Abstract
Purpose
Infertility is one of the systemic problems in diabetic men. The purpose of the present study is investigation of the effects of the Alpinia officinarum (AO) hydro-alcoholic extract on the reproductive system damages in diabetic male rats.
Methods
Twenty four male rats were randomly assigned into 4 groups (n = 6); i.e., control, diabetic control, and diabetic rats treated orally with AO extract (200 and 500 mg kg−1). A single dose (60 mg kg-1) of streptozotocin (STZ) was injected intraperitoneally (IP) to induce diabetes. After 8 weeks of treatment, blood samples, testis, and cauda epididymis were excised to evaluate specific hormonal changes, sperm parameters, and testis morphology.
Results
Diabetic control rats showed remarkably lower body and testicular weights, testicular volumes, and sperm parameters compared with the control group (p <0.05). Diabetic control rats also exhibited significantly decreased serum testosterone and follicle stimulating hormone (FSH). Sperm parameters were considerably enhanced in diabetic animals gavaged with AO extract. Testosterone levels were significantly elevated by administrating 500 mg kg−1 AO extract to the diabetic control rats (p <0.05). The morphological assessment of testis of treatment group (500 mg kg−1) indicated remarkable differences (p <0.05) by increasing the seminiferous tubules diameter (STD) and thickness of the seminiferous epithelium (TSE) compared with diabetic control rats.
Conclusion
As demonstrated by the results, AO extract ameliorated sperm damage and improved sperm morphology besides improving histological damage in the testis in diabetic rats. In addition, the dose of 500 mg kg−1 worked more efficiently than 200 mg kg−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM), as important worldwide healthcare challenge, causes many systemic problems including impaired male reproductive function [1, 2]. Preclinical and clinical studies have shown that DM leads to male subfertility and/or infertility due to decreased spermatogenesis, testicular tissue damage and changes in steroidogenesis [3,4,5]. Excessive generation of mitocondrial reactive oxygen species (ROS) due to hyperglycemia can cause many problems in several tissues [6]. The precise mechanism of oxidative stress induced infertility in diabetic men remains a challenging issue. Nevertheless, sperm motility is often disturbed by high levels of ROS via the stimulation of lipid peroxidation of the spermatozoa plasma membrane and destruction of the structure of the lipid matrix [7].
Animal models are also often used to investigate the protective effect of various compounds in fertility impairment induced by diabetes. Type 1 diabetic rats are often generated using STZ as a chemical, which destroys insulin secreting cells [8]. Reduction in sperm number and motility, serum testosterone deficiency, sexual failure, and testicular injuries are reportedly observed during STZ induced diabetic model [5, 7, 9, 10]. Furthermore, the concentration of sperm in the cauda epididymis has been reported to decrease even fortnight following STZ induction [11]. Medicinal plants are rich sources of phytochemicals with antioxidant properties, which can decrease oxidative stress induced diabetic complications [12,13,14].
AO belongs to the ginger family (Zingiberaceae) and was extensively utilized in ancient and medieval Europe. Some groups of important compounds; namely, glycosides, diarylheptanoids and flavonoids have been found in this plant [15]. The rhizomes, referred to as galangal, contain high concentrations of the flavonol galangin [16] and have traditionally been used for several decades because of their antiulcer, antidiarrheal, anti-cancer [17], analgesic, anti-inflammatory [18], antimicrobial [19], antiparasitic, anti-arthritic, antitubercular activity [20] and antiplatelet effects [21].
The rhizome of AO is utilized in traditional medicine to reinforce male sexual power [22]. The spermatogenic effects of AO in a diabetic model have not been reported before. Thus, this research is conducted in STZ- induced DM rats to study the possible protective impact of AO extract on reproductive damage.
Materials and methods
Chemicals
STZ [cat. no. S0130], citric acid [cat. no. 251275] and sodium citrate dehydrate [cat. no. W302600] were obtained from Sigma Aldrich Chemical Co. (Germany). FSH [cat. no.926], Luteinizing hormone (LH) [cat. no.1018] and testosterone [cat. no.616] kits were obtained from Padtan Gostar Isar Co. (Iran).
Plant material and extraction method
AO rhizomes were obtained from the market and then characterized by a taxonomist at Tehran University of Medical Sciences Herbarium. The voucher specimen (No.PMP-231) was available there. 600 g of the air dried powdered AO were macerated with 70% ethanol (3 L) for 12 h in a dark room. The solvent extraction was repeated thrice. After filtering through a Whatman filter, the AO extract was concentrated to dryness on a rotatory evaporator and weighed. The percent yield of the obtained extract was calculated. All the extracts were dissolved in 0.9% normal saline solution to form the specified concentration, which was used throughout treatment processes [23].
Phytochemical screening
Chemical tests for the screening of total phenolic and flavonoid content were done on the hydroalcoholic extract of AO rhizome in accordance with the standard procedures.
Folin-Ciocalteu method (standard: gallic acid) and aluminum method (standard: rutin) were used for screening of total phenolic and flavonoid, respectively [24].
Animals
Twenty four male Wistar albino rats, which weighed 180–250 g and aged 8 weeks old, were kept under controlled standard environmental conditions with ad lib food and water supplies during the experiments. The experimental procedures were performed under the Medical Research Ethics Commission of Qom University of Medical Sciences (Code: MUQ.REC.1395.11).
Diabetes induction
Experimental type I DM was created in the male rats by IP injection of STZ (60 mg kg−1) [25] dissolved in 0.1 M cold solution of citrate buffer (pH = 4.5) in overnight fasting rats [26, 27]. The glucose solution (10%) was available for animals to drink 6 h after STZ injection for 24 h to prevent fatal hypoglycemia, then regular water was used [28]. The glucose amount was determined in tail vein blood samples on the 3rd day of STZ injection employing a glucometer (EasyGluco, China). Rats were assigned as diabetic when fasting blood sugar amounts be more than 250 mg/dl [29].
Experimental design
The animals utilized in this research were categorized into four groups (n = 6) including group I: control (non-diabetic) rats, which treated with 10 mL kg− normal saline 0.9%; group II: diabetic control rats, which treated with 10 mL kg−1 normal saline 0.9%; group III: diabetic rats gavaged by AO hydroalcoholic extract (200 mg kg−1); group IV: diabetic rats gavaged by AO hydroalcoholic extract (500 mg kg−1). Upon completion of the biochemical analyses, the evaluation of sperm parameters and histopathological studies were carried out as follows.
Biochemical parameters
The rats were fasted overnight on the 57th day of treatment. Cardiac puncture was used for blood sampling under mild general anesthesia. The samples were centrifuged (2000 rpm, 20 min.) for serum separation then kept at −20 °C for different biochemical evaluations.
A commercial ELISA Kit (PGI, Iran) was used to determine testosterone, FSH, and LH levels, following ̕ the instruction from the manufacturer.
Sperm parameter evaluation
Sperm count
Upon completion of the experiment (after 8 weeks), the cauda epididymis was removed, chopped to tiny parts in 1 mL of 0.9% normal saline, and then pressed to allow the spermatozoa to enter the medium.100 μL of the above liquid were then diluted with 100 μl of distilled water and the number of sperms was evaluated employing a hemocytometer and microscope. One drop of the above sample was placed onto Neubauer hemocytometer lam (HBG Co., Germany). Sperms distributed in the chambers were counted using an Olympus Microscope (Tokyo, Japan). The number of sperms per milliliter were reported as sperm count [30].
Sperm motility
Fresh semen (one droplet) and warm 0.9% normal saline (two droplet) were placed onto a pre-warmed (37 °C) slide to assess sperm motility. Randomly selected spermatozoa (at least 200) were graded under an optical magnification of 400 times, based on the manual criteria of World Health Organization as follows [31]: Grade A sperms (rapid forward): swimming forward quickly along a line; grade B sperms (slow forward): swimming forward along a curve, crooked line or linear slowly; grade C sperms (not forward): not moving forward but shaking their tails; grade D sperms with minimum movement (immobile).
Sperm viability
Eosin staining was utilized to evaluate rat sperms viability; that is, the determination of dead (stained) sperms and live (unstained) [32, 33]. 20 μl of the eosin solution was added to an equal amount of sperm suspension and placed at room temperature for 5 min. Sperm viability (%) was determined by the evaluation of 200 randomly selected sperms under optical magnification of 200 times [34].
Sperm morphology
A drop of semen sample collected from right epididymis was smeared on a slide and stained using Diff-Quick for sperm morphological studies [1, 35]. Sperms with deformities in the head, neck, and tail of randomly selected 200 sperm were considered to have abnormal morphology.
Histological evaluation
Histopathological study
The animals, in several experiment groups, were killed on the 57th day under light anesthesia and the excised testes were washed in 0.9% normal saline. The length, width, volume and weight of the testes in each group were measured [36]. A small piece of testis tissue was taken for histological analysis. Once biopsies were fixed by formalin solution (10%), they were inserted in paraffin. The sliced Tissue blocks (5 μm sections) were stained with hematoxylin-eosin for histological evaluation. Assessment of histological change, atrophic tubules and reduced number of spermatocytes in non-consecutive, randomly selected ×40 histological fields were carried out using a light microscope and the data were reported in five atretic grades, as absent of atrophy, slight, moderate, severe and most severe atrophic appearance of testicular tissue and reduced number of spermatocytes [37].
Histomorphometric study
Two histomorphometric parameters, STD and TSE, were assessed in all experimental groups. Twenty five tubule cross sections in most circular tubules of each group were measured for estimating the mean diameter of the seminiferous tubules. Measurements were performed using an ocular micrometer in a light microscope and a 10× objective lens. The mean STD and TSE were determined in micrometers for each testis [38].
Statistical analysis
Data analysis was done with IBM® SPSS software (version 19, Chicago, IL, USA), and expressed as mean ± standard error of mean (SEM). One-way ANOVA followed by the Tukay Multiple Comparison tests were utilized for statistical significance determination. P < 0.05 was set as the level of statistical significance.
Results
Extraction and phytochemical analysis
The percent yield of dried hydroalcoholic extract was found to be 14.21% w/w of the dried rhizome. Phytochemical screening performed on the hydroalcoholic extract of AO showed that it contained 28.6 and 12.17% of total phenol and flavonoid, respectively.
Biochemical analysis
Biochemical parameters including LH, FSH and testosterone were assessed in several groups (Table 1). The testosterone and FSH levels significantly decreased in the diabetic control group in comparison with the control group (p < 0.05). Moreover, LH elevated in diabetic control rats compared to the control group (p < 0.05).
None of the hormonal changes (LH, FSH and testosterone) were significant in the extract group (200 mg kg−1) compared with diabetic control rats. However, the hormonal changes were significantly different in the control group (p < 0.05).
A remarkable increase (p < 0.05) in testosterone was observed after treatment with AO (500 mg kg−1) compared with the diabetic control group. However, a significant difference was not seen compared to the control group. Furthermore, the LH level decreased significantly (p < 0.05) in the treatment group (500 mg kg−1) compared with diabetic control group but the difference is not significant in comparison to the control group. The AO (500 mg kg-1) treated rats did not display a major elevation in FSH level compared with the diabetic control group and the difference was significant in comparison to the control group (p < 0.05). The data also revealed that treatment with AO (500 mg kg−1) did not cause a significant difference in comparison to AO (200 mg kg−1).
Sperm parameters
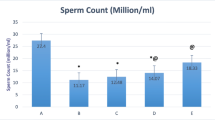
Induction of diabetes with STZ significantly (p < 0.05) decreased sperm count, viability and motility as well as increasing abnormal sperm morphology in comparison to the control group. Nevertheless, AO (500 mg kg−1) remarkably improved these parameters compared to the diabetic control group (p < 0.05) (Table 2). None of the parameters; except for the sperm morphology, represented a significant difference compared with the control group. AO (200 mg kg−1) treated rats also indicated significant improvement in sperm count, motility, and morphology compared with the diabetic control group (p < 0.05), but sperm viability did not indicate any significant improvement compared with the diabetic control group. In addition, all the sperm parameters indicated a significant difference in AO (200 mg kg−1) compared with the control group (p < 0.05). A significant (p < 0.05) improvement in sperm motility, morphology, and viability was also seen in AO (500 mg kg−1) compared to AO (200 mg kg−1).
Total body and testis weights volume
As indicated in Table 3, all the parameters (body weight, testis weight, and volume) significantly declined (p < 0.05) in the diabetic control group, whereas AO (200 and 500 mg kg−1) significantly increased these parameters in comparison with the diabetic control group (p < 0.05). On the other hand, none of the parameters in AO (500 mg kg−1) displayed a significant difference compared with the control group, but the dose of 200 mg kg−1 showed significant difference with the control group (p < 0.05). Moreover, high dose dependent improvement was seen in the body and testis weight (p < 0.05).
Histological study
Histological observations of tissue samples in the control group (A1&A2) showed healthy appearance of seminiferous tubules. The STD and TES were also normal (Fig. 1, Table 4). It was observed that STD and TES remarkably reduced in the testicular samples of diabetic control group (B1&B2) compared to the control group and severe atrophy was seen in some seminiferous tubules. In addition, the spaces between seminiferous tubules were quite clear. Complete loss of spermatogenic series associated with one or two layers of vacuolated sertoli cells were apparent in some seminiferous tubules. Only few spermatozoa were observed inside their lumina (Fig. 1, Table 4). In both AO treated groups, the seminiferous tubules atrophy was smaller than that of the diabetic control group. However, TSE in AO treated group (200 mg kg−1) was the same as that in the diabetic control group. Furthermore, spermatocytes number in AO treated groups (200 and 500 mg kg−1) indicated a smaller reduction. As the data show, the testes of rats treated with 200 mg kg−1 (C1&C2) and 500 mg kg−1 of AO (D1&D2) showed moderate and minor degeneration of seminiferous tubules, respectively (Fig. 1, Table 4).
Histopathological assessments of the testicular tissue samples stained using hematoxylin and eosin in the different experimental groups. Control group (A1 & A2), diabetic control group (B1 & B2), 200 mg kg−1Alpinia officinarum treated group (C1 & C2), 500 mg kg−1 Alpinia officinarum treated group (D1 & D2) (H&E, 40× and 100×). Spermatogonial cell (arrowhead), Sertoli cell nucleus (arrow), Seminiferous Tubule Diameter (STD) (line), Thickness of seminiferous epithelium (TSE) (two headed arrow)
Discussion
The present investigation indicated that sperm parameters significantly dropped in the diabetic control group compared to the control group. However, these features and parameters could be significantly enhanced by the hydroalcoholic extract of AO. According to the findings of this work, diabetes remarkably decreases body weight, testicular weight, and volume. Previous studies have shown that STZ injection increases micronuclei frequency and sperm abnormality through activation of several cellular damaging pathways via ROS overproduction [6]. ROS overproduction increases the number of abnormal sperms, apoptosis of germ cells, and lipid peroxidation, finally reflecting male infertility [39]. AO, which has a high phenolic acid and flavonoid content, may have scavenging activity [40], reduce oxidative stress and improve sperm parameters since ROS plays a significant role in diabetic reproductive damage [1]. Aloud et al. reported that galangin, a flavonoid in AO, improves antioxidant status in STZ- induced diabetic rats [41].
The cells can be protected against free radicals by these compounds, which have the hydroxyl groups in their structures and thus contribute to the antioxidant defense system [42]. Lipid free radicals can be neutralized and the decomposition of hydroperoxides in them can be prevented by these compounds [43, 44]. AO extract (total daily dose of 300 mg) is effective on semen parameters (sperm number and morphology) in males, who suffer from idiopathic infertility, based on the results of recent studies [45]. In another work, it was demonstrated that supplementation with dried AO (5, 7.5 and 10%) improved sperm parameters and sex hormonal changes in male rats with lead acetate induced damage on reproductive function [46]. Several investigations have also reported spermatogenic effects of Alpinia galangal (greater galangal) extract (100 and 300 mg/day) in the animal model [47, 48]. Furthermore, a clinical study indicated that greater galangal powder (764 mg) significantly increased motile spermatozoa compared to placebo [49]. Flavonol galangin is one of the main common chemical constituents in lesser and greater galangal, which has an antioxidant effect [50].
Testosterone level decreased in diabetic animals, according to our findings. This is supported by previous reports and may be caused by Leydig cell dysfunction in diabetic rats [2, 51, 52]. Furthermore, an LH increase in diabetic rats can be justified by the feedback mechanism of testosterone decrease in them. Another work indicated that insulin binding diminished in the testicular membrane of STZ-induced diabetic rats. Considering the stimulatory impact of insulin on testosterone production in Leydig cells, it can be expected that the Leydig cell function be disturbed in the diabetic rats [53]. Our results also indicate FSH level reduction in diabetic rats, which can affect sperm output and fertility as well [54].
In the current investigation, AO had little effect on hormone modification despite the improvement in sperm parameters and testicular weight and volume and only the 500 mg kg−1dose could increase testosterone level as well as significantly decreasing LH level compared to diabetic control rats. Also, testosterone, LH, and FSH improved in the 200 mg kg−1 group, but not significantly. Considering these results, AO extract probably has a recovery effect on the hormonal profile only at higher doses (500 mg kg−1). Therefore, sperm parameters and testis tissue improvement in low dose (200 mg kg−1) observed in this research may be due to modulation of the metabolic status and prevention of hyperglycemic induced tissue damages [55].
Histopathological evaluation illustrated that spermatogonia cell number, STD and TSE were on a normal scale in the control group and marked histological changes including tubular atrophy, reduction of STD, TSE and number of spermatocytes in diabetic control rats, which is consistent with previous reports [56]. Overproduction of free radicals in diabetes can prevent androgen production by Leydig cells and may be responsible for histological changes in testis [37]. However, increased STD and TSE and reduced atretic features were observed upon administration of AO extract; especially 500 mg kg−1 dose, in diabetic animals. The protective effects of AO are probably due to the reduction of the oxidative damage of testis and the changes in the concentrations of LH and testosterone.
Finally, further research is required for the identification of the main constituent of this extract, which is responsible for improving spermatogenesis in the diabetic animal model and the determination of its mechanism. There were some limitations including lack of chromatographic fractionation of the crude extract, isolation of the marker compound and further spermatogenic activity evaluation, which should be carried out in next study.
The present study reveals that AO extract improves spermatogenic status in STZ induced diabetic rats for the first time. Regarding the anti-hyperglycemic and spermatogenic effects of AO extract, this plant can be considered for adjuvant therapy in diabetic patients.
In summary, the present observations suggest that AO hydroalcoholic extract can improve sperm parameters (count, motility, morphology, and viability) in STZ- induced diabetic rats, which may be mediated by antioxidant components. In addition, the higher dose of this extract (500 mg kg−1) seems to be more efficient in the improvement of sperm parameters and testicular damages.
References
Öztaş E, Yılmaz TE, Güzel E, Sezer Z, Okyar A, GJSPJ Ö. Gliclazide alone or in combination with atorvastatin ameliorated reproductive damage in streptozotocin-induced type 2 diabetic male rats. Saudi Pharmaceut J. 2019;27(3):422–31.
Heeba GH, Hamza AAJL. Rosuvastatin ameliorates diabetes-induced reproductive damage via suppression of oxidative stress, inflammatory and apoptotic pathways in male rats. Life Sci. 2015;141:13–9.
La Vignera S, Calogero A, Condorelli R, Lanzafame F, Giammusso B, EJME V. Andrological characterization of the patient with diabetes mellitus. Minerva Endocrinol. 2009;34(1):1–9.
Mohasseb M, Ebied S, Yehia MAH, Hussein N. Testicular oxidative damage and role of combined antioxidant supplementation in experimental diabetic rats. J Physiol Biochem. 2011;67(2):185–94.
Kanter M, Aktas C, Erboga M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol Nutr Food Res. 2013;57(9):1578–85.
Rabbani SI, Devi K, Khanam S. Inhibitory effect of glimepiride on nicotinamide-streptozotocin induced nuclear damages and sperm abnormality in diabetic Wistar rats.Indian J. Exp. Biol. 2009;47(10):804–10.
Ghanbari E, Nejati V, Najafi G, Khazaei M, Babaei M. Study on the effect of royal jelly on reproductive parameters in streptozotocin-induced diabetic rats. Int Fertil steril. 2015;9(1):113.
Ding G-L, Liu Y, Liu M-E, Pan J-X, Guo M-X, Sheng J-Z, et al. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl. 2015;17(6):948.
Afifi M, Almaghrabi OA, Kadasa NM. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. Biomed Res Int. 2015;2015:153573.
Shi G-J, Zheng J, Wu J, Qiao H-Q, Chang Q, Niu Y et al. Protective effects of Lycium barbarum polysaccharide on male sexual dysfunction and fertility impairments by activating hypothalamic pituitary gonadal axis in streptozotocin-induced type-1 diabetic male mice. Endocr J. 2017;64(9):907–22.
Sookhthezari A, Alirezaei M, Kheradmand A, Dezfoulian OJSJ. Streptozotocin-induced diabetic effects on the sperm fertility parameters. Glycated Hemoglob Total Choles Mice. 2016;23(10):980–8.
Jain GC, Jangir RN. Modulation of diabetes-mellitus-induced male reproductive dysfunctions in experimental animal models with medicinal plants. Pharmacogn Rev. 2014;8(16):113.
Yildirim M, Degirmenci U, Akkapulu M, Comelekoglu U, Balli E, Ozcan TM et al. The effect of Rheum ribes L. on oxidative stress in diabetic rats. J Basic Clin Physiol Pharmacol. 2020. https://doi.org/10.1515/jbcpp-2020-0058.
Almalki DA, Alghamdi SA, AI-Attar AM. Comparative study on the influence of some medicinal plants on diabetes induced by streptozocin in male rats. J Bri. 2019. https://doi.org/10.1155/2019/3596287.
Rajendiran V, Natarajan V, Devaraj SN. Anti-inflammatory activity of Alpinia officinarum hance on rat colon inflammation and tissue damage in DSS induced acute and chronic colitis models. Food Sci Human Wellness. 2018;7(4):273–81.
Ma Y-L, Zhao F, Yin J-T, Liang C-J, Niu X-L, Qiu Z-H, et al. Two approaches for evaluating the effects of galangin on the activities and mrna expression of seven cyp450. Molecules. 2019;24(6):1171.
Reid K, Wright V, Omoregie SJ. Anticancer properties of Alpinia officinarum (lesser galangal)–A mini review. Int J Adv Res. 2016;4:300–6.
Honmore VS, Kandhare AD, Kadam PP, Khedkar VM, Sarkar D, Bodhankar SL, et al. Isolates of Alpinia officinarum Hance as COX-2 inhibitors: Evidence from anti-inflammatory, antioxidant and molecular docking studies. Int Immunopharmacol. 2016;33:8–17.
Eumkeb G, Sakdarat S, S S. Reversing β-lactam antibiotic resistance of Staphylococcus aureus with galangin from Alpinia officinarum Hance and synergism with ceftazidime. Phytomedicine. 2010;18(1):40–5.
Honmore VS, Rojatkar SR, Nawale LU, Arkile MA, Khedkar VM, Natu AD, et al. In vitro and ex vivo antitubercular activity of diarylheptanoids from the rhizomes of Alpinia officinarum Hance. Nat Prod Res. 2016;30(24):2825–30.
Köse LP, Gülcin I, Gören AC, Namiesnik J, Martinez-Ayala AL, Gorinstein S. LC–MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crop Prod. 2015;74:712–21.
Khorasani MA. Makhzan ul- Advia. 3rd ed. Tehran university publications; 1996. pp. 479–80
Ahangarpour A, Heidari H, Junghani MS, Absari R, Khoogar M, Ghaedi E. Effects of hydroalcoholic extract of Rhus coriaria seed on glucose and insulin related biomarkers, lipid profile, and hepatic enzymes in nicotinamide-streptozotocin-induced type II diabetic male mice. Res Pharm Sci. 2017;12(5):416.
Nickavar B, Esbati N. Evaluation of the antioxidant capacity and phenolic content of three Thymus species. J Acupunct Meridian Stud. 2012;5(3):119–25.
Samie A, Sedaghat R, Baluchnejadmojarad T, Roghani M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci. 2018;210:132–9.
Kumar R, Pate DK, Prasad SK, Sairam K, Hemalatha S. Antidiabetic activity of alcoholic leaves extract of Alangium lamarckii Thwaites on streptozotocin–nicotinamide induced type 2 diabetic rats. Asian Pac J Trop Med. 2011;4(11):904–9.
Parmar GR, Pundarikakshudu K, Balaraman R. Antidiabetic and antihyperlipidemic activity of Euphorbia thymifolia L. extracts on streptozotocin-nicotinamide induced type 2 diabetic rats. J Appl Pharmaceut Sci. 2017;7(08):078–84.
Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70(1):5.47.1–5.20.
Weinberg E, Maymon T, Moses O, Weinreb M. Streptozotocin-induced diabetes in rats diminishes the size of the osteoprogenitor pool in bone marrow. Diabetes Res Clin Pract. 2014;103(1):35–41.
Ahangarpour A, Oroojan AA, Radan M. Effect of aqueous and hydro-alcoholic extracts of lettuce (Lactuca sativa) seed on testosterone level and spermatogenesis in NMRI mice. Iran J Reprod Med. 2014;12(1):65.
Haredy SA, Imam TS, Ahmed-Farid OA. Combination of Ficus carica leaves extract and ubiquinone in a chronic model of lithium induce reproductive toxicity in rats: Hindrance of oxidative stress and apoptotic marker of sperm cell degradation. JJPBS. 2017;12:64–73.
Keyhanmanesh R, Hamidian G, Alipour MR, Ranjbar M, Oghbaei H. Protective effects of sodium nitrate against testicular apoptosis and spermatogenesis impairments in streptozotocin-induced diabetic male rats. Life Sci. 2018;211:63–73.
Ommati MM, Heidari R, Jamshidzadeh A, Zamiri MJ, Sun Z, Sabouri S, et al. Dual effects of sulfasalazine on rat sperm characteristics, spermatogenesis, and steroidogenesis in two experimental models. Toxicol Lett. 2018;284:46–55.
Navarro-Casado L, Juncos-Tobarra M, Chafer-Rudilla M, De Onzono LÍ, Blazquez-Cabrera J, Miralles-Garcia JJ. Effect of experimental diabetes and STZ on male fertility capacity. Study in Rats. J Androl. 2010;31(6):584–92.
Halvaei I, Roodsari HRS, Harat ZN. Acute effects of Ruta graveolens L. on sperm parameters and DNA integrity in rats. J Reprod Infertil. 2012;13(1):33.
Ahangarpour A, Oroojan AA, Heidari H, Ehsan G, Nooshabadi R, Nooshabadi MRR. Effects of hydro-alcoholic extract of Rhus coriaria (Sumac) seeds on reproductive complications of nicotinamide-streptozotocin induced type-2 diabetes in male mice. World J Mens Health. 2014;32(3):151–8.
Khaneshi F, Nasrolahi O, Azizi S, Nejati V. Sesame effects on testicular damage in streptozotocin-induced diabetes rats. Avicenna J Phytomed. 2013;3(4):347.
Soudamani S, Yuvaraj S, Malini T, Balasubramanian K. Experimental diabetes has adverse effects on the differentiation of ventral prostate during sexual maturation of rats. Anat Rec A Discov Mol Cell Evol Biol. 2005;287(2):1281–9.
Shoorei H, Khaki A, Khaki AA, Hemmati AA, Moghimian M, Shokoohi M, et al. The ameliorative effect of carvacrol on oxidative stress and germ cell apoptosis in testicular tissue of adult diabetic rats. Biomed Pharmacother. 2019;111:568–78.
Kaushik D, Yadav J, Kaushik P, Sacher D, Rani R. Current pharmacological and phytochemical studies of the plant Alpinia galanga. Zhong Xi Yi Jie He Xue Bao. 2011;9(10):1061–5.
Aloud AA, Chinnadurai V, Govindasamy C, Alsaif MA, Al-Numair KS. Galangin, a dietary flavonoid, ameliorates hyperglycaemia and lipid abnormalities in rats with streptozotocin-induced hyperglycaemia. Pharm Biol. 2018;56(1):302–8.
Saggu S, Sakeran MI, Zidan N, Tousson E, Mohan A, Rehman H, et al. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem Toxicol. 2014;72:138–46.
Javanmardi J, Stushnoff C, Locke E, Vivanco JM. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83(4):547–50.
Abbas ZK, Saggu S, Sakeran MI, Zidan N, Rehman H, Ansari AA. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J Biol Sci. 2015;22(3):322–6.
Kolangi F, Shafi H, Memariani Z, Kamalinejad M, Bioos S, Jorsaraei SGA, et al. Effect of Alpinia officinarum Hance rhizome extract on spermatogram factors in men with idiopathic infertility: A prospective double-blinded randomised clinical trial. Randomized Controlled Trial. 2019;51(1):e13172.
Negm SH, Ragheb EM. Effect of (m) hance on sex hormones and certain biochemical parameters of adult male experimental rats. J Sci Dent. 2019;10(9):315–22.
Mazaheri M, Shahdadi V, Boron AN. Molecullar and biochemical effect of alcohlic extract of Alpinia galanga on rat spermatogenesis process. Iran J Reprod Med. 2014;12(11):765.
Qureshi S, Shah A, Ageel AM. Toxicity studies on Alpinia galanga and Curcuma longa. Planta Med. 1992;58(02):124–7.
Fedder MD, Jakobsen HB, Giversen I, Christensen LP, Parner ET, Fedder J. An extract of pomegranate fruit and galangal rhizome increases the numbers of motile sperm: a prospective, randomised, controlled, double-blinded trial. Randomized Controlled Trial. Plos One. 2014;9(10). https://doi.org/10.1371/journal.pone.0108532.
Kolangi F, Shafi H, Memariani Z, Kamalinejad M, Naeimi M, Mozaffarpur SAJT et al. Improvement in semen quality and occurrence of pregnancy treated with an herbal medication: a case report. Traditional and Integrative Medicine. 2019;4(3):123–29.
Zhao H, Xu S, Wang Z, Li Y, Guo W, Lin C, et al. Repetitive exposures to low-dose X-rays attenuate testicular apoptotic cell death in streptozotocin-induced diabetes rats. Toxicol Lett. 2010;192(3):356–64.
Hussein Z, Al-Qaisi J. Effect of diabetes mellitus type 2 on pituitary gland hormones (FSH, LH) in men and women in Iraq. Al-Nahrain J Sci. 2012;15(3):75–9.
Jelodar G, Khaksar Z, Pourahmadi M. Endocrine profile and testicular histomorphometry in adult rat offspring of diabetic mothers. J Physiol Sci. 2009;59(5):377.
Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH-and LH-linked mechanisms. J Androl. 2004;25(5):706–19.
Ebokaiwe AP, Ijomone OM, Osawe SO, Chukwu CJ, Ejike CE, Zhang G, et al. Alteration in sperm characteristics, endocrine balance and redox status in rats rendered diabetic by streptozotocin treatment: attenuating role of Loranthus micranthus. Redox Rep. 2018;23(1):194–205.
Kianifard D, Sadrkhanlou RA, Hasanzadeh S. The ultrastructural changes of the sertoli and leydig cells following streptozotocin induced diabetes. Iran J Basic Med Sci. 2012;15(1):623.
Acknowledgments
This research project was approved and financially supported by Qom University of medical sciences (Qom, Iran). The authors deeply thank Miss Azam Khalaj and Danesh laboratory for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heidari, H., Abdollahi, M., Khani, S. et al. Effect of Alpinia officinarum extract on reproductive damages in streptozotocin induced diabetic male rats. J Diabetes Metab Disord 20, 77–85 (2021). https://doi.org/10.1007/s40200-020-00711-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00711-0