Abstract
Purpose
Association between vitamins C (VC)/ E (VE) and cancer survival is inconsistent. This systematic review is aimed to summarize trials for effects of VC/VE on cancer survival.
Methods
Relevant English trials were retrieved from PubMed, Cochrane Library, Embase, Web of Science, Scopus databases, and Clinicaltrials.gov through 21/June/2022. Inclusion criteria were all trials which assessed sole/combinations intake of VC/VE on survival rate, mortality, or remission of any cancer. Exclusion criteria were observational and animal studies.
Results
We reached 30 trials conducted on 38,936 patients with various cancers. Due to severe methodological heterogeneity, meta-analysis was impossible. High dose VC + chemotherapy or radiation was safe with an overall survival (OS) 182 days − 21.5 months. Sole oral or intravenous high dose VC was safe with non-significant change in OS (2.9–8.2 months). VE plus chemotherapy was safe, resulted in stabling diseases for 5 years in 70- 86.7% of patients and OS 109 months. It was found 60% and 16% non-significant reductions in adjusted hazard ratio (HR) deaths or recurrence by 200 mg/d tocotrienol + tamoxifen in breast cancer, respectively. Sole intake of 200–3200 mg/d tocotrienol before resectable pancreatic cancer was safe and significantly increased cancer cells’ apoptosis. Combination VC and VE was non-significantly reduced 7% in rate of neoplastic gastric polyp.
Conclusion
Although our study is supported improvement of survival and progression rates of cancers by VC/VE, more high quality trials with large sample sizes are required to confirm.
PROSPERO Registration number
CRD42020152795.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, 19.3 million new cases and about 10 million deaths related to cancer were reported in 2020. According to the Global and Regional Estimates of the Incidence and Mortality for Cancers (GLOBOCAN 2020), the global burden of cancer is predicted to rise 47% over the next two decades (28.4 million cases) due to the aging population and increase in the risk factors associated with globalization and socioeconomic development [1]. As cancer is the leading cause of death before the age of 70 years and its incidence and prevalence rates are both significantly in developed and developing countries, efforts to provide efficient complementary cancer care is vital for global cancer control. Antioxidants are proposed as potential therapeutic agents against cancer [1, 2].
Existing evidence demonstrates that patients with cancer experience vitamin C (VC) deficiency due to reduced oral intake, co-occurrence of infection and inflammation, and vitamin loss during the treatment process; administration of VC, thus, should be included in oncologic care as a potential adjuvant therapy in order to improve quality of life in patients [2, 3]. The metabolism of tumor cells could increase the rate of reactive oxygen species (ROS). This is while VC plays anti-tumorigenic roles via scavenging ROS, inhibiting oxidative stress, and exerting local antioxidant effects through killing tumor cells or restricting their growth and metastasis [4,5,6]. Besides, vitamin c deficiency becomes more severe after therapies such as surgery, chemotherapy and radiation [2, 7]. Therefore, quality of life in patients with cancer is affected by the oxidative stress-associated side effects including gastrointestinal disorders, anemia, fatigue, mental disorders and lipid abnormalities [8]. Extensive search in literature supports the idea that intravenous injection of high doses VC could enhance the efficiency of anti-cancer drugs or ameliorate their side effects [9, 10]. Moreover, some studies link higher VC intake with reduced cancer mortality rate [11]. However, the therapeutic effects of high-doses VC is still controversial and the interaction between this nutrient and tumor cells remains unclear and might be more complex than previously thought [12, 13].
Vitamin E (VE), a potent fat-soluble antioxidant that could stop the production of ROS, is another candidate for adjuvant therapy in cancer. This vitamin could be affect cancer cells apoptosis, reduce chemotherapeutic-induced ROS and enhance the therapeutic effects of anti-cancer agents [14,15,16]. Moreover, γ-tocotrienol could help reverse multidrug resistance in cancer patients [17]. Noteworthy, various VE isoforms are revealed to have different pharmacological properties; i.e. tocotrienol is reported to possess superior anti-inflammatory and antioxidant properties compared with α-tocopherol [18]. Furthermore, potential anti-radiation damaging properties are limited to tocotrienols [18]. However, the anti-tumor properties of VE are still unclear and more studies are needed.
Several systematic reviews have assessed the anti-cancer properties of VC or VE [19, 20], however, most of them are conducted on both observational studies and clinical trials [21,22,23,24,25]. This is while unbiased interpretation of the additive value of VC and VE on the survival rate or progression of cancer is required to conduct systematic review on a specific type of studies. The current comprehensive systematic review was conducted on clinical trials alone to investigate the effectiveness of single or combined VC and VE consumption on various types of cancer.
Methods
This systematic review was registered in International Prospective Register of Systematic Reviews (PROSPERO) with the registration code CRD42020152795. A comprehensive systematic search was carried out in PubMed, Cochrane Library, Embase, Web of Science and Scopus databases to find trials published up to 21 June 2022, assessing the efficacy of sole or combined dietary antioxidative vitamin C and E on cancer survival. In addition, we searched grey literature; https://clinicaltrials.gov to avoid publication bias. The search terms were “cancer”, “survival”, “vitamin C”, “vitamin E”, along their Medical Subject headings (MeSH) terms and Emtree limited to human. The trials were limited to English language ones. Details of the utilized search strategy in Embase is shown as a supplementary file; Table S1.
The study was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline [26] (PRISMA 2020). Firstly, two researchers independently screened the title and abstract of the retrieved studies based on the inclusion and exclusion criteria. Then, the full text of eligible studies was attained. If the full text was not available, the authors were contacted via e-mail. Thereafter, the hand searching of the reference list of the included studies was performed. The data extraction and quality assessment of the included studies are done by two independent researchers. Possible disagreements were resolved by discussion and consensus with a third researcher or the corresponding author. The study was approved by the Ethics Committee of Vice-Chancellor in Research Affairs-Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1397.926).
All types of trials such as controlled (CTs) and randomized controlled trials (RCTs) that met the following criteria were included, (1) all trials that assessed the effects of dietary antioxidative vitamins VC (ascorbic acid) and VE alone or in combination with tumor responsiveness, including survival rate, mortality, remission and stage of any cancer, (2) those reporting the relative risk (RR) or odds ratio (OR) of the mentioned outcomes, and (3) those comparing the effects of VC and VE with placebo or standard treatment of cancer. Exclusion criteria consisted of (1) observational studies, reviews, experimental, animal models, reports, and letters, (2) all trials on the combination of other antioxidants with VC and VE.
The following data were extracted; authors, year of publication, study design, the characteristics of the participants (total number, age, gender), sample size of each group (intervention or control), type of consumed vitamin, dosage and duration of each treatment (intervention or placebo), survival, death or progression rates, and JADAD score. Any discrepancy in data extraction was resolved through discussion or consulting with an expert. The methodological quality of each included CTs/RCTs and also their risk of bias were appraised using the JADAD scoring system, and ”Cochran risk of bias” by two independent reviewers[27, 28]. Clinical trials with JADAD score of < 3 were considered as low quality. Due to heterogeneity among studies performing meta-analysis was not possible.
Results
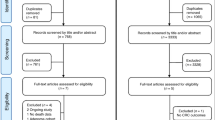
According to the PRISMA flowchart (Fig. 1), 30 articles were included in the present systematic review [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Characteristics of the included trials and the results of their Cochrane quality assessment are shown in Tables 1 and 2, respectively. PRISMA checklist is available as a Supplementary file; Table S2.
The included studies were different phases of clinical trials conducted on 38,936 patients with various primary, advanced or metastatic cancers. Patients in 25 of these studies [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] were supplemented with VC, four studies [54,55,56,57] with VE and one [58] with combined VC and VE. The participants in most of the studies were adults from both genders, aged 19–93 years. The majority of the 25 trials assessed the effects of the combination of VC and chemotherapy or radiation. Sole intake of VC was studied in 9 trials without any control group [34, 40, 41, 44,45,46,47, 51, 53] and with placebo in another 4 trials [48,49,50, 52]. Most trials included patients with advanced/metastatic stages of cancers; eight studies, however, included patients in primary stages [30, 33, 44, 48, 50, 56,57,58]. Details of the included studies are described in the following.
Sole intake of VC (oral or IV) was reported to be safe, causing cancer progression in most studies, non-significant reduction in death RR or improved OS [35, 40, 41, 45,46,47,48,49,50,51,52], except for a non-randomized trial using simultaneous IV and oral VC. The combination significantly increased the survival rate (300 days) [53]. Sole intake of oral VC resulted in significant increase in regression RR: 3.3 (1.1, 9.5), or non-significant reduction in progression RR: 0.5 (0.2, 1.1) in patients with histologic multifocal atrophic gastritis [48]. As for patients with newly diagnosed breast cancer, however, a non-significant increase in death RR: 1.52 (0.72, 3.23) with no change in survival rate was reported [50]. The combination of VC and chemotherapy or standard anti-cancer regimen in patients with primary cancer was safe and associated with a significant number of complete remission, significant increase in median overall survival (OS) and significant decrease in death hazard ratio (HR): 0.47 (0.26, 0.84) [30, 33, 44].
Sole intake of VC (mostly IV VC) was reported to be safe with no sign of regression and even cancer progression in most patients. It also resulted in non-significant difference in median OS and significant increase in survival in others [34, 40, 41, 45,46,47, 49, 52, 53].
The combination of high doses of VC and chemotherapy or radiation was reported to be safe, increasing the median progression-free survival (PFS) from 89 days in patients with stage IV pancreatic cancer [43] to 21.5 months in patients with glioblastoma [36]. Median OS was from 182 days in patients with stage IV pancreatic cancer [43] to 21.5 months in patients with glioblastoma [36].
Comparing low or high doses of VC versus placebo, the study also reported a non-significant increase (52%) in adjusted HR death [50]. Significant increase in the RR of cancer regression was reported in the Correa et al. [48] study, in which atrophic gastritis was treated using 1 g/twice daily oral VC: 3.3 (95% CI: 1.1, 9.5). High doses of VC alone resulted in a non-significant change in the median OS from 2.9 to 8.2 months after treating patients with advanced colorectal and other terminal cancers for up to 26 months [49, 51]. Most of the trials had low quality except 6 trials [48, 49, 52, 56,57,58].
Among the four VE trials, cancer patients were treated with combined VE and chemotherapy in two [54, 56], VE and placebo in one [57] and VE alone without any control group in one trial [55]. VE dosages were ranged from 200 to 3200 mg/d which used up to 12 years. The combination of VE and chemotherapy was safe, resulting in no changes in the disease progress for 5 years in 70-86.7% of patients with refractory ovarian cancer, with a median PFS of 6.9 months and median OS of 109 months [54, 56]. In addition, a 60% non-significant reduction was noted in adjusted HR death and a 16% non-significant reduction in HR recurrence among patients in early stages of breast cancer [56]. The consumption of 400 IU/d VE for about 8 years versus placebo resulted in a 16% non-significant decrease in mortality rate [57]. Sole intake of 200–3200 mg/d VE before resectable pancreatic cancer was reported safe, resulting in a significant increase in the apoptosis of cancer cells [55]. Half of the trials, conducted on 35,773 subjects, were of high quality [56, 57].
A single trial was designed to treat colorectal polyps with a combination of VC and VE versus placebo for two years [58]. The treatment led to a 14% non-significant reduction in the number of any polyps and 7% in neoplastic polyps. The study had a high quality, with a JADAD score of 5.
Discussion
The systematic review suggested that the existing literature denotes the beneficial effects of VC, VE and their combinations on the death, progression and survival rate of cancers. In addition, the intake of antioxidative vitamins C and E in phase I/II trials of different cancers are safe and tolerable.
The key role of oxidative stress in the pathogenesis of cancers is well established. ROS and other free radicals can mediate the phenotypic and genotypic changes in the cells, from mutation to neoplasia through causing oxidative damage to DNA and chromosomes [7]. Certain internal and external defense mechanisms help to stop ROS formation [59]. On the other hand, the internal antioxidant defense system can be reinforced through external sources of antioxidants. Thereby, adopting a healthy lifestyle through the consumption of fruits and vegetables as natural sources of antioxidants is recommended to help modify oxidative stress and prevent cancer [60,61,62]. From among the antioxidative vitamins, different forms of VC and VE, ranging from dietary supplements to infusion in pharmacological doses, are consumed by cancer patients [63].
VC and cancer survival
VC is a water-soluble vitamin with active transport abilities. Thereby, its intake and distribution in the body is simple. Moreover, VC intake is essential to prevent deficiency due to its high turnover rate, especially during the illness, and its storage in body being impossible. Low levels of VC are noted in both plasma and tissue of cancer patients [7, 64]. Overall, two mechanisms for the anti-cancer activity of VC, including the redox mechanism (pro-oxidant activity) and co-factor activity can result in oxidative damage and up-regulation of epigenetic demethylases/ decreasing hypoxic stress [63]. Based on the pro-oxidant activity, VC can reduce the transition of metal ions such as Fe3+ and Cu2+ via chelation and hydrogen peroxide (H2O2) generation. The latter is cytotoxic, in the presence of oxygen, and can result in increased cell cycle arrest, up-regulation of p53, reduced levels of ATP, mitochondrial dysfunction, cell apoptosis and inhibited expression of antioxidant genes such as nuclear factor erythroid 2–related factor 2 (NrF-2) [65,66,67]. Potential antitumor effects of VC are shown by extracellular conversion of ascorbic acid into dehydroascorbic acid for H2O2 generation [40]. Thereby, treatment with dehydroascorbic acid may circumvent the antitumor properties of VC. Adequate access of tumor cells to VC throughout its effective distribution in the tumor environment is critical for achieving such antitumor effects.
The toxic effects of VC, used alone or in combination with chemotherapy or radiation, on cancers cell, even in low concentrations (1 µM), are shown in in vitro studies [68, 69]. The potential antitumor activities of VC are shown when it is consumed along with chemotherapeutic agents such as etoposide, cisplatin, 5-fluorouracil, doxorubicin, and paclitaxel [40]. Other properties include the ability of VC to sensitizing cancer cells to chemotherapy drugs such as gemcitabine and generating a synergistic cytotoxic response [70]. In the majority of the included studies, VC was consumed alongside chemotherapy agents. Current study, therefore, reports the synergistic effect when different dosages of VC, ranging from 0.2 g/kg/week to 100 g/week for up to 52 weeks, are used in combinations with chemotherapy agents in patients with non-small-cell lung cancer, glioblastoma, colorectal, acute myeloid leukemia, non-Hodgkin’s lymphoma, and advanced ovarian or pancreatic cancers [29,30,31,32,33, 36,37,38, 42, 43]. VC administration in all of the abovementioned trials was shown to not only be safe and tolerable, but also result in increased median survival from 89 days to 15.3 months, and reduced progression rate. VC in combination with certain anticancer agents helped reduce the mass of advanced pancreatic tumor by 10% in Monti et al. study [43].
Infusion of high doses of VC alone for 12 weeks did not lead to remission in prostate cancer patients [34]. Similar effects were reported in some of the included trials, in which oral or intravenous VC was administered alone [40, 48, 51, 54]. The majority of trials conducted on the consumption of VC alone reported its safety without beneficial effects on the remission and progression rates [35, 41, 46,47,48,49,50, 52]. The reason behind such effects could be the short duration of intervention [40, 48, 51], or the negative effect of earlier immunosuppressive treatment [54]. VC administration is also shown to increase its concentration as well as reduce the adverse events induced by the chemotherapeutic agents such as hepatotoxicity, cardiomyopathy, and lipid oxidation [71].
Some of the included trials did not show any significant difference in the median OS following oral VC administration compared with placebo [49, 52]. In 1990s, the researchers had shown a significant difference between oral and intravenous VC pharmacokinetics. Consequently, in vitro studies represented valuable information regarding the intravenous VC (IVC) mechanism, whereas preclinical studies offered useful evidence on the IVC efficacy [67, 72]. The clinical evidence of the positive effects of VC is perceived by an open-label trial [33] conducted on patients with acute myeloid leukemia and treated with Decitabine in the presence/absence of VC. They reported improved median OS following the administration of Decitabine + low doses of IVC. Enhanced up-regulation of ten-eleven-translocation (TET) proteins is believed to be the underlying mechanism. TET enzymes are important for DNA methylation and their function is attenuated in patients with acute leukemia [33]. Overall, evidence suggests positive interaction between IVC and other cancer agents; details of this co-administration, however, are diverse and occasionally uncertain. Moreover, the response of various cancers to VC supplementations is probably dissimilar due to their diverse underlying mechanisms. Accordingly, future research should focus on the VC regimens in specific cancers or subtypes.
Overall, our results are in line results of previous systematic review on IVC [22]. In a systematic review conducted by van Gorkom, [23], 19 studies (including clinical trials and observational studies) were evaluated regarding the effectiveness and safety of VC administration in cancer. The results of the systematic review did not demonstrate a clinically relevant beneficial effect of VC supplementation on the overall survival and clinical status of most cancer patients. This may be due to the low quality of included studies as well as the heterogeneous nature of the patients groups. In another systematic review by Jacob et al. [24], the antitumor effects and toxicity of VC treatment was evaluated in 34 trials and observational studies. None of the five included RCTs was reported to result in any statistically significant improvement in survival or reduction in toxicity with VC compared to control group. Beneficial therapeutic effects of VC, however, were observed in the included uncontrolled, case reports and observational studies.
VE and cancer survival
VE is a lipid-soluble antioxidative vitamin that can suppress the proliferation, growth, and migration of cancer cells [57]. The potential anti-cancer properties of VE may be attributed to its capabilities in enhancing apoptosis and cell cycle arrest as well as suppressing two important transcription activators, namely NF-kβ and Signal Transducer and Activator of Transcription 3 (STAT3) involved in angiogenesis and metastasis [73]. Moreover, VE can interfere with the production of ROS through buthionine sulfoximine (BSO) production following cancer cell death [74].
Despite of various in vitro and preclinical studies evaluating the anticancer effects of VE [73], limited clinical studies were found in this regard [54,55,56,57]. As a result, controversial anti-cancer effects are reported for VE supplementation. Intake of up to 3200 mg/d VE for two weeks before pancreatic exocrine cancer resection was reported to be safe with no effects on the survival rate [55]. Thomsen et al. [54] in their trial, however, reported low toxicity rates following the administration of high doses of delta tocotrienol (900 mg daily), an analogue of VE + Bevacizumab until symptoms of grade III toxicity appeared or the patients with refractory ovarian cancer decided not to continue the treatment anymore. Median PFS and median OS were reported to be 6.9 and 109 months, respectively. Bevacizumab is an anti-tumor drug used in many cancers. Combining Bevacizumab with chemotherapy in a phase III trial helped retain the Bevacizumab activity for 1.4 months after tumor progression [75]. This finding suggests that the anticancer effect observed in Thomsen et al. [54] trial should have been secondary to the synergistic effects of delta tocotrienol and Bevacizumab. Despite the potent antitumor properties of combined tocotrienol and tamoxifen, a trial on breast cancer patients reported a non-significant increase (60%) in 5-year survival rate following the consumption of combined tocotrienol with tamoxifen compared with tamoxifen alone [56].
Lippman et al. [57] in their trial reported a non-significant reduction in HR deaths following the consumption of 400 IU α-tocopherol (VE analogue) versus not taking it in patients with primary prostate cancer or other malignancies. Possible reasons behind this finding include lower efficacy of high doses of α-tocopherol than its lower doses, and the adverse effects of high dose α-tocopherol on cytochrome p450. In addition, the authors reported the protective effects of VE on smoker cancer patients compared with non-smoker ones. This is while less than 60% of the study participants were smokers [57]. Our findings are in line with Alkhenizan et al. meta-analysis reporting the non-significant effect of VE intake on cancer mortality regardless of the type of cancer [25]. They included twelve RCTs with 167,025 participants to assess the effects of VE intake alone or in combination with other supplements on cancer prevention. They reported a statistically significant reduction in the incidence of prostate cancer, which is not among the objectives of the current study. As a result, the limited number of studies and the variety of their findings makes drawing concrete decisions about the positive effects of VE supplements on cancer outcomes impossible. Well-designed trials with large sample size on VE therapy alone or as an adjuvant are therefore needed to confirm abovementioned findings.
Co-intake of VC, VE and cancer survival
There is dearth of data regarding the effects of VC and VE co-supplementation on cancer outcome. A single trial was found on co-treatment of cancer patients with VC and α-tocopherol [58]. Compared with placebo, the combinations of VC and VE in patients with adenomatous colorectal polyps resulted in a non-significant difference in the mortality and recurrence rates [58]. Certain factors influencing these results are overestimation due to misdiagnosis of polyps in the first exam, higher rate of colonoscopy examinations in the follow-up period for individuals in the vitamin group, and low vitamin compliance. Several laboratories have detected mutagens in the stool of colon cancer sufferers, confirming that VE and VC supplementation can reduce the number of fecal mutagens [76, 77]. Clinical evidence on this finding, however, is scarce and more studies are needed in this regard.
To our knowledge, the current study is the first systematic review of trials assessing the effects of VC and VE supplementation on cancer survival rate. We report not only their safety and tolerability but also their effectiveness on the survival and progression rates. The study, however, suffers from several limitations. The main limitation is related to the quality of existing evidence. The small sample size and the absence of control group in the majority of the included trials might have interfered with the interpretation of the results. In addition, the differences noted in the studied population and cancer type and stage (ranging from primary to advanced forms) resulted in methodological heterogeneity and thus made meta-analysis impossible.
Conclusion
In conclusion, this systematic review aimed at assessing the beneficial effects of VC and VE supplementation on cancer responsiveness. Although VC and VE intake helped improve the survival and progression rates, the majority of trials were designed as uncontrolled trials with a small sample size and no appropriate control group. Thereby, high-quality well-designed controlled trials are required to confirm our results.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- BSO :
-
Buthionine sulfoximine
- CT :
-
Controlled trial
- GLOBOCAN :
-
Global and Regional Estimates of the Incidence and Mortality for Cancers
- H2O2 :
-
Hydrogen peroxide
- HR :
-
Hazard ratio
- IVC :
-
Intravenous VC
- MeSH :
-
Medical Subject headings
- NrF-2 :
-
Nuclear factor erythroid 2–related factor 2
- OR :
-
Odds ratio
- OS :
-
Overall survival
- PFS :
-
Progression-free survival
- PRISMA :
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- PROSPERO :
-
International Prospective Register of Systematic Reviews
- ROS :
-
Reactive oxygen species
- RR :
-
Relative risk
- STAT3 :
-
Signal Transducer and Activator of Transcription 3
- TET :
-
Ten-eleven-translocation
- VC :
-
Vitamin C
- VE :
-
Vitamin E
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Abiri B, Vafa M, Vitamin C, Cancer. The role of vitamin C in disease progression and quality of life in cancer patients. Nutr Cancer. 2021;73:1282–92.
Morrin HR, Pullar JM, Spencer E, Vissers MCM, Robinson BA, Dachs GU. Low vitamin C status in patients with cancer is associated with patient and tumor characteristics. Nutrients. 2020;12:2338. https://doi.org/10.3390/nu12082338.
Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005;102:13604–9.
Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104:8749–54.
Fu J, Wu Z, Liu J, Wu T, Vitamin C. A stem cell promoter in cancer metastasis and immunotherapy. Biomed Pharmacother. 2020;131:110588. https://doi.org/10.1016/j.biopha.2020.110588.
Mehdi WA, Zainulabdeen JA, Mehde AA. Investigation of the antioxidant status in multiple myeloma patients: effects of therapy. Asian Pac J Cancer Prev. 2013;14:3663–7.
Vollbracht C, Schneider B, Leendert V, Weiss G, Auerbach L, Beuth J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo. 2011;25:983–90.
Codini M. Why Vitamin C could be an excellent complementary remedy to conventional therapies for breast cancer. Int J Mol Sci. 2020;21:8397. https://doi.org/10.3390/ijms21218397.
Li P, Han B, Jia H, Mo S, Deng K, Huang Y, et al. High-dose vitamin C tends to kill colorectal cancer with high MALAT1 expression. J Oncol. 2020; 2020:2621308. https://doi.org/10.1155/2020/2621308.
Zhang D, Xu P, Li Y, Wei B, Yang S, Zheng Y, et al. Association of vitamin C intake with breast cancer risk and mortality: a meta-analysis of observational studies. Aging. 2020;12:18415–35.
Roa FJ, Peña E, Gatica M, Escobar-Acuña K, Saavedra P, Maldonado M, et al. Therapeutic use of vitamin C in cancer: physiological considerations. Front Pharmacol. 2020;11:211. https://doi.org/10.3389/fphar.2020.00211.
Zasowska-Nowak A, Nowak PJ, Ciałkowska-Rysz A. High-dose vitamin C in advanced-stage cancer patients. Nutrients. 2021;13:735. https://doi.org/10.3390/nu13030735.
Moore C, Palau VE, Mahboob R, Lightner J, Stone W, Krishnan K. Upregulation of pERK and c-JUN by γ-tocotrienol and not α-tocopherol are essential to the differential effect on apoptosis in prostate cancer cells. BMC Cancer. 2020;20:428. https://doi.org/10.1186/s12885-020-06947-6.
Figueroa Gonzalez D, Young F. Gamma tocopherol reduced chemotherapeutic-induced ROS in an ovarian granulosa cell line, but not in breast cancer cell lines in vitro. Antioxid (Basel). 2020;9:51. https://doi.org/10.3390/antiox9010051.
Wei CW, Yu YL, Chen YH, Hung YT, Yiang GT. Anticancer effects of methotrexate in combination with α–tocopherol and α–tocopherol succinate on triple–negative breast cancer. Oncol Rep. 2019;41:2060–6.
Ding Y, Fan J, Fan Z, Zhang K. γ-Tocotrienol reverses multidrug resistance of breast cancer cells through the regulation of the γ-Tocotrienol-NF-κB-P-gp axis. J Steroid Biochem Mol Biol. 2021;209:105835. https://doi.org/10.1016/j.jsbmb.2021.105835.
Peh HY, Tan WS, Liao W, Wong WS. Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther. 2016;162:152–69.
Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223–31.
Chen J, Jiang W, Shao L, Zhong D, Wu Y, Cai J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. Int J Food Sci Nutr. 2016;67:744–53.
Fritz H, Flower G, Weeks L, Cooley K, Callachan M, McGowan J, et al. Intravenous vitamin C and cancer: a systematic review. Integr Cancer Ther. 2014;13:280–300.
Nauman G, Gray JC, Parkinson R, Levine M, Paller CJ. Systematic review of intravenous ascorbate in cancer clinical trials. Antioxid (Basel). 2018;7:89. https://doi.org/10.3390/antiox7070089.
van Gorkom GNY, Lookermans EL, Van Elssen CHMJ, Bos GMJ. The Effect of Vitamin C (Ascorbic Acid) in the treatment of patients with cancer: a systematic review. Nutrients. 2019;11:977. https://doi.org/10.3390/nu11050977.
Jacobs C, Hutton B, Ng T, Shorr R, Clemons M. Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? a systematic review. Oncologist. 2015;20:210–23.
Alkhenizan A, Hafez K. The role of vitamin E in the prevention of cancer: a meta-analysis of randomized controlled trials. Ann Saudi Med. 2007;27:409–14.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. https://doi.org/10.1016/0197-2456(95)00134-4.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Ou J, Zhu X, Chen P, Du Y, Lu Y, Peng X, et al. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J Adv Res. 2020;24:175–82. https://doi.org/10.1016/j.jare.2020.03.004.
Allen BG, Bodeker KL, Smith MC, Monga V, Sandhu S, Hohl R, et al. First-in-human phase i clinical trial of pharmacologic ascorbate combined with radiation and temozolomide for newly diagnosed glioblastoma. Clin Cancer Res. 2019;25:6590–7.
Mikirova N, Casciari J, Hunninghake R. Continuous intravenous vitamin C in the cancer treatment: re-evaluation of a Phase I clinical study. FFHD. 2019;9:180–204.
Wang F, He MM, Wang ZX, Li S, Jin Y, Ren C, et al. Phase I study of high-dose ascorbic acid with mFOLFOX6 or FOLFIRI in patients with metastatic colorectal cancer or gastric cancer. BMC Cancer. 2019;19:460. https://doi.org/10.1186/s12885-019-5696-z.
Zhao H, Zhu H, Huang J, Zhu Y, Hong M, Zhu H, et al. The synergy of Vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk Res. 2018;66:1–7.
Nielsen TK, Højgaard M, Andersen JT, Jørgensen NR, Zerahn B, Kristensen B, et al. Weekly ascorbic acid infusion in castration-resistant prostate cancer patients: a single-arm phase II trial. Transl Androl Urol. 2017;6:517–28.
Polireddy K, Dong R, Reed G, Yu J, Chen P, Williamson S, et al. High dose parenteral ascorbate inhibited pancreatic cancer growth and metastasis: mechanisms and a phase I/IIa study. Sci Rep. 2017;7:17188. https://doi.org/10.1038/s41598-017-17568-8.
Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. O2⋅- and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;31:487-500.e8.
Hoffer LJ, Robitaille L, Zakarian R, Melnychuk D, Kavan P, Agulnik J, et al. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: a phase I-II clinical trial. PLoS One. 2015;10:e0120228. https://doi.org/10.1371/journal.pone.0120228.
Kawada H, Sawanobori M, Tsuma-Kaneko M, Wasada I, Miyamoto M, Murayama H, et al. Phase I clinical trial of intravenous L-ascorbic acid following salvage chemotherapy for relapsed B-cell non-Hodgkin’s lymphoma. Tokai J Exp Clin Med. 2014;39:111–5.
Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6(222):222ra18. https://doi.org/10.1126/scitranslmed.3007154.
Stephenson CM, Levin RD, Spector T, Lis CG. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;72:139–46.
Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013;71:765–75.
Mikirova N, Casciari J, Rogers A, Taylor P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med. 2012;10:189. https://doi.org/10.1186/1479-5876-10-189.
Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7:e29794. https://doi.org/10.1371/journal.pone.0029794.
Berenson JR, Yellin O, Woytowitz D, Flam MS, Cartmell A, Patel R, et al. Bortezomib, ascorbic acid and melphalan (BAM) therapy for patients with newly diagnosed multiple myeloma: an effective and well-tolerated frontline regimen. Eur J Haematol. 2009;82(6):433–9. https://doi.org/10.1111/j.1600-0609.2009.01244.x.
Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19:1969–74.
Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J Korean Med Sci. 2007;22:7–11.
Riordan HD, Casciari JJ, González MJ, Riordan NH, Miranda-Massari JR, Taylor P, et al. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J. 2005;24:269–76.
Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–8.
Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med. 1985;312:137–41.
Poulter JM, White WF, Dickerson JW. Ascorbic acid supplementation and five year survival rates in women with early breast cancer. Acta Vitaminol Enzymol. 1984;6:175–82.
Murata A, Morishige F, Yamaguchi H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int J Vitam Nutr Res Suppl. 1982;23:103–13.
Creagan ET, Moertel CG, O’Fallon JR, Schutt AJ, O’Connell MJ, Rubin J, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. a controlled trial. N Engl J Med. 1979;301:687–90.
Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1978;75(9):4538–42. https://doi.org/10.1073/pnas.75.9.4538.
Thomsen CB, Andersen RF, Steffensen KD, Adimi P, Jakobsen A. Delta tocotrienol in recurrent ovarian cancer. A phase II trial. Pharmacol Res. 2019;141:392–6.
Springett GM, Husain K, Neuger A, Centeno B, Chen DT, Hutchinson TZ, et al. A phase I safety, pharmacokinetic, and pharmacodynamic presurgical trial of vitamin E δ-tocotrienol in patients with pancreatic ductal neoplasia. EBioMedicine. 2015;2:1987–95.
Nesaretnam K, Selvaduray KR, Abdul Razak G, Veerasenan SD, Gomez PA. Effectiveness of tocotrienol-rich fraction combined with tamoxifen in the management of women with early breast cancer: a pilot clinical trial. Breast Cancer Res. 2010;12:R81. https://doi.org/10.1186/bcr2726.
Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51.
McKeown-Eyssen G, Holloway C, Jazmaji V, Bright-See E, Dion P, Bruce WR. A randomized trial of vitamins C and E in the prevention of recurrence of colorectal polyps. Cancer Res. 1988;48:4701–5.
Tabatabaei-Malazy O, Nikfar S, Larijani B, Abdollahi M. Influence of ascorbic acid supplementation on type 2 diabetes mellitus in observational and randomized controlled trials; a systematic review with meta-analysis. J Pharm Pharm Sci. 2014;17:554–82.
Ghosn B, Benisi-Kohansal S, Ebrahimpour-Koujan S, Azadbakht L, Esmaillzadeh A. Association between healthy lifestyle score and breast cancer. Nutr J. 2020;19:4. https://doi.org/10.1186/s12937-020-0520-9.
Zamani B, Daneshzad E, Azadbakht L. Dietary total antioxidant capacity and risk of gastrointestinal cancers: a systematic review and meta-analysis of observational studies. Arch Iran Med. 2019;22:328–35.
Khodaeian M, Tabatabaei-Malazy O, Qorbani M, Farzadfar F, Amini P, Larijani B. Effect of vitamins C and E on insulin resistance in diabetes: a meta-analysis study. Eur J Clin Invest. 2015;45:1161–74.
Vissers MCM, Das AB. Potential mechanisms of action for vitamin C in cancer: reviewing the evidence. Front Physiol. 2018;9:809. https://doi.org/10.3389/fphys.2018.00809.
Nagamma T, Baxi J, Singh PP. Status of oxidative stress and antioxidant levels in smokers with breast cancer from western Nepal. Asian Pac J Cancer Prev. 2014;15:9467–70.
Rouleau L, Antony AN, Bisetto S, Newberg A, Doria C, Levine M, et al. Synergistic effects of ascorbate and sorafenib in hepatocellular carcinoma: New insights into ascorbate cytotoxicity. Free Radic Biol Med. 2016;95:308–22.
Yang Y, Lu X, Liu Q, Dai Y, Zhu X, Wen Y, et al. Palmitoyl ascorbate and doxorubicin co-encapsulated liposome for synergistic anticancer therapy. Eur J Pharm Sci. 2017;105:219–29.
Parrow NL, Leshin JA, Levine M. Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics. Antioxid Redox Signal. 2013;19:2141–56.
Cieslak JA, Strother RK, Rawal M, Du J, Doskey CM, Schroeder SR, et al. Manganoporphyrins and ascorbate enhance gemcitabine cytotoxicity in pancreatic cancer. Free Radic Biol Med. 2015;83:227–37.
Xia J, Xu H, Zhang X, Allamargot C, Coleman KL, Nessler R, et al. Multiple myeloma tumor cells are selectively killed by pharmacologically-dosed ascorbic acid. EBioMedicine. 2017;18:41–9.
Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, et al. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med. 2011;50:1610–9.
Carr AC, Cook J. Intravenous vitamin C for cancer therapy-identifying the current gaps in our knowledge. Front Physiol. 2018;9:1182. https://doi.org/10.3389/fphys.2018.01182.
Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826:443–57.
Cui XY, Skretting G, Jing Y, Sun H, Sandset PM, Sun L. Hypoxia influences stem cell-like properties in multidrug resistant K562 leukemic cells. Blood Cells Mol Dis. 2013;51:177–84.
Jiang Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv Nutr. 2017;8:850–67.
Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37.
Gratz SW, Wallace RJ, El-Nezami HS. Recent perspectives on the relations between fecal mutagenicity, genotoxicity, and diet. Front Pharmacol. 2011;2:4. https://doi.org/10.3389/fphar.2011.00004.
Bonelli L, Puntoni M, Gatteschi B, Massa P, Missale G, Munizzi F, et al. Antioxidant supplement and long-term reduction of recurrent adenomas of the large bowel. A double-blind randomized trial. J Gastroenterol. 2013;48:698–705.
Funding
This research has been supported by Tehran University of Medical Sciences & health services grant numbered 40713-192-03-97. The funder had no role in any part of study; design, data collection, analysis, interpretation or writing.
Author information
Authors and Affiliations
Contributions
ShM, HSE and OTM did literature bibliography, reviewed data, wrote draft and conceived the paper. MQ, LA, PKh and BL did literature bibliography, reviewed data, and drafted the paper. OTM and LA conceived, supervised, and edited the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
This study was approved by the Ethics Committee of Vice-Chancellor in Research Affairs-Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1397.926).
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohseni, S., Tabatabaei-Malazy, O., Ejtahed, HS. et al. Effect of vitamins C and E on cancer survival; a systematic review. DARU J Pharm Sci 30, 427–441 (2022). https://doi.org/10.1007/s40199-022-00451-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-022-00451-x