muthenna P, Raghu G, Kumar PA, Surekha MV, Reddy GB. Effect of cinnamon and its procyanidin-B2 enriched fraction on diabetic nephropathy in rats. Chem Biol Interact. 2014;222:68–76.
Okuda J, Miwa I, Maeda K, Tokui K. Rapid and sensitive, colorimetric determination of the anomers of D-glucose with D-glucose oxidase, peroxidase, and mutarotase. Carbohydr Res. 1977;58(2):267–70.
Morgan Al CR. Immunoassay of insulin: two antibody system: plasma insulin levels of normal. Subdiabetic Diabetic Rats Diabetes. 1963;12(2):115–26.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–74.
Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–9.
Uchida K, et al. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci U S A. 1998;95(9):4882–7.
Bhuyan KC, Bhuyan DK, Podos SM. Lipid peroxidation in cataract of the human. Life Sci. 1986;38(16):1463–71.
Nimgulkar C, et al. Combination of spices and herbal extract restores macrophage foam cell migration and abrogates the athero-inflammatory signalling cascade of atherogenesis. Vascul Pharmacol. 2015;72:53–63.
Ranasinghe P, et al. Cinnamomum zeylanicum (Ceylon cinnamon) as a potential pharmaceutical agent for type-2 diabetes mellitus: study protocol for a randomized controlled trial. Trials. 2017;18(1):446.
Ranasinghe P, et al. Effects of Cinnamomum zeylanicum (Ceylon cinnamon) on blood glucose and lipids in a diabetic and healthy rat model. Pharmacogn Res. 2012;4(2):73–9.
Sharma S, Mandal A, Kant R, Jachak S, Jagzape M. Is cinnamon efficacious for glycaemic control in type-2 diabetes mellitus? J Pak Med Assoc. 2020;70(11):2065–9.
AlizadehBehbahani B, Falah F, Lavi Arab F, Vasiee M, TabatabaeeYazdi F. Chemical Composition and Antioxidant, Antimicrobial, and Antiproliferative Activities of Cinnamomum zeylanicum Bark Essential Oil. Evid Based Complement Alternat Med. 2020;2020:5190603.
Meena Vangalapati NSS, Surya Prakash DV, Sumanjali A. A Review on Pharmacological Activities and Clinical effects of Cinnamon Species. Res J Pharm Biol Chem Sci. 2012;3(1).
Abdelmageed ME, Shehatou GS, Abdelsalam RA, Suddek GM, Salem HA. Cinnamaldehyde ameliorates STZ-induced rat diabetes through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(2):243–58.
Hosni AA, Abdel-Moneim AA, Abdel-Reheim ES, Mohamed SM, Helmy H. Cinnamaldehyde potentially attenuates gestational hyperglycemia in rats through modulation of PPARgamma, proinflammatory cytokines and oxidative stress. Biomed Pharmacother. 2017;88:52–60.
Singh P, et al. Potential Dual Role of Eugenol in Inhibiting Advanced Glycation End Products in Diabetes: Proteomic and Mechanistic Insights. Sci Rep. 2016;6:18798.
Faam MT, Sarmidi MR. A proanthocyanidin from cinnamomum zeylanicum stimulates phosphorylation of insulin receptor in 3T3–L1 adipocytes. J Teknol. 2006;44(1):53–68.
Frangogiannis NG. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99.
Shimizu M, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46(1):32–6.
Niknezhad F, et al. Improvement in histology, enzymatic activity, and redox state of the liver following administration of Cinnamomum zeylanicum bark oil in rats with established hepatotoxicity. Anat Cell Biol. 2019;52(3):302–11.
Manrique Franco K, Aragon Valera C, Gutierrez Medina S, Sanchez-Vilar Burdiel O, RoviraLoscos A. Acute pericarditis associated to onset of diabetes mellitus. Endocrinol Nutr. 2012;59(10):608–9.
Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55(3):798–805.
Im K, et al. Effects of the polyphenol content on the anti-diabetic activity of Cinnamomum zeylanicum extracts. Food Funct. 2014;5(9):2208–20.
Li R, et al. Protective effect of cinnamon polyphenols against STZ-diabetic mice fed high-sugar, high-fat diet and its underlying mechanism. Food Chem Toxicol. 2013;51:419–25.
Han B, Baliga R, Huang H, Giannone PJ, Bauer JA. Decreased cardiac expression of vascular endothelial growth factor and redox imbalance in murine diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2009;297(2):H829-835.
Acknowledgements
This work was supported by grants from Department of Biotechnology, Government of India to PS and GBR. CUK was supported by a research fellowship from the Indian Council of Medical Research, Government of India. We thank Khaja Ather Hussain for histological analysis and also Mr. Manohar Reddy for statistical analysis.
Funding
This work was supported by grants from Department of Biotechnology, Government of India to PS and GBR. CUK was supported by a research fellowship from the Indian Council of Medical Research, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We affirm that our work is original and is not submitted to any other journal except as an abstract in conferences. All authors have read and approved the manuscript and are aware of its submission.
Ethics approval
Obtained ICMR-National Institute of Nutrition Institutional Animal Ethical Clearance.
Conflicts of interest/Competing interests
No conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, C.U., Reddy, S.S., Suryanarayana, P. et al. Protective effect of cinnamon on diabetic cardiomyopathy in nicotinamide-streptozotocin induced diabetic rat model. J Diabetes Metab Disord 21, 141–150 (2022). https://doi.org/10.1007/s40200-021-00948-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00948-3