Abstract
Acknowledging the significant impact of temperature deviations on vaccine efficacy, this study examines the prevalence of exposure to non-recommended temperatures, particularly focusing on freeze-sensitive vaccines. This study aims to identify critical points of temperature breaches during transportation from storage to last-mile delivery. In this cross-sectional study, the temperature integrity of vaccines has been examined during transportation across three states in India (Karnataka, Maharashtra, and Rajasthan), utilizing data loggers to track and record temperatures in real time. By analyzing instances of exposure to temperatures beyond the recommended 2–8 °C range, the study aims to identify the critical junctures within the vaccine supply chain susceptible to temperature excursions. The methodology entailed a systematic data logger analysis at each stage of transportation up to the last mile. In this study, descriptive statistics with the analysis of variance test has been applied to investigate the significance of variations in the duration of temperature breaches. The novelty of this study lies in its comprehensive examination of vaccine temperature integrity across critical transportation phases in India, utilizing real-time data-logging technology. Based on the results, the study provides recommendations using the ABCD strategic framework, encompassing awareness, best practices, continuous monitoring, and data management and documentation. This framework will be instrumental in addressing the identified challenges and significantly improving the temperature monitoring flexibility. The findings will inform targeted interventions, optimizing the cold chain to enhance the preservation of vaccine potency and identifying flexible solutions for maintaining the required temperatures from storage to transportation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vaccination has been recognized as a critical public health intervention, significantly reducing morbidity and mortality, and aiding in the control, elimination, or eradication of numerous diseases (Das et al., 2023; Dhawan et al., 2023; Gül et al., 2023; Patel et al., 2024). The effectiveness of most vaccines is intrinsically linked to their temperature integrity; exposure to temperatures outside the recommended range (2–8 °C) results in rapid loss of potency (Kartoglu & Ames, 2022; Lin et al., 2020; Preston & Randolph, 2021). Heat exposure negatively impacts almost all vaccines, but freezing temperatures are particularly detrimental to freeze-sensitive vaccines, such as diphtheria–pertussis–tetanus, tetanus toxoid, Hemophilus influenza type b (Hib), and inactivated poliovirus (Gedi, 2022; Guignard et al., 2019). Freezing causes the adjuvants in these vaccines to clump, adversely affecting their immunogenic potency (Asamoah, 2020). Such incidents, documented in both developing and developed nations, underscore a significant vulnerability in the vaccine supply chain, potentially undermining the integrity and efficacy of these vital biological products (Sarker et al., 2021; Shore et al., 2022; Wadhwa & Rao, 2004; Weinman et al., 2021; Zaoui et al., 2023).
A review spanning 7 years (2007–2014) revealed that in low- and middle-income countries, vaccines were exposed to freezing temperatures during transportation in 28.1% of the cases (Hanson et al., 2017). In India, a specific study found vaccines exposed to sub-zero temperatures for 18.1% of the time during transportation (Murhekar et al., 2013). The research gap identified for this study has been showcased from the limited empirical evidence on real-time temperature monitoring and its effectiveness in preventing temperature deviations during the transportation of vaccines, particularly in the context of the varied climatic conditions across India (Pambudi et al., 2022). While existing studies have addressed the components of cold chain logistics, there has been a notable dearth in comprehensive research that integrates data-logging technology with a strategic framework for enhancing temperature control from storage to last-mile delivery (Wanganoo et al., 2021). This gap signifies a critical need for detailed investigations that not only quantify the extent of temperature excursions but also propose actionable strategies tailored to the complexities of vaccine logistics in diverse geographical settings.
In response to these findings, our study has been initiated to assess temperature integrity during vaccine transportation between stores and outreach sessions in India. This study focuses on assessing the integrity of vaccine temperatures during transportation across three states in India. By analyzing data related to temperature at various levels, from the dispatch of vaccines from manufacturers to their final administration at health facilities and outreach services, this study aims to do the following: (i) ascertain the occurrence of temperature excursions beyond the designated range for selected vaccines during their transport phase, (ii) identify specific points and practices in the vaccine distribution system where temperature problems occur, and (iii) implement necessary actions to rectify detected problems and to identify issues warranting the support of new policies.
To investigate temperature deviations across the vaccine delivery process, from storage to transportation, this study has been designed to gather data from public sector vaccine supply chains in Rajasthan and Karnataka. The objective was to gain a thorough understanding of the temperature monitoring challenges in these regions. In addition, to conduct a more holistic analysis and capture the real dynamics of temperature monitoring during vaccine transportation, the study collected data from private sector players in Maharashtra. This comprehensive approach allowed for a detailed examination of the temperature control practices and challenges in both public and private sectors of vaccine last-mile distribution. This study has consolidated pivotal insights derived from both descriptive analysis and analysis of variance (ANOVA). These insights provide the foundation for proposing strategic recommendations using the ABCD framework, encompassing awareness, best practices, continuous monitoring, and data management and documentation. This approach not only underscores the robustness of the study’s methodology but also reinforces the applicability of its recommendations in enhancing the vaccine transportation processes.
Methodology
Overview of the Vaccine Distribution System
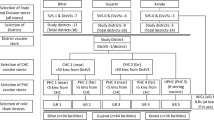
In India, under the Universal Immunization Programme, vaccine distribution typically begins with manufacturers supplying vaccines to state or division stores. From here, a cascade system ensures delivery to district stores, and subsequently to sub-district stores [community health centers (CHCs) and primary health centers (PHCs)], often followed by distribution from CHCs to PHCs (Fig. 1). This transfer process utilizes cold boxes of varying sizes equipped with ice packs to maintain the required temperature across the cold chain for the vaccines mentioned below.
This study includes a comprehensive list of various vaccines such as BCG, Bevac, and Biovac-A, including Prevenar, rotavirus vaccines, and Zyvac. This inventory encompasses a wide spectrum of vaccines, including those used for routine immunization such as DPT and hepatitis B and specific ones such as Gardasil and Havrix, indicating the breadth of vaccine types studied. Each vaccine on the list represents a unique profile regarding storage requirements, stability, and temperature sensitivity, underscoring the complexity of vaccine management and distribution.
Study Design and Sites
Conducted in Karnataka, Maharashtra, and Rajasthan from September to December 2023, this cross-sectional study purposively selected states based on their immunization coverage and governance levels. In each state, two districts were randomly chosen. Temperature monitoring during vaccine transfers was feasible in these districts, including seven locations each in state of Maharashtra (Pune district), Rajasthan (Jhareda, Suroth within Karauli district and Bambori, Choti Sadri within Pratapgarh district), and Karnataka (Hanamanal and Hoolegere within Koppal district). The study covered 10 cold chain stores across these districts. Vaccine transfers from state to division store were documented in Rajasthan, while in Maharashtra, manufacturers supplied vaccines directly to division stores. In total, three transfers from division to district stores and seven transfers from district to sub-district stores were observed alongside vaccine transfers between sub-district stores, particularly from CHCs to PHCs in Rajasthan and Karnataka. Vaccine transfers typically occurred every month to cold chain stores with outreach sessions held weekly. The study documented temperature integrity for 182 vaccine transfer episodes, encompassing 164 interfacility transfers and 18 outreach sessions. All necessary administrative permissions were obtained from the national and state health authorities.
Field Preparation
To ensure the safety and efficacy of temperature-sensitive medical specimens, including vaccines, medicines, and blood samples, it is imperative to employ a robust temperature monitoring system during refrigerated storage and transport (Maity et al., 2022; Ramírez-Faz et al., 2020). One such innovative solution is the use of a data logger, specifically designed for continuous temperature monitoring and alerting (Mabrouki et al., 2021; Thiyaneswaran et al., 2022). This system plays a critical role in safeguarding the integrity of valuable medical cargo throughout its journey.
The data logger system used in this study named Global Tracker Mini by B-Medical Systems has combined two devices for optimal performance. The monitoring device is installed directly on the vaccine box or carrier, while a sensor is strategically placed inside the storage unit. Utilizing the Bluetooth Low Energy technology, the system features a data logger for external communication, seamlessly interfacing with the sensor located internally. This dual-sensor approach ensures precise and reliable temperature monitoring, providing critical alerts to protect these sensitive medical materials under various conditions of transport and storage. The data loggers were connected via mobile phone and allowed for real-time transmission of data in a standard format.
Configuration of Data Loggers
The configuration interface reveals an array of settings that can be customized to meet specific monitoring requirements. At the heart of the system’s configuration are the temperature range settings, which include predefined options as well as a custom setting for precise control. The system’s internal low-temperature threshold has been set to 2 °C, which is within the broader permissible range of − 40–79 °C. Correspondingly, the internal high-temperature threshold is established at 8 °C, fitting within an acceptable range of − 39–80 °C. These thresholds are critical to ensure that the monitored vaccines remain within the safe temperature range, protecting their integrity and efficacy.
Alarm delays for both low- and high-temperature breaches are configured to trigger after 1 min. However, the system allows for these to be set anywhere between 1 min and 24 h (1440 min). This feature ensures prompt alerts, enabling immediate corrective actions to mitigate any risk to the shipment.
In addition, the configuration details the frequency range for temperature data collection. The ambient collection frequency is set at 15-min intervals, while the internal collection frequency, which likely pertains to the temperature inside the refrigerated space, is more frequent, set at 5-min intervals. Both settings contribute to a comprehensive data log that can be used for detailed temperature trend analysis.
An additional safety feature is the buzzer repeat interval, which is configured to sound every minute, with the flexibility set up to a maximum of 240 min. This ensures continuous auditory monitoring, which is especially useful in environments where immediate human response to temperature deviations is necessary.
Finally, the system features the Bluetooth Low Energy technology for connectivity, with a discoverable duration set to 2 min. This setting allows for quick and efficient wireless communication with other devices or sensors, enhancing the system’s monitoring capabilities.
These comprehensive configuration settings underscore the system’s advanced capabilities in providing continuous, detailed monitoring to ensure the safety and efficacy of sensitive medical specimens throughout their transportation and storage lifecycle.
Capacity Building of the Staff
Capacity building of the staff is an essential component in the effective implementation of advanced temperature monitoring systems for vaccine transportation. To this end, comprehensive training was provided to personnel at various storage facilities and those responsible for carrying vaccines to the last mile in two phases. The initial phase of the training program was focused on building the skills of staff members who were unfamiliar with the device. The subsequent phase targeted staff already acquainted with the device, enhancing their proficiency and knowledge of its usage. This training focused on the operational mechanisms of the temperature monitoring device, ensuring that each individual was proficient in using the equipment. Participants were educated on setting thresholds, interpreting alerts, and responding to potential temperature breaches. In addition, they received guidance on maintaining the devices and troubleshooting common issues. By empowering the staff with this knowledge and skill set, the integrity of the vaccine supply chain was significantly strengthened, contributing to the maintenance of vaccine efficacy until the point of delivery. This initiative not only enhances the technical capacity of the staff but also instills a greater sense of accountability and precision in the handling of temperature-sensitive medical products.
Data Collection
Data were systematically retrieved in PDF format from each data logger via the dedicated portal. The PDF reports from the field were comprehensive, detailing various categories of temperature data. This included date-specific minimum, maximum, and average temperatures, both internal to the vaccine container and in the ambient environment. Furthermore, it provided hourly temperature readings and more granular minute-by-minute recordings at specified intervals (every 5 or 10 min for internal temperatures and every 30 min for ambient temperatures).
The retrieved datasets contained extensive data points; however, the analysis required filtering to include only those records that provided minute-by-minute temperature readings with corresponding timestamps. The recorded temperatures, originally captured in degree Celsius, were converted into a numerical format suitable for the analytical processes of the study. This conversion was critical to facilitate the subsequent data analysis phases, ensuring accurate and meaningful interpretation of the temperature conditions experienced during vaccine transport.
Data Analysis
Descriptive Analysis
In our study, we focused on the thorough extraction and examination of temperature data, particularly paying attention to the internal and ambient temperatures recorded by certain devices. This data, sourced from an Excel file, underwent a rigorous process to ensure accuracy and relevance. Initially, we applied a strict filtering method, selecting only the temperature data that corresponded with active shipping periods, as indicated by specific dates and times. This step was critical to guarantee the precision of our dataset.
The descriptive analysis (Wang et al., 2020) conducted on a shipment basis aimed to investigate the performance and reliability of the temperature monitoring devices throughout the shipments, with particular focus on detecting any temperature deviations.
The use of descriptive statistical methods allowed us to assess the central tendencies, dispersion, and other statistical characteristics of the temperature readings. We interpreted the results with an emphasis on their implications for the integrity of transported vaccines. Our analysis aimed at identifying patterns and anomalies within the recorded temperature ranges, which could be crucial in pinpointing specific instances or trends of temperature fluctuations that could affect the efficacy of the vaccines. By merging and comparing the findings from individual shipments and devices, our study sought to evaluate the overall impact of these devices within a given state. This approach provided us with a broader understanding of the effectiveness of temperature monitoring within the logistics and supply chain management sectors, highlighting areas of success and potential improvement.
ANOVA
ANOVA has been employed for inferential analysis, in which the data is analyzed in contexts of one category-independent variable and one quantitative-dependent variable (Dhillon et al., 2023; Tapati et al., 2023; Vishvakarma et al., 2021). The independent variable should possess a minimum of three levels, indicating the presence of at least three distinct groups or categories (Das et al., 2022; Dugard et al., 2022). In one-way ANOVA analysis, the independent variable is compared with the dependent variable that helps to explore the substantial impact of the independent variable on the dependent variable (Alhawatmeh et al., 2021; Brahimi et al., 2023; Hussain & Ali, 2019). In the case of three different states, it is difficult to determine the particular pairs of the states’ temperature breach durations that have been significantly differing. By employing a post hoc test under ANOVA, we can identify the pair of states that exhibit a significant difference. Yildirim and Sisman (2023) stated that ANOVA is used to forecast an individual-dependent variable based on one or more predictor variables and determine the effectiveness of those predictors. In addition, Hassan et al. (2024) highlighted that ANOVA is “more reliable because it considers many parameters at the same time plus considering the frequency trend” (p. 1). ANOVA analyzes response outcomes and determines each variable’s contribution (Madani et al., 2023; Rajakumar et al., 2023). Therefore, in this study, ANOVA has been used to analyze the significant difference in temperature breach duration across states.
Results and Discussion
Descriptive Analysis and Variance Analysis
In this study, descriptive statistics and advanced analysis tools have been employed to scrutinize temperature breach data across shipments in three different states: Karnataka, Maharashtra, and Rajasthan. Table 1 presents a comprehensive analysis of all three states. There are a total of 214 shipments in the three states. The average trip duration is 21,290 min, while the average temperature breach duration is 9448 min, representing a 44.37% temperature breach (hot breach and cold breach). This analysis highlights the importance of effective management of temperature control mechanisms for each shipment. For Karnataka, the analysis revealed a higher incidence of cold breaches, underscoring the need for improved heating mechanisms during shipments. In Maharashtra and Rajasthan, hot breaches were more prevalent, suggesting the requirement for better cooling solutions. Table 1 demonstrates that Karnataka faced more temperature breaches per shipment than Maharashtra and Rajasthan. Therefore, there is a need for a comprehensive approach to temperature regulation. These findings are instrumental in informing logistics strategies for the transportation of temperature-sensitive vaccines. Effective logistics can improve the overall robustness of temperature-sensitive supply chains by resolving the specific issues raised during shipments.
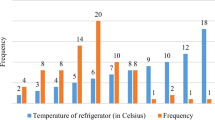
Figure 2 showcases a snapshot of temperature breaches during shipments in Maharashtra. In the same shipment, the cold and hot breaches occurred concurrently. Recorded breaches indicate that 32.21% (5795 min) were hot breaches, while 15.58% (2805 min) were cold breaches for shipments, covering 67% and 33% of total temperature breaches, respectively. This suggests that the temperature threshold was not consistently maintained. Figure 3a presents the shipment-wise minimum internal temperature variation and Fig. 3b presents the shipment-wise maximum internal temperature variation. The recorded average minimum temperature is 4.92 °C and the average maximum internal temperature is 19.38 °C. There are a total of five shipments in which the minimum internal temperature goes beyond 0 °C. This presents the need for robust cooling mechanisms to ensure the integrity of vaccines during transit and emphasizes the need for robust thermal protection of vaccines that are sensitive to higher temperatures.
The analysis underscores a pressing concern for shipments in Maharashtra, indicating that measures to control and mitigate temperature excursions are necessary. Given the critical nature of ensuring product quality, especially for temperature-sensitive vaccines, these insights call for a revisit of the strategies for maintaining the vaccine temperatures during transportation. This visual tool serves as a compelling illustration for logistics managers, emphasizing the need to focus on solutions that specifically address the risk of temperature breaches during transit.
Figure 4 presents a snapshot of temperature breaches during shipments in Rajasthan. There are 26.23% hot breaches, where the temperature exceeded the prescribed upper limit, indicating that the shipment experienced a warmer-than-acceptable threshold limit of 8 °C. Meanwhile, 12.63% cold breaches were recorded for this shipment, indicating that the lower temperature threshold was not maintained properly during transit. In addition, the analysis of the dataset from Rajasthan reveals insightful details about the internal temperature variations encountered during various shipments (Fig. 5a and b). Each shipment experienced distinct temperature fluctuations, as evidenced by the recorded minimum internal and maximum internal temperatures, where the recorded average minimum internal temperature is 5.2 °C and the average maximum internal temperature is 18.16 °C, whereas the median of the internal minimum temperature is 2.62 °C and that of the maximum internal temperature is 17.75 °C. There is a total of four shipments in which the minimum internal temperature goes beyond 0 °C, which presents the need for enhanced cooling mechanisms to ensure that the vaccines are preserved within the specified temperature range. In addition, there are 98 shipments in which the minimum internal temperature exceeds 8 °C, which emphasizes the need to employ efficient cooling procedures to protect temperature-sensitive vaccines from potential harm caused by extreme temperatures.
Figure 6 presents the temperature breaches during shipments in Karnataka. It shows that 37.13% cold breaches were recorded for this shipment, raising concerns about the possible compromise of temperature-sensitive vaccines due to little control of the lower temperature threshold during shipments. Furthermore, 18.85% are hot breaches, where the temperature exceeded the prescribed upper limit, indicating that the shipment gets easily impacted by the outside temperature, and there is a need to take measures to ensure strong insulation or cooling mechanisms to protect the vaccines being carried. Figure 7a and b presents the minimum and maximum internal temperature variations during shipments in Karnataka, respectively. The recorded average minimum temperature is 1.52 °C and the average maximum internal temperature is 18.4 °C. There are a total of 23 shipments in which the minimum internal temperature goes beyond 0 °C, which presents the need for enhanced cooling mechanisms to preserve the carried vaccines. Moreover, there are 33 shipments in which the internal temperature goes above 8 °C, which emphasizes the importance of adopting efficient cooling strategies to protect vaccines from hot temperature breaches.
These insights highlight that the primary issue in the region is maintaining temperatures above the lower threshold, as cold breaches are more prevalent than hot breaches across the shipments. This data is crucial for logistics providers, indicating a need for enhanced insulation and heating mechanisms within the transportation units, especially for vaccines that are sensitive to low temperatures. These findings indicate that few difficulties can act as obstacles to maintaining temperatures within the threshold. In addition, in Karnataka, cold breaches surpass hot breaches in shipments, and logistics providers can prioritize improving insulation and heating mechanisms in transportation units, particularly for vaccines that are susceptible to low temperatures. Adopting a data-driven approach will help develop precise solutions that consider the specific climatic variations and operational details at every stage of the cold chain. This approach will help ensure the preservation and effectiveness of temperature-sensitive shipments during transportation in Karnataka.
Table 2 presents a state-wise temperature analysis of the data that provides a comprehensive overview of the temperature breaches during shipments. The analysis encompasses key parameters such as minimum and maximum internal temperature and minimum and maximum ambient temperature. Each shipment in the dataset has been meticulously analyzed to identify the number of cold and hot temperature breaches. This analysis reveals the frequency and severity of temperature deviations during transit, crucial for understanding the logistical challenges faced in maintaining optimal temperature conditions.
The data indicate varying degrees of total temperature breaches, cold breaches, and hot breaches across different shipments in different states. Figures 2, 4, and 6 provide a clear and comparative view of cold and hot breaches across shipments, illustrating the fluctuating nature of temperature control challenges in each shipment. The temperature variations are critical, especially for shipments containing temperature-sensitive vaccines. This visualization is instrumental for stakeholders in logistics and supply chain management, offering a vivid depiction of the areas where improvements in temperature regulation are most needed.
Cold temperature breaches present instances where the temperature drops below the safe threshold, posing risks to the integrity and quality of the transported vaccines, especially critical for temperature-sensitive vaccines. Similarly, hot breaches were recorded, where the temperature exceeded the upper threshold limit. These instances are equally concerning as they can lead to the degradation of products, particularly those sensitive to higher temperatures.
The wide range of temperatures highlights the challenging conditions faced during transit and underscores the importance of efficient temperature control mechanisms. These data are particularly valuable for logistics providers, emphasizing the need for enhanced temperature management strategies to maintain product integrity and quality throughout the transportation process. By addressing the identified breaches, logistics providers can enhance their transportation methods, ensuring better preservation of product quality and adherence to safety standards.
For variance analysis, a one-way ANOVA test is performed to determine whether there are any significant differences in temperature breach duration across various states. In this study ANOVA test is performed on three subsets of state-wise analyzed data, including total temperature breach duration, cold breach temperature duration, and hot breach temperature duration. Further, a post hoc test is conducted to determine which set of states the breach duration differs from and if the difference in temperature breach is significant.
Using the data set of the total temperature breach duration, the ANOVA test results show that the F ratio (2.054) is statistically significant (0.131) at a 5% level of significance (Table 3); this demonstrates that the total breach duration in the three states (Maharashtra, Rajasthan, and Karnataka) is not statistically different. However, the analysis results indicate that the total breach durations in these states are similar, and the observed differences are likely less due to random variation. The results of the post hoc analysis present that the total breach duration in Rajasthan is lower than that in Karnataka and higher than that in Maharashtra, and Karnataka has a higher total breach duration than Maharashtra. The differences are not statistically significant (Table 4).
The analysis results using the data set of the cold temperature breaches duration present that the F ratio (11.379) for the ANOVA test is statistically significant (0.000) at a 5% level of significance (Table 5). This states that the total cold breach durations in at least one of the three states (Maharashtra, Rajasthan, and Karnataka) is statistically significant. The post hoc analysis results present that the difference between Rajasthan and Karnataka (-66.818) is statistically significant (0.000) at a 5% significance level (Table 6). The cold breach duration in Rajasthan is significantly lower than that in Karnataka. The difference in cold breach duration between Rajasthan and Maharashtra (4.100) is not statistically significant (0.941) at a 5% level of significance. The same between Maharashtra and Karnataka (70.917) is statistically significant (0.000) at a 5% significance level, illustrating that the cold breach duration in Karnataka is significantly higher than that in Maharashtra.
Using the data set of the total duration of hot temperature breaches, the ANOVA test results show that the F ratio (1.232) is statistically significant (0.294) at a 5% level of significance (Table 7). The result states that the hot breach duration in the three states (Maharashtra, Rajasthan, and Karnataka) is not statistically different. The analysis results indicate that the hot breach durations in all states are similar, and the observed differences are likely less. The results of the post hoc analysis present that the total hot breach duration in Rajasthan is higher than that in Karnataka and Maharashtra, and Maharashtra has a higher hot breach duration than Karnataka, but the differences are not statistically significant (Table 8).
Strategic Recommendations
Based on the findings of this comprehensive study on temperature monitoring during vaccine transportation in India, key areas have been identified where improvements will be crucial for maintaining the recommended temperature range. The following strategic recommendations have been proposed by developing the ABCD strategic framework to address these challenges and enhance the vaccine supply chain efficacy by improving temperature monitoring flexibility (Fig. 8). These are aimed at mitigating the risks of temperature deviations, particularly during the critical last-mile delivery. The following ABCD framework recommendations have been grounded in the research data. They are designed to optimize the cold chain process, ensuring vaccine-safe and effective delivery.
Awareness
Promoting awareness of flexible vaccine shipment protocols ensures a comprehensive understanding of the dynamic nature of vaccine transportation. Enhancing awareness helps to focus on adapting protocols according to real-time data and dynamic conditions, promoting a proactive vaccination transportation approach to resolve challenges.
-
(a)
Flexibility in protocols: A focused increase in awareness and knowledge sharing and greater flexibility in the last-mile delivery and outreach protocols can improve effective shipment management. Various regions face challenges due to diverse geography, transportation infrastructure, and population accessibility. Temperature integrity, strategic resource allocation, and adaptable policies can enhance protocol flexibility. Therefore, flexibility in protocols can improve transportation approaches and shipment schedules based on real-time data, ensuring optimal conditions for the transportation of vaccines.
-
(b)
Capacity Building and Training: Capacity building and training as a strategy can enhance the awareness, knowledge, and expertise of those involved across various phases of vaccine transportation. Implementing a thorough capacity-building and training program for healthcare professionals, suppliers, and distributors may help them develop the required abilities and create a culture of awareness, continuous enhancement, and adaptability. This strategic initiative improves the competence of vaccine suppliers, distributors, and healthcare professionals and increases collaborative dedication to maintain vaccine safety and effectiveness throughout the shipment process.
Best Practices
The temperature-regulated cold chain is an important component in preserving the quality of vaccinations. Implementing optimal techniques is essential to improve efficiency, protect the efficacy of vaccines, and reduce risks. Implementing best practices enables managing cold chain activities effectively and efficiently, ensuring product effectiveness and quality maintenance; therefore, it fulfills the need of policy makers to understand and develop adequate policies.
-
(a)
Flexibility in Shipments: Implementing flexible shipment techniques can be an efficient strategy that involves adapting to constantly evolving environmental conditions and unanticipated challenges. Incorporating flexibility into shipping strategies, including real-time tracking and temperature monitoring, temperature-controlled packaging, optimized route planning, and pre-conditioned shipment containers, can provide effective cold chain management. These strategies can efficiently handle uncertainties, ultimately improving the overall success of the temperature-sensitive supply chain.
-
(b)
Utilizing Temperature Monitoring Technologies: Integrating modern temperature monitoring devices is an essential best practice to enhance the cold chain process. Utilizing advanced tracking devices and sensors provides immediate access to the temperature conditions of sensitive products (i.e., vaccines) across the entire cold chain management process. Automated systems can consistently track and record temperature data, enabling accurate and up-to-date data about the state of the cold chain. Therefore, employing temperature monitoring technological devices as best practices makes it simple to reduce the probability of temperature breaches and maintain the quality and safety of temperature-sensitive vaccines.
-
(c)
Assessment of Risk and Mitigation: Risk assessment and adopting measures to minimize risks involves identifying vulnerabilities at various stages of the cold chain processes, such as storage, transportation, and handling, and taking steps to minimize the identified risks. Equipment malfunctions, unexpected logistical obstacles, and temperature fluctuations threaten the quality and stability of temperature-sensitive products. Therefore, organized risk assessment and mitigation can protect the cold chain from disruptions. This strategy ensures the quality and effectiveness of products sensitive to temperature and improves the cold chain functionalities.
-
(d)
Mobile and Remote Access: In today’s interconnected world, mobile and remote access are important for enabling individuals to maintain productivity and connectivity, although their physical presence is not at their primary workplace. The significance of remote access has increased the importance of mobile access due to the increasing prevalence of flexible work arrangements. Utilizing new technologies enabling immediate monitoring via mobile devices or remote access platforms is important for improving operational efficiency and responsiveness. By adopting mobile and remote access strategies, it will be easy to make quick and informed decisions, prevent potential disruptions, and resolve any issues that arise during the cold chain journey. This adaptive approach optimizes the logistics processes and significantly contributes to the overall reliability and effectiveness of the cold chain management system.
Continuous Monitoring
Continuous monitoring is the constant and continuous real-time data analysis that enables an immediate response to emerging challenges and vulnerabilities. Effective cold chain management in the medical domain requires continuous monitoring. It tracks the processes across the supply chain in real-time, enabling fast responses to variances. By adopting an active strategy, the integrity of sensitive objects, such as vaccinations, can be improved.
-
(a)
Controlled temperature range: A rigorous protocol should be followed for managing the temperature range, ensuring it is at a temperature that perfectly maintains the vaccine carrier’s internal environment within the required range of 2–8 °C. This approach will involve leveraging the latest technological advancements in temperature control and monitoring, ensuring the ice packs provide stable thermal conditions over extended periods. This step is critical in preventing the vaccines’ undue freezing or overheating, which can compromise their efficacy.
-
(b)
Real-time monitoring alerts: This proactive strategy enables prompt actions that ensure the vaccinations are maintained within the assigned temperature range during transportation. From this constant observation, real-time monitoring alerts notify if deviations from optimal conditions occur. Integrating continuous monitoring and real-time monitoring alarms can improve the overall strength of vaccine shipment protocols, ensuring the effectiveness and safety of the vaccines.
-
(c)
Protocols for Communication: Protocols for communication strategy can help immediately identify any deviations or disruptions, enabling prompt implementation of corrective measures. The emphasis relies on adapting communication protocols in response to shifting circumstances; therefore, it aids in improving the reliability and responsiveness of the vaccine shipment process. Continuous monitoring fosters a proactive approach to tackle challenges and maintain effective communication, enhancing the vaccine distribution operations’ overall efficacy.
-
(d)
Enforcement and Consequences: Enforcement and consequence are crucial in emphasizing the importance of adhering to predefined protocols in the cold chain management, and policymakers play a vital role in this aspect. They are instrumental in developing and implementing policies that ensure quick responses to potential risks; therefore, they reduce the likelihood of adverse outcomes. In addition, policymakers can advocate for the integration of continuous monitoring systems with automated alarms, ensuring that deviations are promptly notified to responsible individuals. This ensures a proactive approach to managing temperature excursions. Furthermore, they can mandate regular data analysis and audits, which enhance the efficacy of continuous monitoring. These audits provide critical insights into the overall integrity of shipments, enabling better decision-making and policy adjustments. Therefore, the involvement of policymakers is essential in improving the implementation of quality standards and contributing to more a stable and efficient cold chain management.
Data Management and Documentation
Efficient data management ensures the accuracy, accessibility, and availability of data to individuals who require it while preserving its authenticity and privacy. Documentation helps preserve an in-depth understanding of their data resources, fosters optimal techniques, and facilitates compliance with rules and regulations. Data management and documentation enhance the quality of development, and it is important for creating evidence.
Documentation and reporting strategies involve systematic organization, storage, and retrieval of information related to temperature monitoring, shipment processes, and risk assessments that provide a transparent and traceable cold chain management. Using advanced data management systems ensures that the documentation process is accurate and extensive. Therefore, proper documentation and reporting help healthcare professionals improve accountability, decision-making, vaccine safety, and efficacy by using precise practices.
The following strategic recommendations can help address the challenges of effective cold chain management and enhance vaccine supply chain efficacy:
-
(a)
Optimized Vaccine Loading Protocol Based on Real-Time Temperature Monitoring: A methodological approach should be followed for loading vaccines into the carrier, emphasizing that this should only occur when the internal temperature is definitively within the safe range. This practice is crucial in minimizing the risk of temperature breaches that could occur due to premature vaccine placement. Future practices will dictate a more precise and controlled addition of vaccines to carriers, based on real-time temperature monitoring data. This approach will minimize the risk of temperature breaches, ensuring vaccines are added only when the carrier’s internal environment is verified to be within the optimal range.
-
(b)
Stringent box/carrier opening policies: A set of strict guidelines should be recommended for accessing the vaccine carrier, highlighting that it should remain closed except when necessary. This measure is vital in maintaining a consistent internal temperature; therefore, it protects the vaccine’s integrity. Implementing strict access protocols for vaccine carriers will be crucial. Future guidelines should involve sensor-based monitoring systems to track and limit access to the vaccine carrier; therefore, they should maintain a stable internal temperature essential for vaccine viability.
-
(c)
Strategies for addressing cold breaches: In instances where the internal temperature falls below 0 °C, a controlled method should be suggested for briefly exposing the carrier to ambient temperatures to gently raise the internal temperature to the lower threshold of the safe range. In response to cold breaches, future strategies should involve automated temperature regulation systems within carriers, allowing for a controlled temperature adjustment to bring the internal environment back within the safe range.
-
(d)
Systematic cold breach analysis: A regular monitoring and analysis system should be developed and followed for cold breach occurrences to investigate if they are linked to the over-conditioning of ice packs, leading to their freezing. This analysis will be key to identifying and rectifying issues in the cold chain. Advanced data analytics will play a key role in monitoring and analyzing cold breach patterns. This will involve the use of sophisticated algorithms to assess whether cold breaches are linked to ice pack conditioning issues, leading to a more proactive approach in the cold chain management.
-
(e)
Proactive hot breach management: In case of a hot breach, the need for immediate action should be emphasized, recommending the prompt closure of the vaccine carrier to mitigate the impact of external warm temperatures. The approaches should employ automated systems for an immediate response to hot breaches. This will include real-time alerts and automated closure mechanisms for vaccine carriers to mitigate the impact of external warm temperatures quickly.
-
(f)
Tailored cold chain point assessment for hot breaches: There is the need to encourage detailed evaluation of hot breaches at different stages of the cold chain, considering local climatic conditions and operational factors that may contribute to rapid ice pack warming. Future cold chain management should involve a more accurate and methodological analysis of hot breaches, using data-driven insights to account for local climatic variations and operational specifics at each cold chain stage. This will enable tailored strategies to optimize ice pack usage and carrier insulation in different geographic settings.
Implications
Academic Implications
This study marks a significant advancement in vaccine distribution research, establishing a new benchmark for the academic community. It highlights the crucial aspect of temperature integrity during the vaccine transportation phase, especially at the last-mile delivery points, contributing to a previously under-documented area of the vaccine supply chain. By providing a rigorous framework for investigating temperature deviations and their impact on vaccine efficacy, this research opens new avenues for academic inquiry into optimizing cold chain systems. It serves as a foundational model, encouraging further detailed examination of the vaccine logistics process. For researchers and academicians, the methodologies and insights offered by this study advocate for a comprehensive approach to maintaining vaccine potency; therefore, it enriches the dialog within public health and global health security domains.
Practical Implications
The findings of this study underscore the significant role policymakers and healthcare systems play in enhancing logistical operations for vaccine transportation. Identifying the specific stages disposed to temperature breaches enables the strategic improvement of cold chain logistics, incorporating advanced monitoring technologies, and refining session site storage practices. Such logistical enhancements are imperative for sustaining vaccine integrity during transit, ensuring the effectiveness of immunization campaigns, and controlling vaccine-preventable diseases. Moreover, the study’s recommendations for preventing temperature breaches have global implications, addressing vaccine equity challenges and ensuring that vaccines retain their potency upon reaching remote or resource-limited areas. This contributes to global efforts aimed at ensuring comprehensive vaccine access, underlining the study’s contribution to improving health outcomes and vaccine accessibility worldwide.
Conclusion
This study demonstrates the critical incidences of temperature deviations, particularly showcasing the vulnerability of freeze-sensitive vaccines. Through extensive data collection across three Indian states including Karnataka, Maharashtra, and Rajasthan, using real-time temperature tracking data loggers, significant instances have occurred where vaccine temperatures deviated beyond the recommended 2–8 °C range during transportation. This comprehensive cross-sectional analysis has revealed key junctures in the vaccine supply chain, from storage to last-mile delivery, that are prone to temperature excursions.
The application of descriptive statistics and ANOVA tests has been pivotal in quantifying the significance of the duration and frequency of these temperature breaches. The findings from this statistical analysis underscore the necessity for targeted interventions in the cold chain process to maintain vaccine potency. Furthermore, the study introduces the ABCD strategic framework, comprising awareness, best practices, continuous monitoring, and data management and documentation, as a robust approach to tackle the challenges identified. This framework promises to substantially enhance the flexibility and efficiency of temperature monitoring in the vaccine distribution process. It is a step toward ensuring that vaccines remain within their efficacy-preserving temperature range throughout their journey from storage to the end recipient using flexible monitoring solutions.
The recommendations proposed by this study aim to make a significant contribution to optimizing cold chain management. They offer a blueprint for implementing flexible and effective temperature control measures; therefore, they safeguard the integrity and potency of vaccines, which is crucial for public health, especially in low- and middle-income countries such as India. This research sets a precedent for future studies in vaccine distribution and cold chain management, emphasizing the need for continuous innovation and improvement in these critical areas.
This study, while providing valuable insights into the challenges of maintaining temperature integrity during vaccine transportation, is subject to certain limitations. The geographical focus on Karnataka, Maharashtra, and Rajasthan, although diverse, does not encompass the full range of climatic conditions present across India, potentially limiting the generalizability of the findings. The reliance on real-time temperature tracking data loggers, while innovative, may not capture the complete spectrum of temperature excursions, particularly those of very short duration that could still impact vaccine efficacy.
Given the limitations identified, future studies could expand the geographical scope to include a wider range of climatic conditions across different regions in India and beyond, providing a more comprehensive understanding of the challenges faced in vaccine transportation. In addition, there is a need for longitudinal studies that track the evolution of cold chain management practices and technologies over time offering insights into the long-term effectiveness of interventions. Moreover, exploring the integration of more advanced technologies, such as IoT-based monitoring systems, could offer finer granularity and higher accuracy in temperature tracking. This research opens the door for developing adaptable strategies for cold chain management, contributing to the global efforts in enhancing vaccine accessibility and efficacy.
References
Alhawatmeh, H., Alsholol, R., Dalky, H., Al-Ali, N., & Albataineh, R. (2021). Mediating role of resilience on the relationship between stress and quality of life among Jordanian registered nurses during COVID-19 pandemic. Heliyon, 7(11), e08378.
Asamoah, A. (2020). Knowledge, attitude and practice of cold chain management among health practioners in the Sekyere central district (Doctoral dissertation, University of Cape Coast).
Brahimi, A., Kadri, D., & Fekir, M. (2023). The impact of the organizational climate on employee loyalty. Case study: Sonelgaz Ain Defla Distribution Management. Business Excellence and Management, 13(4), 85–100.
Das, B. K., Jha, D. N., Sahu, S. K., Yadav, A. K., Raman, R. K., & Kartikeyan, M. (2022). Analysis of variance (ANOVA) and design of experiments. In Concept Building in Fisheries Data Analysis (pp. 119–136). Singapore: Springer Nature Singapore.
Das, P., Shukla, S., Bhagwat, A., Purohit, S., Dhir, S., Sushil, Jandu, H. S., Kukreja, M., Kothari, N., Sharma, S., Das, S., Taneja, G., & Ghosh, R. S. (2023). Modeling a COVID-19 vaccination campaign in the State of Madhya Pradesh, India. Global Journal of Flexible Systems Management, 24(1), 143–161
Dhawan, V., Aggarwal, M. K., Dhalaria, P., Kharb, P., Sharma, D., Dinesh, K. K., Dhir, S., Sushil, S., Taneja, G., & Ghosh, R. S. (2023). Examining the Impact of key factors on COVID-19 vaccination coverage in India: A PLS-SEM approach. Vaccines, 11(4), 868.
Dhillon, M. K., Rafi-Ul-Shan, P. M., Amar, H., Sher, F., & Ahmed, S. (2023). Flexible green supply chain management in emerging economies: A systematic literature review. Global Journal of Flexible Systems Management, 24(1), 1–28.
Dugard, P., Todman, J., & Staines, H. (2022). Approaching multivariate analysis: A practical introduction. Taylor & Francis.
Gedi, E. M. (2022). Evaluation of the storage and cold chain management of vaccines in the primary health facilities in Arusha City, Northern Tanzania (Doctoral dissertation, university of nairobi).
Guignard, A., Praet, N., Jusot, V., Bakker, M., & Baril, L. (2019). Introducing new vaccines in low-and middle-income countries: Challenges and approaches. Expert Review of Vaccines, 18(2), 119–131.
Gül, A., Alak, S. E., Gül, C., Karakavuk, T., Can, H., Karakavuk, M., Köseoğlu, A. E., Döşkaya, M., Hameş, E. E., Ün, C., Gürüz, A. Y., & Döşkaya, A. D. (2023). The Importance of Vaccines in a Sustainable Healthy Society. In A sustainable green future: Perspectives on energy, economy, industry, cities and environment (pp. 183–212). Cham: Springer International Publishing.
Hanson, C. M., George, A. M., Sawadogo, A., & Schreiber, B. (2017). Is freezing in the vaccine cold chain an ongoing issue? A Literature Review. Vaccine, 35(17), 2127–2133.
Hassan, A., Samy, G., Hegazy, M., Balah, A., & Fathy, S. (2024). Statistical analysis for water quality data using ANOVA (Case study–Lake Burullus influent drains). Ain Shams Engineering Journal, 15, 102652.
Hussain, M. S., & Ali, M. (2019). A multi-agent based dynamic scheduling of flexible manufacturing systems. Global Journal of Flexible Systems Management, 20(3), 267–290.
Kartoglu, U., & Ames, H. (2022). Ensuring quality and integrity of vaccines throughout the cold chain: The role of temperature monitoring. Expert Review of Vaccines, 21(6), 799–810.
Lin, Q., Zhao, Q., & Lev, B. (2020). Cold chain transportation decision in the vaccine supply chain. European Journal of Operational Research, 283(1), 182–195.
Mabrouki, J., Azrour, M., Dhiba, D., Farhaoui, Y., & El Hajjaji, S. (2021). IoT-based data logger for weather monitoring using arduino-based wireless sensor networks with remote graphical application and alerts. Big Data Mining and Analytics, 4(1), 25–32.
Madani, T., Boukraa, M., Aissani, M., Chekifi, T., Ziadi, A., & Zirari, M. (2023). Experimental investigation and numerical analysis using Taguchi and ANOVA methods for underwater friction stir welding of aluminium alloy 2017 process improvement. International Journal of Pressure Vessels and Piping, 201, 104879.
Maity, S., Aakriti, J., Manandhar, S., Anchan, S. B., Bhat, A., Shetty, M. U., & Nayak, Y. (2022). Emvolio-A battery operated portable refrigerator preserves biochemical and haematological integrity of biological samples in preclinical studies. F1000Research, 11, 223.
Murhekar, M. V., Dutta, S., Kapoor, A. N., Bitragunta, S., Dodum, R., Ghosh, P., Swamy, K. K., Mukhopadhyay, K., Ningombam, S., Parmar, K., Ravishankar, D., Singh, B., Singh, V., Sisodiya, R., Subramanian, R., & Takum, T. (2013). Frequent exposure to suboptimal temperatures in vaccine cold-chain system in India: Results of temperature monitoring in 10 states. Bulletin of the World Health Organization, 91(12), 906–913. https://doi.org/10.2471/blt.13.119974
Pambudi, N. A., Sarifudin, A., Gandidi, I. M., & Romadhon, R. (2022). Vaccine cold chain management and cold storage technology to address the challenges of vaccination programs. Energy Reports, 8, 955–972.
Patel, J., More, S., Sohani, P., Bedarkar, S., Dinesh, K. K., Sharma, D., Dhir, S., Sushil, S., & Ghosh, R. S. (2024). Reshaping the equitable and inclusive access to healthcare: A qualitative study. Clinical Epidemiology and Global Health, 26, 101544.
Preston, K. B., & Randolph, T. W. (2021). Stability of lyophilized and spray dried vaccine formulations. Advanced Drug Delivery Reviews, 171, 50–61.
Rajakumar, S., Hemavathi, S., El-Marghany, A., & Warad, I. (2023). Synthesis and adsorption capacity of biochar derived from Tamarindus indica shell for the removal of heavy metal. Global Nest Journal, 25, 73–81.
Ramírez-Faz, J., Fernández-Ahumada, L. M., Fernández-Ahumada, E., & López-Luque, R. (2020). Monitoring of temperature in retail refrigerated cabinets applying IoT over open-source hardware and software. Sensors, 20(3), 846.
Sarker, M. R., Moktadir, M. A., & Santibanez-Gonzalez, E. D. (2021). Social sustainability challenges towards flexible supply chain management: Post-COVID-19 perspective. Global Journal of Flexible Systems Management, 22(Suppl 2), S199–S218.
Shore, C., Brown, L., Hopp, W. J., & National Academies of Sciences, Engineering, and Medicine (2022). Causes and Consequences of Medical Product Supply Chain Failures. In Building Resilience into the Nation's Medical Product Supply Chains. National Academies Press (US).
Tapati, R., Saha, A., Dhakre, D. S., & Gupta, R. K. (2023). Determinants of entrepreneurial behaviour of women dairy farmers by using one way ANOVA analysis in Sepahijala district of Tripura (Vol. 18(1), pp. 36–42)
Thiyaneswaran, B., Anguraj, K., Kumarganesh, S., & Ghosh, S. (2022). IOT based smart cold chain temperature monitoring and alert system for vaccination container. Przeglad Elektrotechniczny, 98(8), 208.
Vishvakarma, N. K., Sharma, R. R. K., & Kumar, A. (2021). An empirical analysis of impact of organizational strategies on critical success factors of business process reengineering. Global Journal of Flexible Systems Management, 22(1), 55–73.
Wadhwa, S., & Rao, K. S. (2004). A unified framework for manufacturing and supply chain flexibility. Global Journal of Flexible Systems Management, 5(1), 29–36.
Wang, W., Wu, Q., Yang, J., Dong, K., Chen, X., Bai, X., Chen, X., Chen, Z., Viboud, C., Ajelli, M., & Yu, H. (2020). Global, regional, and national estimates of target population sizes for COVID-19 vaccination: descriptive study. BMJ, 371, m4704.
Wanganoo, L., Shukla, V. K., & Panda, B. P. (2021). NB-IoT powered last-mile delivery framework for cold supply chain. In Data Driven approach towards disruptive technologies: Proceedings of MIDAS 2020 (pp. 257–268). Springer, Singapore.
Weinman, B., Levine, G. H., McCarthy, J., & Sims, G. (2021). The American medical product supply chain. Food and Drug Law Journal, 76(2), 235–269.
Yildirim, U. K., & Sisman, Y. (2023). Compliance analysis in polynomial surface determination with ANOVA. Intercontinental Geoinformation Days, 6, 228–232.
Zaoui, S., Foguem, C., Tchuente, D., Fosso-Wamba, S., & Kamsu-Foguem, B. (2023). The viability of supply chains with interpretable learning systems: The case of COVID-19 vaccine deliveries. Global Journal of Flexible Systems Management, 24(4), 633–657.
Funding
This research is funded by B Medical Systems, India (Grant No. CW15945N).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that Prof. Sushil, serving as the Editor in Chief, and Prof. Sanjay Dhir, serving as an Associate Editor of this journal, are co-authors of this manuscript. Their involvement in the authorship has been fully disclosed, and the manuscript has undergone the journal’s standard peer review process managed by independent editors to ensure objectivity and fairness.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lamba, H.K., Sharma, D., Dhir, S. et al. Ensuring Vaccine Temperature Integrity: Monitoring from Storage to Last-Mile Delivery. Glob J Flex Syst Manag 25, 559–578 (2024). https://doi.org/10.1007/s40171-024-00401-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40171-024-00401-3