Abstract
Background and purpose
Hyperglycemia induced oxidative stress and inflammation lead to development of diabetic cardiomyopathy. Diabetic patients are more at risk for myocardial infarction than non-diabetics. The current study has investigated the involvement of PPARγ activation in effects of crocin as a natural carotenoid against cardiac infarction in diabetic rats.
Materials and methods
Diabetes was induced in male Wistar rats by streptozotocin injection (55 mg/kg, i.p) 15 min after the administration of nicotinamide (110 mg/kg). Then saline, crocin (40 mg/kg, orally) and GW9662 (1 mg/kg, as PPARγ antagonist) were injected for 4 weeks. Isoprenaline was administrated on the 27th and 28th days to induce infarction. Cardiac injury markers, antioxidant enzymes content, blood glucose level, lipid profile, pro and anti-inflammatory cytokines, and PPARγ gene expression were measured.
Results
GSH, CAT content, CK-MB isoenzyme, LDH level, IL-10 and PPARγ gene expression in myocardial tissue were decreased in diabetic rats receiving isoprenaline and inflammatory cytokines TNFα and IL-6 and also plasma lipids were increased. Crocin administration significantly ameliorated inflammatory cytokines levels, CK-MB, and LDH contents and also it could enhance antioxidant capacity and PPARγ expression. However, GW9662 administration reversed the effects of crocin.
Conclusion
Overexpression of PPARγ in crocin treated rats and inhibition of crocin effects by GW9662 reflected the potential involvement of PPARγ pathway in the protective effects of crocin.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a chronic and metabolic disorder that is increasing in both developed and developing countries [1]. Cardiovascular diseases due to diabetes are the major cause of death among the population [2]. Insufficient secretion of insulin leads to diabetes symptoms including hyperglycemia, hyperlipidemia, inflammation and oxidative stress [3]. Hyperglycemia promotes free radicals generation and disturbs antioxidant system and also membrane function in diabetic heart [4]. Based on research findings, high risk of ischemic heart disorders are related to diabetes [5]. Imbalance between coronary blood supply and myocardial demand causes myocardial necrosis which has been recognized as myocardial infarction (MI). Hyperglycemia, lipid peroxidation, and oxidative stress lead to MI, which ultimately results in myocardial damage and heart failure [6].
Isoprenaline (ISO) is a synthetic β-adrenoceptor agonist which causes peroxidation of membrane phospholipids and entirely myocardial tissue damage by generating cytotoxic free radicals [7]. As diabetic cardiomyopathy is related to inflammation and oxidative stress [8], inhibition of inflammation and free radical generation or protection of the endogenous antioxidant system have been shown to ameliorate cardiac dysfunction [9]. Crocin is a natural carotenoid compound and color pigment which is water-soluble and mainly found in saffron and also gardenia flowers [10]. A number of studies revealed that crocin have antioxidant [11, 12], neuroprotective [13], anti-hyperlipidemic [14, 15] and anti-diabetic [16] properties.
Crocin and saffron have been shown to exert protective effects by activation of peroxisome proliferator activated receptors (PPAR) [13]. The PPARs have been recognized as nuclear receptors family and their subtype such as PPARγ is mainly involved in insulin sensitivity, glucose and lipid metabolism [17, 18]. PPARγ agonists and antagonists have been used for many pharmacological and pathological studies, and findings have shown that PPARγ exerts free radical scavenger [19], anti- inflammatory [20], anti-diabetic and anti-atherosclerotic effects [21]. Although the crocin effect on diabetes and myocardial infarction models has been reported, however the effective mechanisms are yet to be explored. Hence, we investigated the involvement of PPARγ activation in protective effects of crocin in diabetic rats in model of MI.
Materials and methods
Animals
Male Wistar rats, weighing 200–230 g were purchased from Ahvaz Jundishapur University of Medical Sciences, Animal House Center, Ahvaz, Iran. Rats were housed in an air-conditioned colony room at 23 °C ± 2 °C and under a 12 h light-dark cycle (lights on 07:00 h). Rats received drinking water and a standard pellet diet. The experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publications No.80–23, revised 1996) and the study was approved by the Ethic Committee for Animal Experiments at Jundishapur University of Medical Sciences (IR.AJUMS.ABHC.REC.1397.005).
Induction of diabetes
After acclimatization to the laboratory, the animals were weighed and blood samples were taken from tail veins for the measurement of blood glucose levels. Streptozotocin (55 mg/kg, 0.1 M cold citrate buffer, pH = 4.5) was injected intraperitoneally to induce diabetes in overnight fasting rats 15 min after the administration of nicotinamide (110 mg/kg, intraperitoneally) [22].
Diabetes was confirmed 10 days post injection, blood glucose level 250 mg/dl was considered as an index of successful induction of diabetes and such rats showing this value were included in the study. Upon confirmation for diabetes, rats were randomly divided into experimental groups and used for further experiments.
Treatment groups
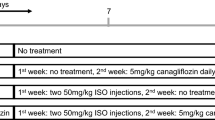
Animals were divided into six groups and each group consisted of eight rats. Group 1 received normal saline (Control group). Group 2 received nicotinamid (110 mg/kg, i.p) and STZ (55 mg/kg, i.p) then followed by normal saline (D group). Groups 3 received nicotinamide and STZ then followed by normal saline and isoprenaline (100 mg/kg) (D + ISO group). Group 4 received nicotinamide, STZ and followed by crocin (40 mg/kg, orally) and isoprenaline (D + C + ISO group). Group 5 received nicotinamide, STZ then followed by GW9662 (1 mg/kg, intraperitoneally) and isoprenaline (D + GW + ISO group). Group 6 received nicotinamide, STZ then followed by crocin, GW9662 and isoprenaline (D + C + GW + ISO group). Animals were under treatment for 4 weeks and isoprenaline was injected subcutaneously on the 27th and 28th days.
Experimental protocol design is illustrated in Fig. 1.
Lipid profile
After anesthesia, blood samples were collected and the serum was obtained by centrifugation at 14000 g for 15 min at room temperature. Then the level of VLDV, LDL, HDL, Cholesterol and Triglycerides were determined using commercial kits.
Antioxidant enzymes assay
To determine catalase (CAT) and glutathione (GSH) content, the heart tissue from different groups were frozen and then homogenized in phosphate buffered saline (PBS; 50 mM at pH = 7.4) using a homogenizer (Heidolph Silenterosher M, Germany), and centrifuged for 15 min at 14000 g. The obtained supernatant was used for enzyme level determination using appropriate kits. (Zellbio Lab, Ulm, Germany).
Cardiac marker enzymes assay
Tissue level of myocardial creatine kinase (CK-MB) and lactate dehydrogenase (LDH) were measured using commercial kits, purchased from Pars Azmoon Co., Tehran, Iran.
Inflammation assessment
The levels of inflammatory and anti-inflammatory cytokines in myocardial homogenate were measured using commercially available ELISA assay kits for tumor necrosis factor-α (TNF-α), interleukin-6 (IBL, Hamburg, Germany) and interleukin-10 (Zellbio, Ulm, Germany).
Quantitative real time RT-PCR analysis
The total RNA from the homogenized tissues was extracted using RNeasy plus mini kit (Qiagen Co, Germany). Complementary DNA was synthesized using 1 μg of the total RNA and also cDNA synthesis kit (Qiagen, Germany),. A light cycler PCR (Roche, Diagnostics) was used to determine the PPARγ and housekeeping genes levels. The following gene-specific primer was used: PPARγ, F: 5’ TTCGGCTTTCTGTTGAAT, R: CAAAGGAATGGGAGTGGTCA, GAPDH, R: CGGAGATGATGACCCTTTTGG F: TGCTGGTGCTGAGTATGTCGTG.
Statistical analysis
All data obtained from the experimental groups were presented as means ± SEM. The data analyzed using one-way ANOVA followed by the tukey post hoc test. P < 0.05 was considered as statistically significance.
Results
The level of blood glucose
As shown in Table 1, the blood glucose level was significantly (P < 0.05) increased in D + GW + ISO group compared to diabetic group. Crocin treated rats showed significant reduction in blood glucose level compared to diabetic rats and diabetic receiving ISO (P < 0.01, p < 0.01, respectively). Co-treatment of GW9662 with crocin demonstrated higher level of glucose compared to D + C + ISO group.
Lipid profile
Based on our biochemical results shown in Fig. 1, VLDL, LDL, Cholesterol and TG level were significantly increased in D + ISO and D + GW + ISO groups compared to untreated diabetic group. Crocin treatment significantly (P < 0.01, P < 0.01, P < 0.05, P < 0.01, respectively) reduced the level of these biochemical factors compared to D + ISO. In addition, it significantly improved HDL level Fig. 2.
Effect of crocin on lipid profile in diabetic rats receiving ISO. a VLDL, b LDL, c HDL, d Cholesterol, e Triglyceride. The data are expressed as the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 compared to D group; # P < 0.05, ## P < 0.01, ### P < 0.01 compared to D + ISO, χχ P < 0.01, χχχ P < 0.001 compared to D + C + ISO using one-way ANOVA followed by the tukey post hoc test
Antioxidant enzymes and cardiac injury markers
The results showed a significant decrease in GSH and CAT content in D + ISO and D + GW + ISO groups compared to diabetic group (P < 0.01, P < 0.001 respectively). Crocin administration significantly (P < 0.01, P < 0.001 respectively) elevated GSH and CAT content when compared with both groups D + ISO and D + GW + ISO. However, PPARγ antagonist injection showed opponent effects against crocin in D + C + GW + ISO groups (Fig. 3a, b).
Effect of crocin on antioxidant enzymes and cardiac injury markers in diabetic rats receiving ISO. a GSH, b CAT, c CK-MB, d LDH. The data are expressed as the mean ± SEM (n = 8). * P < 0.05, **P < 0.01, ***P < 0.001compared to D group; # P < 0.05, ## P < 0.01, ### P < 0.001 compared to D + ISO; χχ P < 0.01, χχχ P < 0.001 compared to D + C + ISO using One-way ANOVA followed by the tukey post hoc test
Also, the level of CK-MB was significantly decreased in D + ISO and D + GW + ISO groups compared to diabetic group, treatment with crocin significantly improved this cardiac injury marker level. In addition, crocin significantly augmented LDH level in the heart tissue compared to D (P < 0.05) and D + ISO (P < 0.01) groups. In D + GW + ISO group, level of both cardiac injury markers were significantly (P < 0.01, P < 0.001) decreased when compared with D + C + ISO group.
Inflammatory cytokines
Pro-inflammatory cytokines TNFα and IL-6 were significantly higher in both groups D + ISO and D + GW + ISO when compared with diabetic group while IL-10 level was significantly (P < 0.05, P < 0.01 respectively) lower. Crocin administration significantly attenuated TNFα and IL-6 compared to D and D + ISO groups. Level of IL-10 in crocin treated rats was significantly (P < 0.05) elevated than diabetic and D + ISO groups (P < 0.01). Moreover, Comparison between D + GW + ISO group and D + C + ISO demonstrated that TNFα and IL-6 were significantly increased in D + GW + ISO; also these cytokines were higher in D + C + GW + ISO group while IL-10 was lower compared to D + C + ISO, indicates that GW9662 has inhibited the protective effects of crocin (Fig. 4a-c).
Effect of crocin on inflammatory cytokines and PPARγ gene expression level in diabetic rats receiving ISO. a TNFα, b IL-6, c IL-10 d PPARγ. The data are expressed as the mean ± SEM (n = 8). *P < 0.05, **P < 0.01compared to D group; # P < 0.05, ## P < 0.01, ### P < 0.001 compared to D + ISO, χχ P < 0.01, χχχ P < 0.001 compared to D + C + ISO using One-way ANOVA followed by the tukey post hoc test
PPARγ gene expression
As shown in Fig. 4, PPARγ expression level was significantly (P < 0.05, P < 0.01 respectively) decreased in D + ISO and D + GW + ISO groups compared to diabetic group. Crocin significantly (P < 0.05) enhanced the expression of PPARγ compared to D, D + ISO and D + GW + ISO groups. When GW9662 was administrated along with crocin, it attenuated the level of PPARγ expression (Fig. 4d).
Discussion
This study was performed to determine whether the PPARγ activation is involved in protective effects of crocin in diabetic rats subjected to myocardial infarction.
The current research provided evidences that crocin improved inflammation and enhanced antioxidant capacity, while GW9662 as a PPARγ antagonist reversed these effects of crocin, reflects the potential involvement of PPARγ pathway in protective effects of crocin in diabetic rats subjected to MI.
It is well known that deficiency in insulin secretion or ineffectiveness in insulin action lead to diabetes mellitus. Therefore, hyperglycemia and also hyperlipidemia are two major consequences of diabetes [23]. It has been shown that dyslipidemia can promote the progression of MI in diabetic patients by increasing free radicals formation. As a model, it has been demonstrated that isoprenaline as a β-adrenergic agonist could cause myocardial infarction by generating highly cytotoxic free radicals and diminishing the oxygen supply to cardiac muscle cells [24]. Moreover, administration of isoprenaline led to morphological changes in cardiacmyocytes which resulted in hyperlipidemia as a major factor of myocardial infarction in rats [25,26,27]. Since insulin resistance and dyslipidemia occur in diabetes [28], we also observed in our study that LDL, VLDL, TG and cholesterol were significantly increased while HDL was reduced in diabetic rats challenged by ISO and CW9662. The previous studies reported that pioglitazone and rosiglitazone as PPARγ agonists licensed for the management of hyperglycemia in diabetes [29] and pioglitazone reduced TG, VLDL, LDL, while elevated HDL [30]. Also, it has been demonstrated that stimulation of β-adrenergic receptor promoted PPARγ activator to regulate lipid metabolism in adipose tissue. In our study, crocin administration significantly improved lipid profile. Therefore, it can be effective in reducing the complications of diabetes. In line with our results, other studies showed that crocin could exert lipid–lowering effects in high fat diet rats [15] and it improved lipid profile in patients with metabolic syndrome (MetS) [31]. Shirali et al. reported that crocin modified insulin sensitivity and lipid profile through strengthening sensitization of insulin receptors [32].
Myocardial infarction results in several pathological conditions such as necrosis, apoptosis and edema [29]. In order to confirm other harmful effects of diabetes and MI, we measured the level of CK-MB isoenzyme and LDH as cardiac injury markers and also antioxidant enzymes CAT and GSH in the heart tissue.
CK-MB and LDH during myocardial ischemia release in serum because the integrity of myocardial membrane is disrupted. Hence, we showed that administration of isoprenaline and GW9662 in diabetic rats significantly decreased the level of CK-MB, LDH, CAT, and GSH enzymes activity in the myocardium, while crocin improved CK-MB and LDH content and also maintained the antioxidant capacity in the heart tissue. In general, free radicals produced by toxic agents are removed by the intracellular antioxidant enzymes and GSH and CAT have been shown to defend myocardium from oxidative damage [30] and their activities were decreased in the diabetic rats [33]. It has been revealed that myocardial CK-MB isoenzyme activity was decreased in diabetic rats subjected to myocardial ischemia [33]. Also, both LDH and CK-MB has been shown to increase in serum in myocardial infarction induced by ISO.
According to previous researches, crocin was known to decrease oxidative stress in diabetes and ischemia models [11, 34]. Moreover, crocin could elevate GSH content and attenuated level of CK-MB in a cardio toxicity model with diazinon [35] and enhanced GSH, CAT and SOD in liver toxicity [36]. In a diabetic nephropathy model, crocin augmented antioxidant enzymes [37]. Furthermore, diabetes and myocardial infarction are related to inflammatory mediators that promote the release of interleukins from monocytes and macrophages. These Inflammatory factors stimulate more inflammatory reactions and block blood capillaries by exerting cytotoxic effects that could lead to tissue damage [38].
Increased expression of adhesive molecules during inflammation causes leukocyte activity and release of pro-inflammatory cytokines in the heart which resulting in tissue damage [39]. ISO leads to the development of oxidative stress and inflammation by activating β-adrenergic receptors which cause myocardial infarction. In the current experiment, TNFα, IL-6 and IL-10 as pro-inflammatory and anti-inflammatory cytokines in the heart tissue of rats were measured. Elevated pro-inflammatory cytokines and reduction of IL-10 were observed in diabetic rats challenged by ISO as well as improvement of these changes by crocin that indicating and approving the involvement of the inflammatory and oxidative pathways in diabetic cardiomyopathy.
Previous studies have reported that PPARγ plays an important role in inflammation by negatively regulating gene expression related to pro-inflammatory factors [39].
A number of researches provided evidences for effects of PPARγ agonists in ischemia and inflammation [40, 41]. It has been shown that Thiazolidinediones as PPARγ agonists could reduce vascular endothelial insulin resistance by decreasing inflammatory factors [42]. Pharmacological agonists of PPARγ, rosiglitazone and pioglitazone, exerted anti-inflammatory effects and regulated glucose hemostasis, so are important in treatment of obesity, diabetes and other metabolic disorders [43]. A previous study reported that activation of PPARγ prevented cardiac dysfunction by alleviation of inflammatory cytokines IL-6, TNFα and IL-1β [44]. Our results demonstrated that crocin elevated PPARγ expression and it could attenuate inflammatory cytokines and also enhanced Il-10, while GW9662 as PPARγ antagonist suppressed the protective effects of crocin, indicates that PPARγ may be involved in anti-inflammatory and also anti-oxidative effects of crocin.
Furthermore, it has been reported that activation of PPARγ linked to AMPK (adenosine mono phosphate kinase) could inhibit cardiac disorders, inflammation, and improved lipid and energy metabolism [45]. According to recent studies, crocin has been shown to control metabolic disorders via enhancing AMPK and PPARγ [46, 47].
A previous study has demonstrated that crocin protected against myocardial toxicity induced by doxorubicin by suppressing the inflammatory pathway [48]. Also, croin ameliorated hepatotoxicity through inhibition of oxidative stress and inflammatory mediators [36].
Conclusion
The findings of the current study reveal that crocin as an antioxidant compound protects myocardium against diabetes complications through activating PPARγ, decreasing inflammatory cytokines, elevating antioxidant capacity and improvement of cardiac injury markers activities. Thus, based on the above results, crocin may provide therapeutic approach in diabetic especially in conditions such as myocardial infarction by mediating PPARγ activation.
References
Mehta V, Verma P, Sharma N, Sharma A, Thakur A, Malairaman U. Quercetin, ascorbic acid, caffeine and ellagic acid are more efficient than rosiglitazone, metformin and glimepiride in interfering with pathways leading to the development of neurological complications associated with diabetes: A comparative in-vitro study. B-FOPCU. 2017;55:115–21.
Saklani R, Gupta SK, Mohanty IR, Kumar B, Srivastava S, Mathur R. Cardioprotective effects of rutin via alteration in TNF-α, CRP, and BNP levels coupled with antioxidant effect in STZ-induced diabetic rats. Mol Cell Biochem. 2016;420:65–72.
Korkmaz-Icoz S, Lehner A, Li S, Vater A, Radovits T, Hegedus T, et al. Mild type 2 diabetes mellitus reduces the susceptibility of the heart to ischemia/reperfusion injury: identification of underlying gene expression changes. J Diabetes Res. 2015:396–414.
Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23.
Wang R, Xi L, Kukreja RC. PDE5 inhibitor tadalafil and hydroxychloroquine cotreatment provides synergistic protection against type 2 diabetes and myocardial infarction in mice. J Pharmacol Exp Ther. 2017;361:29–38.
Kumar J, Menon V. Changes in levels of lipid peroxides and activity of superoxide dismutase and catalase in diabetes associated with myocardial infarction. J Exp Biol. 1992;30:122–7.
Zaafan MA, Zaki HF, El-Brairy AI, Kenawy SA. Protective effects of atorvastatin and quercetin on isoprenaline-induced myocardial infarction in rats. B-FOPCU. 2013;51:35–41.
Varga ZV, Giricz Z, Liaudet L, Haskó G, Ferdinandy P, Pacher P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. BBA-Mol Basis Dis. 1852;2015:232–42.
Nakamura T, Nishi H, Kokusenya Y, Hirota K, Miura Y. Mechanism of antioxidative activity of fluvastatin-determination of the active position. Chem Pharm Bull. 2000;48:235–7.
Hazman O, Aksoy L, Buyukben A. Effects of crocin on experimental obesity and type-2 diabetes. Turk J Med Sci. 2016;46:1593–602.
Goyal S, Arora S, Sharma A, Joshi S, Ray R, Bhatia J, et al. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine. 2010;17:227–32.
Dianat M, Esmaeilizadeh M, Badavi M, Samarbaf-zadeh AR, Naghizadeh B. Protective effects of crocin on ischemia-reperfusion induced oxidative stress in comparison with vitamin E in isolated rat hearts. Jundishapur J Nat Parm Prod. 2014;9:e17187.
Batarseh YS, Bharate SS, Kumar V, Kumar A, Vishwakarma RA, Bharate SB, et al. Crocus sativus extract tighten the blood-brain barrier, reduces amyloid-β load and related toxicity in 5XFAD mice. ACS Chem Neurosci. 2017;168:1756–66.
Lee IA, Lee JH, Baek NI, Kim DH. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biol Pharm Bull. 2005;28:2106–10.
Asdaq SMB, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol. 2010;162:358–72.
Kianbakht S, Hajiaghaee R. Anti-hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan-induced diabetic rats. J Med Plants. 2011;3:82–9.
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J Biol Chem. 1995;270:12953–6.
Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh W, et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and-independent pathways. J Biol Chem. 2006;281:8748–55.
Hasegawa T, Okada K, Okita Y, Pinsky DJ. Antioxidant properties of pioglitazone limit nicotinamide adenine dinucleotide phosphate hydrogen oxidase and augment superoxide dismutase activity in cardiac allotransplantation. J Heart Lung Transplant. 2011;301:186–96.
Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Pharm Adv Res. 2011;2:236.
Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH, et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015;243:107–19.
Jadhav J, Puchchakayala G. Hypoglycemic and antidiabetic activity of flavonoids: boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Int J Pharm Sci Res. 2012;4:251–6.
De Oliveira LS, Thomé GR, Lopes TF, Reichert KP, De Oliveira JS, Da Silva PA, et al. Effects of gallic acid on delta–aminolevulinic dehydratase activity and in the biochemical, histological and oxidative stress parameters in the liver and kidney of diabetic rats. Biomed Pharmacother. 2016;84:1291–9.
Loh LK, Sahoo KC, Kishore K, Ray R, Nag TC, Kumari S, et al. Effects of thalidomide on isoprenaline-induced acute myocardial injury: a haemodynamic, histopathological and ultrastructural study. Basic Clin Pharmacol Toxicol. 2007;100:233–9.
Ravichandran L, Puvanakrishnan R, Joseph K. Alterations in the heart lysosomal stability in isoproterenol induced myocardial infarction in rats. Int J Biochem Res. 1990;22:387–96.
Mohanty I, Arya DS, Dinda A, Talwar AA, Joshi S, Gupta SK. Mechanisms of cardioprotective effect of Withania somnifera in experimentally induced myocardial infarction. Basic Clin Pharmacol Toxicol. 2004;94:184–90.
Freedman DS, Gruchow HW, Anderson AJ, Rimm AA, Barboriak JJ. Relation of triglyceride levels to coronary artery disease: the Milwaukee cardiovascular data registry. Am J Epidemiol. 1988;127:1118–30.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25.
Agrawal YO, Sharma PK, Shrivastava B, Ojha S, Upadhya HM, Arya DS. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS One. 2014;9:e111212.
Goyal SN, Reddy NM, Patil KR, Nakhate KT, Ojha S, Patil CR, et al. Challenges and issues with streptozotocin-induced diabetes–a clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem Biol Interact. 2016;244:49–63.
Javandoost A, Afshari A, Nikbakht-Jam I, Khademi M, Eslami S, Nosrati M, et al. Effect of crocin, a carotenoid from saffron, on plasma cholesteryl ester transfer protein and lipid profile in subjects with metabolic syndrome: a double blind randomized clinical trial. ARYA Atheroscler. 2017;13(5):245.
Shirali S, Bathaie SZ. And M. Nakhjavani. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phytother Res. 2013;27(7):1042–7.
Mahajan UB, Chandrayan G, Patil CR, Arya DS, Suchal K, Agrawal YO. The protective effect of Apigenin on myocardial injury in diabetic rats mediating activation of the PPAR-γ pathway. Int J Mol Sci. 2017;18:756.
Yang X, Huo F, Liu B, Liu J, Chen T, Li J, et al. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. J Mol Neurosci. 2017;61:581–9.
Razavi BM, Hosseinzadeh H, Movassaghi AR, Imenshahidi M, Abnous K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem Biol Interact. 2013;203(3):547–55.
Bahashwan S, Hassan MH, Aly H, Ghobara MM, El-Beshbishy H, Busati I. Crocin mitigates carbon tetrachloride-induced liver toxicity in rats. J Taibah Univ Sci. 2015;10(2):140–9.
Altinoz E, Oner Z, Elbe H, Cigremis Y, Turkoz Y. Protective effects of saffron (its active constituent, crocin) on nephropathy in streptozotocin-induced diabetic rats. Hum ExpToxicol. 2015;34:127–34.
Wang Y, Qi X, Wang C, Zhao D, Wang H, Zhang J. Effects of propofol on myocardial ischemia-reperfusion injury in rats with type-2 diabetes mellitus. Biomed Rep. 2017;6:69–74.
Han J, Wang D, Ye L, Li P, Hao W, Chen X, et al. Rosmarinic acid protects against inflammation and cardiomyocyte apoptosis during myocardial ischemia/reperfusion injury by activating peroxisome proliferator-activated receptor gamma. Front Pharmacol. 2017;8:456.
Wang H, Zhu Q, Ye P, Li Z, Li Y, Cao Z, et al. Pioglitazone attenuates myocardial ischemia-reperfusion injury via up-regulation of ERK and COX-2. Biosci Trends. 2012;6:325–32.
Rani N, Bharti S, Bhatia J, Nag T, Ray R, Arya DS. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem Biol Interact. 2016;250:59–67.
Zhang Y, Zhan RX, Chen JQ, Gao Y, Chen L, Kong Y, et al. Pharmacological activation of PPAR gamma ameliorates vascular endothelial insulin resistance via a non-canonical PPAR gamma-dependent nuclear factor-kappa B trans-repression pathway. Eur J Pharmacol. 2015;754:41–51.
Corrales P, Vidal-Puig A, Medina-Gómez G. PPARs and metabolic disorders associated with challenged adipose tissue plasticity. Int J Mol Sci. 2018;19(7):2124.
Peng S, Xu J, Ruan W, Li S, Xiao F. PPAR-γ activation prevents septic cardiac dysfunction via inhibition of apoptosis and necroptosis. Oxid Med Cell. 2017;2017. https://doi.org/10.1155/2017/8326749.
Liu HJ, Liao HH, Yang Z, Tang QZ. Peroxisome proliferator-activated receptor-γ is critical to cardiac fibrosis. PPAR Res. 2016;2016:1–12.
Algandaby MM. Crocin prevents metabolic syndrome in rats via enhancing PPAR-gamma and AMPK. Saudi J Biol Sci. 2020;27:1310–6.
Behrouz V, Dastkhosh A, Hedayati M, Sedaghat M, Sharafkhah M, Sohrab G. The effect of Crocin supplementation on glycemic control, insulin resistance and active AMPK levels in patients with type 2 diabetes. Diabetol Metab Syndr. 2020;12:59.
Elsherbiny NM, Salama FM, Said E, El-Sherbiny M, Al-Gayyar MM. Crocin protects against doxorubicin-induced myocardial toxicity in rats through down-regulation of inflammatory and apoptic pathways. Chem Biol Interact. 2016;24:39–48.
Acknowledgments
This paper was a part of thesis of Neda Dashtbozorgi, a PhD student of Jundishapur University of Medical Sciences. We would like to thanks Research affair of Ahvaz Jundishapur University of Medical Sciences for financial support.
Funding
This study was supported by funds received from the Physiology Research Center of Ahvaz Jundishapur University of Medical Sciences (Grant No. APRC-9702).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Badavi, M., Mard, S.A., Dianat, M. et al. Crocin attenuates oxidative stress and inflammation in myocardial infarction induced by isoprenaline via PPARγ activation in diabetic rats. J Diabetes Metab Disord 19, 1517–1525 (2020). https://doi.org/10.1007/s40200-020-00686-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00686-y