Abstract
Purpose of Review
This review article analyzes current evidence on the neurophysiology of swallowing during development and offers expert opinion on clinical implications and future research directions.
Recent Findings
In the past 5 years, basic and clinical research has offered advances in our understanding of pediatric swallowing neurophysiology. Animal models have elucidated the role of brainstem circuits and the peripheral and central nervous systems in neonatal swallowing. Recent human studies have further showcased that fetal and infant swallowing require cerebral inputs in order to develop functionally. Finally, neurophysiological and neuroimaging studies are starting to better define these cerebral inputs, as well as neuroplastic adaptations that may be needed for optimal feeding development.
Summary
The neural development of swallowing is a complex and dynamic process. Continued research is needed to better understand influences on swallowing neural development, which can be essential for improving prevention, diagnosis, and interventions for pediatric dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oropharyngeal swallowing consists of complex neurophysiological processes involving all levels of the nervous system and many oropharyngeal sensory and motor components that have partly voluntary and partly automatic control. Understanding these processes, their development, and any aberrations, is critical in our efforts to develop interventions that take advantage of the extraordinary neuroplastic capacity of the nervous system. Over the past century, our field has made significant strides in this understanding, primarily from research on adult humans and animal models focused on adults.
Specifically, early studies using animal models revealed insights on the relationship between brainstem circuitry and the pharyngeal swallow response, highlighting the reflexive but complex nature of the pharyngeal swallow [1–4]. Subsequently, microelectrode stimulation research in primates and lagomorphs, among other species, showed that the pharyngeal response could be triggered when microelectrodes stimulated parts of the cortex, laying the groundwork that swallowing also has cortically mediated elements [e.g., 5,6]. These findings were further enhanced by human studies of patients with swallowing disorders (a.k.a., dysphagia) resulting from cortical or subcortical damage [7–12], and by studies using neuroimaging and neuro-stimulation techniques to investigate both healthy and disordered swallowing in vivo [13–19]. These studies highlighted the multitude of sensorimotor mechanisms involved in adult swallowing and corroborated that both central and peripheral contributions are vital for swallowing control. This knowledge formed the basis for the emergence of neurophysiology-based principles in adult swallowing rehabilitation [20–23].
However, much less attention has been given to understanding swallowing neurophysiology across development, that is, in infants and children, which represents a significant gap in our ability to effectively diagnose and intervene in pediatric populations with dysphagia. Pediatric dysphagia is hypothesized to affect 25% of typically developing children, and up to 80% of children with developmental disorders [24–27], and this prevalence continues to increase as more children survive preterm birth and childhood disease [26, 28, 29]. Children with dysphagia are at risk for nutritional deficits, aspiration-related lung disease, developmental delay, decreased independent life participation, and even death [28].
These numbers and consequences underscore the importance of better understanding developmental swallowing neurophysiology in order to develop neurophysiology-based evaluation and interventions for children with dysphagia. In an attempt to highlight this much-needed area of study, in this review, we focus on the most recent advances of basic and clinical research in pediatric swallowing neurophysiology and offer expert insights on implications and future directions.
Infant and Pediatric Feeding and Swallowing
Infant and pediatric feeding and swallowing involve biological and behavioral intricacies that make it substantially different from the adult swallow. Although frequently used interchangeably, the terms feeding and swallowing describe somewhat different but overlapping elements of eating. Feeding is a broader term that encompasses anticipatory events, food acceptance, and interactions between the child and caregiver [30], while swallowing involves the sensorimotor acts necessary for the transportation of a bolus from the mouth to the stomach.
From embryology studies, we know that fetuses with healthy embryonic and fetal development display swallowing as early as 10–14 weeks of gestation, [31] supporting the postulation that swallowing is initially an innate behavior [31, 32••]. Fetal swallowing lays the foundation for later swallowing function and is integral in homeostatic regulation of amniotic fluid volume, acquisition and recirculation of intrauterine solutes, and overall fetal growth [33]. Swallows, suckling, and pharyngeal movements increase in frequency until suckling and swallowing start occurring consistently together in the third trimester [31, 34]. In newborn infants, successful feeding is more complex and requires the integration of respiration, suckling, and swallowing, which must occur within hours of birth for them to meet nutrition and hydration needs. Prerequisite for this integration is the uninterrupted prenatal development of relevant aerodigestive structures (e.g., tongue, lips, palate, pharynx, larynx, esophagus, lungs), the cranial and spinal nerves that control them, and areas of the brain (primarily the hindbrain) [32••]. Furthermore, the role of the caregiver as the feeder and the rapid developmental changes occurring in multiple interacting body systems throughout the first year of life make this process even more dynamic [35]. As infants gain gross motor independence and respiratory stability, and their aerodigestive and cranial structures grow (e.g., the larynx starts descending in the neck, the cheeks flatten, teeth emerge), they gradually shift to more autonomous or voluntary feeding, as evidenced by their increased ability to consume solids at around 4–6 months [30, 35].
Further refinement of feeding and swallowing skills (e.g., biting and chewing, drinking from a cup and straw, eating independently) continues over the first few years of life, leading to relative feeding autonomy by around 3 years [30]. However, emerging evidence, including some of our most recent work, suggests that children may not achieve fully mature swallowing skills until later in childhood [36, 37]. This protracted refinement is consistent with modern sensorimotor theories of development, that support further fine-tuning in most motor systems even in school-age years [38, 39].
Recent Advances in Developmental Neurophysiology of Swallowing
Lessons from Recent Animal Studies
The most rapidly accumulating body of literature on neurophysiology of pediatric swallowing involves animal models. This work has primarily focused on the role of the brainstem and the peripheral nervous system and has validated some of our prior knowledge from the adult-focused work but has also provided new insights.
The role of a swallowing central pattern generator (CPG) within the brainstem has been extensively described in adult-focused work and is considered the primary driver of the pharyngeal swallow response. Specifically, it is well known that two groups of nuclei and interneurons located in the caudal medulla comprise this delicate network. These groups are the dorsal (sensory) swallowing group, including the nucleus tractus solitarius (NTS) and adjacent reticular formation, and the ventral (motor) swallowing group, including the nucleus ambiguous (NA) and surrounding areas [40–43]. It is also known that additional brainstem networks that control other orofacial behaviors and respiration also share some of these neuroanatomical correlates, and that all these networks have to act in coordination for optimal aerodigestive functioning. These brainstem networks are considered critical correlates of the developing neural swallowing control. Recently, Pitts and colleagues [2021] used an in vitro neonatal rat model to examine the role of the swallow CPG, as well as additional areas in the medulla (specifically, the medullary intermediate reticular formation (IRt) and the ventral respiratory column (VRC)), in eliciting cranial nerve responses indicative of a fictive swallow and/or a breathing reflex [44]. Critically, they found that, in addition to neurons within the swallow CPG, new networks within the IRt, near the facial nucleus in the dorsal medulla, were also active during the swallows. These findings indicate that in this neonatal model, the swallow CPG is more extensive than previously thought, which may be an indication of continued maturation or potential for medullary plasticity in the neonate.
Further insights on the role of the peripheral nervous system—and specifically on the impact of nerve lesions on infant swallowing—have been recently offered by the extensive work of Rebecca German and her group in the infant pig. Pigs have similar oropharyngeal anatomy, head positioning, and dynamic integration between sensory inputs and motor responses during swallows as human infants, and therefore offer a good model to study the developing swallowing system. Multiple recent studies from this group have revealed details on peripheral nerve contribution to perinatal swallowing by studying the effects of lesions on two branches of the vagus, the recurrent laryngeal nerve (RLN) and the superior laryngeal nerve (SLN) in infant pigs. RLN damage is frequent in infants who have to undergo cardiovascular surgery, and it may cause unilateral vocal fold paralysis, breathing difficulties, and dysphagia. SLN damage is more common after orofacial surgery/injury and may impact swallow initiation and also lead to dysphagia. Although both types of injuries may temporarily compromise airway safety in term infant pigs, the underlying physiological mechanisms of this compromise differ [45]. More specifically, RLN lesions have been shown to affect hyoid and tongue kinematics [46], volume consumed [47], the neuromuscular control of swallowing [46], and airway safety in infant pigs, and some of these changes can be pervasive. SLN lesions have been associated with higher rates of reduced airway safety as well [48]; however, these changes are mostly explained by temporal dysfunctions in the coordination between suck and swallow cycles [48] or in epiglottic inversion timing [45].
Additional longitudinal studies of the same research group offer insights on swallowing changes over postnatal maturation in the same animal model. Specifically, Stricklen and colleagues [2020] [49] found that peripheral RLN lesions may be most impactful on swallow safety close to birth (on 7 days of life; 1–2 months human equivalent) and mostly resolve with time (at 17 days of life; 6–9 months human equivalent), perhaps due to feeding practice and/or maturation [50]. Follow-up papers by Mayerl and colleagues examined hyoid and thyroid kinematics [2020], and swallow safety [2021] longitudinally (again at 7 and 17 days of life) in preterm and full-term infant pigs, with and without RLN lesions. Results related to hyoid and thyroid kinematics showed that preterm status affected the coordination between the two structures’ movements, but not the extent of their excursion; while RLN lesions were mostly associated with reduced thyroid excursion, and increased variability in hyoid kinematics [51•]. Interestingly, results related to airway safety demonstrated that bolus size (swallowing larger boluses) was more predictive of penetration and aspiration than either lesion status or age (preterm or term status) [52]. According to the authors, this corroborates clinical knowledge that some instances of aspiration in infant feeding may be normal. These studies, using well-controlled and distinct nerve lesions, underscore the importance of peripheral swallow neurodevelopment, but also demonstrate the remarkable ability of the peripheral nervous system to develop adaptations and recover from these perinatal lesions.
However, the reality is that most infants with dysphagia have deficits as a result of genetic or developmental disruptions that occur prenatally and affect a constellation of developing body systems. For this reason, animal models that mimic pathogenesis of dysphagia from a genetic source may be particularly relevant to questions involving systemic impaired swallowing development [32••]. One such genetic syndrome is the DiGeorge/22q11.2 Deletion Syndrome (22q11DS) which includes over 180 clinical features that are thought to emerge from disruption of early stages of neural development, and specifically from altered prenatal hindbrain patterning. In human infants with 22q11DS, these disruptions can compromise the development of craniofacial structures, as well as cranial nerve and brainstem circuits, and lead to significant feeding and swallowing deficits [53]. To help better understand the pathogenesis of developmental dysphagia, Maynard and colleagues recently developed a mouse model of 22q11DS [54], which was validated through genetic and neurological examinations [54]. Welby and colleagues [2020] further confirmed the persistence of feeding and swallowing deficits in adult mice with 22q11DS through the use of videofluoroscopic swallow studies, endoscopy, behavioral feeding videos, and post-mortem lung tissue imaging that revealed aspiration-related lung damage [55]. These studies corroborated the multi-level and persistent nature of dysphagia in this syndrome [54, 56], and indicated that when prenatal neural development is disrupted, the recovery and neuroplastic potential for functional swallowing is reduced. This work is promising because, in addition to providing enhanced insights on genetic influences on the central and peripheral swallowing control, it may also form the foundation for better understanding, diagnosing, and ultimately treating developmental pediatric dysphagia.
Lessons from Recent Clinical and Neuroimaging Studies
In addition to this animal work, recent research on pediatric clinical populations further elucidates contributions of all levels of the nervous system in swallowing development. Starting with the periphery, several clinical studies through the years have shown the importance of orofacial structural and neuromuscular integrity in developmental swallowing control [e.g., 30,57,58]. More contemporary studies have substantiated this by offering evidence for the high occurrence of dysphagia in children with peripheral neuromuscular disease [59], as well as in infants with 22q11.2DS [53].
Moving onto higher neural centers, the contributions of the cerebrum even in infant and pediatric swallowing have also been recently documented. In a 2019 systematic review investigating dysphagia frequency and related factors in children with stroke and cerebral palsy (CP), i.e., central lesions, Sherman and colleagues [2019] found that dysphagia frequency ranged from 24.2 to 88.6% among the reviewed studies [60]. Furthermore, in a sample of 67 neonates and 106 children with acute stroke studied retrospectively, 38.8% of the neonates (average age at stroke = 2.9 days) and 40.6% of the children (average age at stroke = 6.5 years) exhibited confirmed dysphagia [61].
Perhaps the most direct clinical evidence for the role of the cerebrum in infant swallowing is offered by studies that have examined infants with profound central nervous system defects, such as anencephaly. This serious congenital condition involves the partial or full absence of cortical development as well as subcortical structures and at times the cerebellum, and may even affect the development of parts of the brainstem [62, 63•]. In a recent article, Radford and colleagues [2019] reviewed published data on suckling, breathing, swallowing, crying, hiccup, and facial movements of fetuses/infants with anencephaly or hydranencephaly included in older studies. Of the 18 fetuses/infants specifically with anencephaly reported across eight reviewed studies, only one infant who had some preserved cerebral tissue and lived to be 28 months old was reported to be able to swallow, breathe spontaneously, feed from a bottle, and smile [64]. Four of the 18 were able to suckle, although three of these lacked cerebral and cerebellar structures entirely, and one had an abnormal pons but fully developed medulla. Spontaneous breathing was preserved in twelve of the 18 fetuses/infants, including a 17-week fetus with aplastic medulla, pons, cerebellum, and cerebrum. Importantly, none of these patients exhibited fetal swallowing in early fetal stages. These findings provide clear evidence that even for fetuses and infants, swallowing behaviors require at least some cerebral inputs in order to be fully functional. In contrast, suckling and breathing seem to be more reflexive and require fewer cerebral inputs, especially at these early stages of development.
One way to enhance our understanding of these cerebral inputs is through the use of neuroimaging that is now more widely available. It is important to note that neuroanatomical information obtained from routine structural brain CT (computed tomography) or MRI (magnetic resonance imaging) scans may not be fully sufficient to capture microstructural and/or functional disruptions in the pediatric swallowing network and their use has at times led to vague or inconclusive outcomes [65, 66••]. At the same time, the use of advanced neuroimaging techniques, such as task-related functional MRI, positron emission tomography (PET), or magnetoencephalography (MEG), that require active subject participation, can be rather challenging in pediatrics. Therefore, recent neuroimaging studies that examine pediatric swallowing control use techniques, such as resting-state functional connectivity MRI (rs-fcMRI) and diffusion weighted imaging (DWI), that do not require active subject participation but can provide robust quantitative information on the functional and structural integrity of the brain.
For example, DWI was recently utilized to examine the relationship between microstructural integrity of sensorimotor white matter tracts and quantitative measures of neonatal sucking physiology in a study of 10 neonates with brain injury [67]. In this study, the Nfant® swallowing system, i.e., a method to objectively measure suck parameters in real time, was used to assess nutritive sucking, and diffusion-based tractography was used to examine diffusion parameters—such as fractional anisotropy and mean and radial diffusivity—of the main motor, sensory, and corpus callosum tracts [67]. Findings showed that high sucking irregularity was associated with reduced structural integrity of all white matter tracts examined, while abnormalities in sucking smoothness were correlated with reduced integrity of the motor and sensory tracts. The authors also suggested that the relationship between sucking physiology and brain damage could be informative in the inverse direction, i.e., detailed sucking physiology measures could be an indicator of microstructural deficits that are not caught by routine MRI; however, this would require further validation with larger sample sizes.
Emerging Lessons from our Current Work
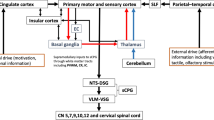
In recent years, our team has sought to add to this body of work by focusing our efforts on a group of children with unilateral brain involvement, i.e., children with unilateral cerebral palsy (UCP). This type of involvement, where predominantly one hemisphere is impacted, offers a good model to understand early neural reorganization, especially for functions that are bilaterally innervated and have both central and peripheral neural underpinnings, such as swallowing. Furthermore, through the use of a multi-level approach, deeper insights of swallowing neural control in children may be offered (Fig. 1).
Our early work in this area was based on a convenience sample of self-feeding school-age children (5.1–17.6 years old) with unilateral and bilateral CP, who participated in summer camps of a Cerebral Palsy Research Center. Through standardized clinical feeding assessments [68, 69, 70••] completed during the camp lunchtimes, we first identified that children with UCP (GMFCS [Gross Motor Function Classification Scale] Levels I and II) exhibit a range of mild to moderate clinical feeding and swallowing difficulties [68, 69, 70••], which have been largely under-recognized. These early studies suggested that even when the brainstem and one hemisphere are largely spared, feeding and swallowing development can still be impacted, therefore underscoring the role of bilaterality in pediatric swallowing control.
This was further elucidated through a study examining the association between brain lesion characteristics and inter-hemispheric (corpus callosum) connectivity indices and clinical swallowing outcomes in 20 self-feeding children with UCP recruited from the same convenience sample [71]. In this study, we added structural MRI and DWI to examine brain correlates of clinical signs of dysphagia that had been identified through the aforementioned standardized feeding assessments. We saw that in children with UCP who had brain lesions primarily affecting the somatosensory cortex/middle cerebral artery (MCA) area (also mostly left-hemisphere lesions) (n = 13), clinical signs of dysphagia were milder than in children who had lesions primarily affecting subcortical/periventricular areas (also mostly right-hemisphere lesions) (n = 7). This finding indicated that type and/or side of lesion plays a role in the development of clinical dysphagia in UCP. Secondly, the structural integrity of the corpus callosum appeared to be important in enabling inter-hemispheric plasticity for the children with MCA lesions, but when intra-hemispheric connections were disrupted, such as in children with subcortical/periventricular lesions, corpus callosum integrity no longer mattered. These findings are partially supported by some of our more recent work which includes the use of resting-state fcMRI to examine the resting-state sensorimotor network of a different sample of 15 children with UCP [72]. In this study, we are finding that children with MCA area lesions affecting the sensorimotor cortex have primarily contralesional resting-state sensorimotor networks, and also relatively milder clinical dysphagia symptomatology compared to children with subcortical lesions [72]. Collectively, these studies provide preliminary evidence indicating that contralesional compensations may be critical in order to develop functional swallowing skills in UCP. Upon further validation, this line of work will help clarify the role of side, type, and site of early brain lesions in swallowing development.
In subsequent collaborative work utilizing a younger sample (ages 5.3–9.1) including mostly children with UCP (8/11 children) and typically developing controls, we further demonstrated physiological deficits in respiratory-swallowing coordination and cough effectiveness in this population [69]. Specifically, we observed that these children exhibit more frequent inspiration after the swallow (i.e., a typically abnormal pattern) and lower cough volume acceleration during voluntary cough, compared to typically developing peers [69]. These findings, albeit involving a small sample size, indicate increased risk for airway compromise, which may result from involvement or discoordination between the swallowing and breathing CPGs at the brainstem level, or from altered higher neurophysiological control.
Building on this work, in the past 3 years we have been conducting a prospective study using behavioral clinical feeding assessments, surface electromyography of orofacial muscles, and neuroimaging to determine both peripheral and central neuroplastic adaptations for swallowing (and speech) in UCP and typical development. This larger scale study includes 20 children with UCP and 20 typically developing controls (7–12 years old). Data analyses thus far reveal additional deficits in mealtime efficiency for the UCP group [73], as well as neuromuscular adaptations, manifesting as submental and perilabial neuromuscular overactivation during swallows, which may represent a peripheral maladaptive or compensatory behavior [74]. Ongoing analysis associating the behavioral with the neuromuscular and neuroimaging data is now in process. Finally, in an offshoot of this work focusing on potential differences in the neuromuscular control of swallowing in two typically developing age groups (7–8 and 11–12 years of age), we are observing more efficient swallowing in older children [37]. These novel findings indicate continued refinement of neuromuscular control of swallowing even in older typically developing children, which is consistent with postulations from modern sensorimotor theories of development [38, 39], and emphasizes the need to better understand typical swallowing neurodevelopment across childhood.
Conclusion and Future Directions
In the past 5 years, basic and clinical research has offered exciting advances in our understanding of the neurophysiology of swallowing in development (Table 1). Studies using animal models corroborated findings from prior adult-focused work, but also provided novel findings on the potential role of additional medullary areas in triggering the pharyngeal response, and on swallowing recovery patterns after perinatal nerve lesions in infants. Furthermore, a new animal model of developmental pediatric dysphagia was developed and validated. Equally important are the several clinical human studies that have provided evidence that pediatric dysphagia can result from structural, neuromuscular, or cerebral aberrations. Importantly, and although fetal and infant swallowing have been long described as innate and reflexive behaviors, clinical evidence stemming from fetuses/infants with anencephaly supports that even fetal and infant swallowing may require at least some cerebral inputs in order to develop functionally. Finally, neuroimaging and neurophysiology studies have started shedding early light to these required cerebral inputs, and to differential central and peripheral swallowing adaptations that may be possible when primarily one hemisphere is affected.
These recent advances also provide valuable insight into remaining gaps which form directions for future work. First, from the work on the newly validated animal model of developmental dysphagia, the potential influence of genetic and prenatal factors in the development of dysphagia has been highlighted. Also, it has become clear that more research is needed in identifying the role of individual genes and/or gene networks in early neural disruptions related to hindbrain patterning that appears particularly disruptive to infant feeding [54]. Through such work, it may be possible to identify genetic correlates of developmental dysphagia, and thus improve diagnostic and prognostic precision in early feeding development. Furthermore, this research highlights the importance of examining prenatal health and the role of prenatal care in potentially preventing these early neural disruptions and warrants further investigation.
In addition, it will also be critical that we gain a deeper understanding of the specific central and peripheral swallowing components and how these mature postnatally. Animal-based work will be vital in this direction, because it allows us to directly identify physiological deficits in brainstem circuits (CPGs), nerves, and muscles, and to test the effects of targeted pharmacological and other invasive interventions; something that is not easy to do in human infants [54]. In regard to human work, the use of neuroimaging and peripheral neurophysiology measures combined with standardized behavioral clinical outcomes has the greatest potential to offer specificity on the neurophysiological and neuromuscular underpinnings of pediatric swallowing control. Such studies will not only help advance the clinical prognosis and diagnosis of pediatric dysphagia, but more importantly have the potential to lead to the development of new neurophysiology-driven treatments, and are highly needed.
Despite challenges inherent in studies of pediatric swallowing neural control, this work is accumulating and is essential. In adults, interventions grounded in neurophysiological principles have led to improvements in swallowing rehabilitation protocols [21,22, 75], and we believe that parallel efforts are possible for pediatric dysphagia. For these efforts to be fruitful, continued research focusing on the areas discussed herein will be critical. As children with significant developmental or acquired challenges continue to be born earlier and live longer, our research efforts in this direction must accelerate to help us not only effectively diagnose and treat, but also potentially prevent pediatric dysphagia.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Amri M, Car A, Jean A. Medullary control of the pontine swallowing neurones in sheep. Exp Brain Res. 1984;55(1):105–10.
Car A, Roman C. Deglutitions and oesophageal reflex contractions induced by electrical stimulation of the medulla oblongata. Exp Brain Res. 1969;11(1):75–92.
Doty RW. Influence of stimulus pattern on reflex deglutition. Am J Physiol. 1951;166(1):142–58.
Jean A, Car A, Roman C. Comparison of activity in pontine versus medullary neurones during swallowing. Exp Brain Res. 1975;22(2):211–20.
Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM, Sessle BJ. Features of cortically evoked swallowing in the awake primate (Macaca fascicularis). J Neurophysiol. 1999;82(3):1529–41.
Sumi T. Reticular ascending activation of frontal cortical neurons in rabbits, with special reference to the regulation of deglutition. Brain Res. 1972;13(46):43–54.
Daniels SK, Foundas AL. Lesion localization in acute stroke patients with risk of aspiration. J Neuroimaging. 1999;9(2):91–8.
Daniels SK, Pathak S, Mukhi SV, Stach CB, Morgan RO, Anderson JA. The relationship between lesion localization and dysphagia in acute stroke. Dysphagia. 2017;32(6):777–84.
Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br Med J (Clin Res Ed). 1987;295(6595):411–4.
Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia. 1993;8(3):195–202.
Meadows JC. Dysphagia in unilateral cerebral lesions. J Neurol Neurosurg Psychiatry. 1973;36(5):853–60.
Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil. 1993;74(12):1295–300.
Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2 15O PET activation. J Neurophysiol. 1999;81(4):1917–26.
Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30(10):3209–26.
Malandraki GA, Johnson S, Robbins J. Functional MRI of swallowing: from neurophysiology to neuroplasticity. Head Neck. 2011;33(S1):S14-20.
Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85(2):938–50.
Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, et al. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92(4):2428–43.
Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18(2):71–7.
Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp Brain Res. 2005;161(1):81–90.
Carnaby GD, LaGorio L, Silliman S, Crary M. Exercise-based swallowing intervention (McNeill Dysphagia Therapy) with adjunctive NMES to treat dysphagia post-stroke: a double-blind placebo-controlled trial. J Oral Rehabil. 2020;47(4):501–10.
Huckabee M-L, Lamvik-Gozdzikowska K. Reconsidering rehabilitation for neurogenic dysphagia: strengthening skill in swallowing. Curr Phys Med Rehabil Rep. 2018;6(3):186–91.
Malandraki GA, Rajappa A, Kantarcigil C, Wagner E, Ivey C, Youse K. The intensive dysphagia rehabilitation approach applied to patients with neurogenic dysphagia: a case series design study. Arch Phys Med Rehabil. 2016;97(4):567–74.
Zimmerman E, Carnaby G, Lazarus Cathy L, Malandraki Georgia A. Motor learning, neuroplasticity, and strength and skill training: moving Ffom compensation to retraining in behavioral management of dysphagia. Am J Speech Lang Pathol. 2020;29(2S):1065–77.
Bhattacharyya N. The prevalence of pediatric voice and swallowing problems in the United States. Laryngoscope. 2015;125(3):746–50.
Borowitz KC, Borowitz SM. Feeding problems in infants and children. Pediatr Clin North Am. 2018;65(1):59–72.
Kovacic K, Rein LE, Szabo A, Kommareddy S, Bhagavatula P, Goday PS. Pediatric feeding disorder: a nationwide prevalence study. J Pediatr. 2021;1(228):126-131.e3.
Linscheid TR. Behavioral treatments for pediatric feeding disorders. Behav Modif. 2006;30(1):6–23.
Lefton-Greif MA, Arvedson JC. Pediatric feeding and swallowing disorders: state of health, population trends, and application of the international classification of functioning, Disability, and Health. Semin Speech Lang. 2007;28(3):161–5.
Lefton-Greif MA, Arvedson JC. Pediatric feeding/swallowing: yesterday, today, and tomorrow. Semin Speech Lang. 2016;37(4):298–309.
Delaney AL, Arvedson JC. Development of swallowing and feeding: prenatal through first year of life. Dev Disabil Res Rev. 2008;14(2):105–17.
Miller JL, Sonies BC, Macedonia C. Emergence of oropharyngeal, laryngeal and swallowing activity in the developing fetal upper aerodigestive tract: an ultrasound evaluation. Early Human Dev. 2003;71(1):61–87.
•• Maynard TM, Zohn IE, Moody SA, LaMantia A-S. Suckling, feeding, and swallowing: behaviors, circuits, and targets for neurodevelopmental pathology. Annu Rev Neurosci. 2020 8;43(1):315–36. This clinical review paper laid out a developmental program of feeding development and described an animal model of developmental pediatric dysphagia.
Ross MG, Nijland MJM. Fetal swallowing: relation to amniotic fluid regulation. Clin Obstet Gynecol. 1997;40(2):352–65.
de Vries JI, Visser GH, Prechtl HF. The emergence of fetal behaviour. II Quantitative aspects Early Hum Dev. 1985;12(2):99–120.
Carruth BR, Ziegler PJ, Gordon A, Hendricks K. Developmental milestones and self-feeding behaviors in infants and toddlers. J Am Diet Assoc. 2004;1(104):51–6.
Hennessey NW, Fisher G, Ciccone N. Developmental changes in pharyngeal swallowing acoustics: a comparison of adults and children. Logoped Phoniatr Vocol. 2018;43(2):63–72.
Hahn Arkenberg R, Mitchell S, Brown B, Goffman L, Malandraki GA. The neuromuscular development of swallowing continues into the school-age years: evidence from a preliminary cross-sectional study. Dysphagia Research Society Meeting. In Virtual; 2021.
Thelen E. Dynamic systems theory and the complexity of change. Psychoanalytic Dialogues. 2005;15(2):255–83.
Haddad JM, Claxton LJ, Keen R, Berthier NE, Riccio GE, Hamill J, et al. Development of the coordination between posture and manual control. J Exp Child Psychol. 2012;111(2):286–98.
Jean A. Brainstem organization of the swallowing network. BBE. 1984;25(2–3):109–16.
Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–69.
Miller AJ. The search for the central swallowing pathway: the quest for clarity. Dysphagia. 1993;8(3):185–94.
Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev. 2008;14(2):77–86.
Pitts T, Huff A, Reed M, Iceman K, Mellen N. Evidence of intermediate reticular formation involvement in swallow pattern generation, recorded optically in the neonate rat sagittally sectioned hindbrain. J Neurophysiol. 2021;125(4):993–1005.
Gould FD, Lammers AR, Mayerl CJ, German RZ. Specific vagus nerve lesion have distinctive physiologic mechanisms of dysphagia. Front Neurol. 2019;10:1301.
Gould FDH, Lammers AR, Mayerl C, Ohlemacher J, German RZ. Muscle activity and kinematics show different responses to recurrent laryngeal nerve lesion in mammal swallowing. J Neurophysiol. 2020;124(6):1743–53.
Gould FDH, Yglesias B, Ohlemacher J, German RZ. Pre-pharyngeal swallow effects of recurrent laryngeal nerve lesion on bolus shape and airway protection in an infant pig Model. Dysphagia. 2017;32(3):362–73.
Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Fukuhara T, Gierbolini-Norat EM, et al. Unilateral superior laryngeal nerve lesion in an animal model of dysphagia and its effect on sucking and swallowing. Dysphagia. 2013;28(3):404–12.
Stricklen BM, Bond LE, Gould FDH, German RZ, Mayerl CJ. Swallow safety in infant pigs with and without recurrent laryngeal nerve lesion. Dysphagia. 2020;35(6):978–84.
Mayerl CJ, Catchpole EA, Edmonds CE, Gould FDH, McGrattan KE, Bond LE, et al. The effect of preterm birth, recurrent laryngeal nerve lesion, and postnatal maturation on hyoid and thyroid movements, and their coordination in infant feeding. J Biomech. 2020;22(105):109786.
• Mayerl CJ, Myrla AM, Gould FDH, Bond LE, Stricklen BM, German RZ. Swallow safety is determined by bolus volume during infant feeding in an animal model. Dysphagia. 2021;36(1):120–9. A study of swallow safety in preterm and full-term infant pigs, with and without RLN lesions. They found that bolus size (swallowing larger boluses) was more predictive of swallow safety than either lesion status or preterm/term status.
Wong NS, Feng Z, Rappazzo C, Turk C, Randall C, Ongkasuwan J. Patterns of dysphagia and airway protection in infants with 22q11.2-Deletion syndrome. The Laryngoscope. 2020;130(11):2532–6.
LaMantia A-S, Moody SA, Maynard TM, Karpinski BA, Zohn IE, Mendelowitz D, et al. Hard to swallow: developmental biological insights into pediatric dysphagia. Dev Biol. 2016;409(2):329–42.
Welby L, Caudill H, Yitsege G, Hamad A, Bunyak F, Zohn IE, Maynard T, LaMantia AS, Mendelowitz D, Lever TE. Persistent feeding and swallowing deficits in a mouse model of 22q11. 2 deletion syndrome. Front Neurol. 2020;11:4.
Maynard TM, Horvath A, P Bernot J, Karpinski BA, Tavares ALP, Shah A, et al. Transcriptional dysregulation in developing trigeminal sensory neurons in the LgDel mouse model of DiGeorge 22q11.2 deletion syndrome. Hum Mol Genet. 2020;29(6):1002–17.
Vijayakumar K, Rockett J, Ryan M, Harris R, Pitt M, Devile C. Experience of using electromyography of the genioglossus in the investigation of paediatric dysphagia. Dev Med Child Neurol. 2012;54(12):1127–32.
Lefton-Greif MA, Carroll JL, Loughlin GM. Long-term follow-up of oropharyngeal dysphagia in children without apparent risk factors. Pediatr Pulmonol. 2006;41(11):1040–8.
Kooi-van Es M, Erasmus CE, de Swart BJM, Voet NBM, van der Wees PJ, de Groot IJM, et al. Dysphagia and dysarthria in children with neuromuscular diseases, a prevalence study. J Neuromuscul Dis. 2020;7(3):287–95.
Sherman V, Greco E, Moharir M, Beal D, Thorpe K, Martino R. Feeding and swallowing impairment in children with stroke and unilateral cerebral palsy: a systematic review. Dev Med Child Neurol. 2019;61(7):761–9.
Sherman V, Martino R, Bhathal I, DeVeber MG, Dlamini N, MacGregor D, et al. Swallowing, oral motor, motor speech, and language impairments following acute pediatric ischemic stroke. Stroke. 2021;52(4):1309–18.
Shewmon DA. Anencephaly: selected medical aspects. Hastings Cent Rep. 1988;18(5):11–9.
Peleg D, Goldman JA. Fetal deglutition: a study of the anencephalic fetus. Eur J Obstet Gynecol Reprod Biol. 1978;8(3):133–6.
• Radford K, Taylor RC, Hall JG, Gick B. Aerodigestive and communicative behaviors in anencephalic and hydranencephalic infants. Birth Defects Res. 2019;111(2):41–52. Review of published data on aerodigestive and communicative behaviors of fetuses/infants with anencephaly or hydranencephaly included in older studies.
Sanchez K, Morgan AT, Slattery JM, Olsen JE, Lee KJ, Anderson PJ, et al. Neuropredictors of oromotor feeding impairment in 12month-old children. Early Human Dev. 2017;1(111):49–55.
Kashou NH, Dar IA, El-Mahdy MA, Pluto C, Smith M, Gulati IK, Lo W, Jadcherla SR. Brain lesions among orally fed and gastrostomy-fed dysphagic preterm infants: can routine qualitative or volumetric quantitative magnetic resonance imaging predict feeding outcomes? Front Pediatr. 2017;5:73.
•• Tamilia E, Parker MS, Rocchi M, Taffoni F, Hansen A, Grant PE, et al. Nutritive sucking abnormalities and brain microstructural abnormalities in infants with established brain injury: a pilot study. J Perinatol. 2019;39(11):1498–508. A pilot neuroimaging study of 10 infants with brain injury, which found a correlation between microstructural white matter damage and sucking deficits.
Mishra A, Sheppard JJ, Kantarcigil C, Gordon AM, Malandraki GA. Novel mealtime duration measures: reliability and preliminary associations with clinical feeding and swallowing performance in self-feeding children with cerebral palsy. Am J Speech Lang Pathol. 2018;27(1):99–107.
Mishra A, Malandraki GA, Sheppard JJ, Gordon AM, Levy ES, Troche MS. Voluntary cough and clinical swallow function in children with spastic cerebral palsy and healthy controls. Dysphagia. 2019;34(2):145–54.
Kantarcigil C, Sheppard JJ, Gordon AM, Friel KM, Malandraki GA. A telehealth approach to conducting clinical swallowing evaluations in children with cerebral palsy. Res Dev Disabil. 2016;1(55):207–17.
•• Mourão LF, Friel KM, Sheppard JJ, Kuo H-C, Luchesi KF, Gordon AM, et al. The role of the corpus callosum in pediatric dysphagia: preliminary findings from a diffusion tensor imaging study in children with unilateral spastic cerebral palsy. Dysphagia. 2017;32(5):703–13. A study examining the association between brain lesion characteristics and corpus callosum connectivity and clinical swallowing outcomes in 20 self-feeding children with UCP. Findings showed that type and/or side of lesion plays a role in severity of clinical dysphagia in UCP and that corpus callosum integrity correlates with feeding/swallowing performance when sensorimotor areas of the left hemisphere are affected.
Malandraki GA, Mourão LF, Lu K-H, Friel KM, Luchesi KF, Sheppard JJ, et al. Functional and structural connectivity in children with unilateral cerebral palsy and clinical dysphagia: a multimodal neuroimaging study. European Society of Swallowing Disorders Congress.In Barcelona, Spain; 2017.
Malandraki GA, Mitchell S, Brown B, Hahn Arkenberg R, Lundine J, Burdo-Hartman W, et al. Feeding skills and eating efficiency are reduced in self-feeding school-age children with unilateral CP: an unrecognized reality. Dysphagia Research Society Meeting. In 2021.
Malandraki GA, Mitchell S, Hahn Arkenberg R, Brown B, Lundine J, Burdo-Hartman W, et al. The neuromuscular control of swallowing and speech in unilateral CP: overactivation and lack of specificity are overlapping traits. Dysphagia Research Society Meeting. In Virtual; 2021.
Crary MA, Carnaby GD, LaGorio LA, Carvajal PJ. Functional and physiological outcomes from an exercise-based dysphagia therapy: a pilot investigation of the McNeill Dysphagia Therapy Program. Arch Phys Med Rehabil. 2012;93(7):1173–8.
Funding
This work was partially supported by the National Institute on Deafness and Other Communication Disorders Early Investigator R21 Grant (Grant 1R21DC015867-01A1, PI: Malandraki) and by an American Academy of Cerebral Palsy and Developmental Medicine Pedal-with-Pete research grant (PI: Malandraki).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Swallowing Disorders
Rights and permissions
About this article
Cite this article
Malandraki, G.A., Arkenberg, R.H. Advances in Swallowing Neurophysiology Across Pediatric Development: Current Evidence and Insights. Curr Phys Med Rehabil Rep 9, 267–276 (2021). https://doi.org/10.1007/s40141-021-00334-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-021-00334-3