Abstract
A new Al–5Ti–0.75B–0.2C master alloy was successfully prepared by self-propagating high-temperature (SHS) reaction from an Al–Ti–B4C system with molten Al. Microstructure and phase characterization of the prepared Al–5Ti–0.75B–0.2C master alloy show that the nearly spherical TiC particles, hexagonal or rectangular TiB2 particles, and block-like TiAl3 particles distribute uniformly in the aluminum matrix. Grain refining test on commercial pure aluminum indicates that Al–5Ti–0.75B–0.2C master alloy exhibits a better grain refining performance than Al–5Ti–1B master alloy. By addition of 0.2 wt% Al–5Ti–0.75B–0.2C master alloy, the average grain size of α-Al can be effectively refined to 160 ± 5 μm from about 3000 μm, and the tensile strength and elongation are increased by about 20% and 14.1% due to the grain refinement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
During the past several decades, Al–Ti–B master alloys which typically consist of the soluble Al3Ti particles and the insoluble TiB2 particles, in particular Al–5Ti–1B, have been widely used in aluminum industry as the grains refiner achieving a fine equiaxed grain structure with improved comprehensive mechanical properties, machinability and cosmetic properties [1–3]. However, TiB2 particles in Al–Ti–B master alloys are fairly coarse and have a tendency to agglomerate and precipitate, reducing the grain refining efficiency and leading to many quality problems of aluminum products, such as porosity and streak in aluminum foils, scratch-like linear surface defects in litho and bright-anodized sheet, internal cracking in extrusion billets [2, 4]. Moreover, Al–Ti–B master alloys have obvious fading behavior due to the poisoning effect of trace elements of Zr, Cr, V, etc., in the aluminum melt [5, 6]. The new Al–Ti–C master alloys are believed to be the most promising grain refiners because TiC particles have small size, small mismatch and are less prone to agglomeration than TiB2 particles [5–7]. However, the intrinsic instability of TiC particles in aluminum melt results in the serious fading behavior and limits the further development of Al–Ti–C master alloys [6]. Therefore, it is necessary to find an effective method to combine the advantages of Al–Ti–B and Al–Ti–C master alloys.
Recently, Nie et al. [5, 6, 8], Tian et al. [9] and Li et al. [10] prepared a series of Al–Ti–C–B master alloy by reaction of molten aluminum with pure Ti, graphite powder and Al–3B master alloy. The results show that Al–Ti–C–B master alloys have a better grain refining performance than Al–Ti–B and Al–Ti–C master alloys. However, the preparation methods still have some problems in the application of Al–Ti–C–B master alloys, such as complicated production step, low energy and production efficiency, high production cost. Moreover, the preparation of Al–3B master alloy can release fluoride toxic gas and pollute the atmosphere.
Compared with the conventional melting and casting, self-propagating high-temperature (SHS) reaction has the advantages of simplicity, economy, high energy efficiency and high purity to fabricate the master alloys [11]. And this method was successfully used for the preparation of Al–Ti–B and Al–Ti–C grain refiners in aluminum melt [11–13]. In this paper, a new Al–5Ti–0.75B–0.2C master alloy was prepared by self-propagating high-temperature (SHS) reaction from an Al–Ti–B4C system with molten Al. In addition, the grain refining performance and its effect on the mechanical properties of commercial pure aluminum were also studied.
2 Experimental Procedures
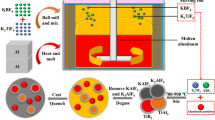
The starting materials used were commercial powders of Al (99.5 wt% in purity, ≤75 μm), Ti (99.5 wt% in purity, ≤40 μm) and B4C (98.0 wt% in purity, ≤5 μm). The proportion of Ti to B4C was fixed to 5:1 in mass ratio, and the aluminum content was fixed to 85 wt% of the total mass of the powders. Powder blends were mixed by ball milling for 10 h and then were pressed into cylindrical compacts (30 mm in diameter and 25 mm in height) with relative density of 80% ± 2% using a stainless steel die under a pressure of 60–65 MPa. About 500-g pure aluminum ingots (99.7 wt% in purity) were melted in a graphite crucible using an electrical resistance furnace at 1100 °C in which the reaction between Ti and B4C can occur. The cylindrical compacts wrapped by aluminum foils were charged into the melt. About 30 s later, the volume of the Al melt began to inflate and now the SHS reaction occurred. About 3 min later, the Al melt surface recovered equanimity and then the SHS reaction is completed. After that the melt was stirred with a graphite stirrer for about 30 min and poured into an iron mold preheated to 250 °C.
Grain refining tests were performed by adding 0.2 wt% Al–5Ti–1B (Sichuan Hua Heng Xiang Metal Sci-tech Co., Ltd) and Al–5Ti–0.75B–0.2C master alloys, respectively, into the aluminum melt with the same procedures. At first, the aluminum ingots (99.7 wt% in purity) were melted in a graphite crucible using an electrical resistance furnace at 720 ± 5 °C. Then, the Al–5Ti–1B and Al–5Ti–0.75B–0.2C master alloys were added into the melt and stirred thoroughly, respectively. The melt was hold for 5 min and poured into a preheated (250 °C) KBI cylindrical steel mold (65 mm in diameter and 25 mm in height) on a fire brick. After solidification, the bottom surface of the refined samples in contact with the brick was ground and etched by a reagent (60% HCl + 30% HNO3 + 5% HF + 5% H2O, vol%). And then, the macrograph of each sample was taken by a digital camera, and the average grain sizes were measured by the linear intercept method. Meanwhile, some of the melts were poured into an iron mold (235 mm × 35 mm × 100 mm) preheated to 250 °C to gain tensile test bars after refining.
Mechanical properties of pure Al before and after refined by different master alloys were determined using a tensile testing machine (WDW-50E) with a crosshead speed of 2.0 mm/min at room temperature, according to the GB/T228-2002 standard. The tensile strength and elongation data of each alloy reported below are average values of three tensile specimens. Microstructure and phase analyses of the prepared Al–5Ti–0.75B–0.2C master alloy were investigated by using scanning electron microscopy (SEM, S-4800, Hitachi, Japan) equipped with an energy-dispersive spectrometer (EDS) and X-ray diffraction (XRD, DX-2700, Fangyuan, China).
3 Results and Discussion
Figure 1 shows the XRD result of the prepared Al–5Ti–0.75B–0.2C master alloy. It can be seen clearly that Al–5Ti–0.75B–0.2C master alloy mainly contains four kinds of phases: α-Al, TiAl3, TiC and TiB2, confirming that TiAl3, TiC and TiB2 can be synthesized by the self-propagating high-temperature (SHS) reaction from an Al–Ti–B4C system in the aluminum melt. However, a small mount of B4C was detected by XRD. According to the equilibrium tetrahedron of Al–Ti–B–C, it is probable that the system has not reached equilibrium [14]. In addition, it may also be caused by the loss of heat which leads to the incomplete reaction between Ti and B4C.
Figure 2 shows the SEM images of Al–5Ti–0.75B–0.2C and Al–5Ti–1B master alloys. It can be seen that both of them are mainly composed of block-like TiAl3 and numerous particles distributing in the aluminum matrix, while the TiAl3 particles in the Al–Ti–B–C master alloy have a smaller size (about 15 μm) than those in the Al–Ti–B–C master alloy. The reason for this is that the SHS reaction time is very short and TiAl3 particles have no enough time to grow up. The research of Ref [11]. also shows that the smallest block-like TiAl3 particles are obtained by the SHS technology. In order to identify the phases and observe their distribution, local areas of the microstructures were magnified (Fig. 2c, d) and EDS analyses were performed on the points ①, ② and ③ in Fig. 2a, c as shown in Fig. 3. As illustrated in the magnified microstructure and EDS results (Fig. 3), the hexagonal or rectangular TiB2 particles with a mean size of about 1 μm and the nearly spherical TiC particles with a mean size of about 0.5 μm are disconnected with each other and dispersed homogeneously in the Al–5Ti–0.75B–0.2C master alloy (Fig. 2c), while TiB2 particles with a mean size of about 3 μm in the Al–5Ti–1B master alloy (Fig. 2d) agglomerated seriously. Notably, some irregular shape TiC x B y particles with a mean size of about 0.5–1 μm formed in the Al–5Ti–0.75B–0.2C master alloy. According to the literature [8], the formed TiC particles in Al melt are usually substoichiometric and can be easily doped with the similar B atoms to form some TiC x B y particles. Therefore, some TiC x B y particles formed in the Al–5Ti–0.75B–0.2C master alloy, and the result is consistent with that reported by Nie et al. [5].
Figure 4 shows the macrostructures of commercial pure aluminum before and after being refined with different master alloys after holding 5 min. It was observed that the macrostructure of unrefined commercial pure aluminum (Fig. 4a) shows a typical grain structure of pure aluminum which is composed of outer columnar grains and centric coarse equiaxed grains, and its average grain size is about 3000 μm. Compared with the commercial pure aluminum without adding grain refiner (Fig. 4a), the macrostructures of commercial pure aluminum refined with 0.2 wt% Al–5Ti–1B and Al–5Ti–0.75B–0.2C master alloys (Fig. 3b, c) exhibit finer equiaxed grains and their average grain size are about 185 ± 5 μm, 160 ± 5 μm, respectively. Compared to Al–5Ti–1B master alloy, Al–5Ti–0.75B–0.2C master alloy exhibits a slightly better grain refinement performance (as shown in the magnified macrostructures of Fig. 4b, c in the top right corner) and the average grain size is reduced by about 13.5%.
It is well known that when master alloys are added into molten aluminum, the aluminum matrix will be melted and release a large number of potential heterogeneous nuclei into the Al melt, which will nucleate α-Al during solidification. According to the literature [5, 10, 15], the distribution, quantity and morphology of the potential heterogeneous nuclei have great influence on the grain refining efficiency of the master alloys. With smaller dimension and better dispersion of inoculating particles, the grain refining efficiency can be improved [16]. In this study, it can be seen that Al–5Ti–0.75B–0.2C master alloy has a better dispersion than Al–5Ti–1B master alloy. The better dispersion of the second phase particles is helpful to hinder the agglomeration and precipitation of the second phase particles, assuring that the particles can be served as the nucleation sites for α-Al during the refining process. In addition, the second phase particles in Al–5Ti–0.75B–0.2C master alloy have a smaller size than those in Al–5Ti–1B master alloy. The number of effective nucleating particles as the nuclei for α-Al is increased significantly, the grain refining performance of Al–5Ti–0.75B–0.2C master alloy can be greatly improved. However, Greer et al. [17] found that the optimum mean particle diameter for minimum grain size and the optimum average particle diameter of TiB2 are about 2 μm. But, their further study [18] shows that a smaller mean particle diameter is beneficial to the good grain refining performance and the optimum mean particle size is about 1 µm. Nie et al. [6] prepared titanium diboride core–shell structure particles with a length of about 800 nm which displays a higher grain refining efficiency than TiB2. The grain refinement mechanism is complex and difficult to understand. Until now, there has been no consensus on the exact mechanism for grain refinement, and the nucleation process cannot be explained purely based on theories. So further study should be made in the future. On the other hand, it is also found that some TiC x B y particles were formed in the Al–5Ti–0.75B–0.2C master alloy. According to Ref. [4], TiC x B y particles have better nucleating ability than TiC x due to the less carbon-atom vacancies and better stability, and TiC x B y particles also have much better refining efficiency than TiB2. Therefore, with the formation of TiC x B y particles in the Al–5Ti–0.75B–0.2C master alloy, an improved grain refining efficiency is obtained. Finally, when Al–5Ti–0.75B–0.2C master alloys are added into the molten aluminum, the smaller block-like TiAl3 particles are easy to dissolve and release solute Ti which will improve the wettability between TiC, TiB2 and TiC x B y particles and Al melt in the grain refining process and enhance the grain refining performance of TiC, TiB2 and TiC x B y particles. And the irregular morphologies of the second phase particles are good for Ti enriching which is helpful to improve the nucleation efficiency [10]. This will make an extra contribution to the high grain refinement performance. Therefore, the excellent grain refining efficiency on pure aluminum is obtained.
The mechanical properties of commercial pure aluminum before and after being refined by Al–5Ti–1B and Al–5Ti–0.75B–0.2C master alloys at room temperature after holding 5 min are also investigated in this work, and the results are shown in Table 1. The tensile strength and elongation of the unrefined pure aluminum are 55 ± 1 MPa and 39% ± 0.3%. After the addition of Al–5Ti–1B and Al–5Ti–0.75B–0.2C master alloys, the tensile strength and elongation are increased to 64.2 ± 1 and 66 ± 1 MPa, and 43% ± 0.3% and 44.5% ± 0.3%, respectively. Compared with pure aluminum, the tensile strength and elongation are increased by about 16.7% and 20%, and 10.3% and 14.1%, respectively. The results indicate that the grain refinement has resulted in a significant enhancement in both tensile strength and elongation of commercial pure aluminum. It is also indicated that Al–5Ti–0.75B–0.2C master alloy has a slightly better ability in improving the mechanical properties of pure aluminum than Al–5Ti–1B master alloy due to the better grain refining performance (as shown in the magnified macrostructures of Fig. 4b, c in the top right corner). Therefore, the prepared Al–5Ti–0.75B–0.2C master alloy by SHS technology is an excellent grain refiner for pure aluminum as well as Al–5Ti–1B master alloy, and the successful usage of Al–5Ti–0.75B–0.2C master alloy opened a new method to fabricate Al–Ti–B–C master alloy which makes it possible in the application of aluminum industrial.
4 Conclusion
A new Al–5Ti–0.75B–0.2C master alloy with a uniform microstructure has been successfully prepared by self-propagating high-temperature (SHS) reaction from an Al–Ti–B4C system with molten Al. It is shown that Al–5Ti–0.75B–0.2C master alloy exhibits an excellent grain refining performance. With addition of 0.2 wt% Al–5Ti–0.75B–0.2C master alloy, the average grain size of pure aluminum can be effectively refined to 160 ± 5 μm and the tensile strength and elongation are increased by about 20% and 14.1% due to the grain refinement. Compared with Al–5Ti–1B master alloy, Al–5Ti–0.75B–0.2C master alloy has a slightly better grain refining performance, which accounts for the better ability in improving the mechanical properties of pure aluminum.
References
Y.J. Zhang, H.W. Wang, N.H. Ma, X.F. Liu, Mater. Lett. 59, 3398 (2005)
Y. Birol, J. Alloys Compd. 422, 128 (2006)
X.F. Liu, X.F. Bian, X.G. Qi, M. Thompson, J.J. Ma, Acta Metall. Sin. (Engl. Lett.) 11, 157 (1998)
G.S. Vinod Kumar, B.S. Murty, M. Chakraborty, J. Alloys Compd. 396, 143 (2005)
J.F. Nie, X.G. Ma, P.T. Li, X.F. Liu, J. Alloys Compd. 509, 1119 (2011)
J.F. Nie, X.G. Ma, H.M. Ding, X.F. Liu, J. Alloys Compd. 486, 185 (2009)
J.F. Nie, H.M. Ding, Y.Y. Wu, X.F. Liu, Scr. Mater. 42, 789 (2013)
J.F. Nie, X.F. Liu, X.G. Ma, J. Alloys Compd. 491, 113 (2010)
W.J. Tian, P.T. Li, T. Gao, J.F. Nie, X.F. Liu, J. Alloys Compd. 561, 48 (2013)
P.T. Li, X.G. Ma, Y.G. Li, J.F. Nie, X.F. Liu, J Alloys Compd. 503, 286 (2010)
V.I. Nikitin, J.I.E. Wanqi, E.G. Kandalova, A.G. Makarenko, L. Yong, Scr. Mater. 42, 561 (2000)
P.J. Li, E.G. Kandalova, A.G. Makarenko, V.I. Nikitin, Y.F. Zhang, A.R. Luts, Mater. Lett. 58, 1861 (2004)
X.T. Liu, H. Hao, J. Alloys Compd. 623, 266 (2015)
M.T. Agne, B. Anasori, M.W. Barsoum, J. Phase Equilib. Diffus. 36, 169 (2015)
X.W. Hu, F.R. Ai, H. Yan, Acta Metall. Sin. (Engl. Lett.) 25, 272 (2012)
E.Z. Wang, T. Gao, J.F. Nie, J. Alloys Compd. 594, 7 (2014)
A.L. Greer, A.M. Bunn, A. Tronche, P.V. Evans, D.J. Bristow, Acta Mater. 48, 2823 (2000)
T.E. Quested, A.L. Greer, Acta Mater. 52, 3859 (2004)
Acknowledgments
This work was financially supported by the Scientific and Technical Project of Sichuan Province (Nos. 13CGZHZX0200, 2014GZX0064, 2015GZ0057).
Author information
Authors and Affiliations
Corresponding author
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195
Rights and permissions
About this article
Cite this article
An, XG., Liu, Y., Ye, JW. et al. Grain Refining Efficiency of SHS Al–Ti–B–C Master Alloy for Pure Aluminum and Its Effect on Mechanical Properties. Acta Metall. Sin. (Engl. Lett.) 29, 742–747 (2016). https://doi.org/10.1007/s40195-016-0452-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-016-0452-8