Abstract
Sarcopenia is now clinically defined as a loss of muscle mass coupled with functional deterioration (either walking speed or distance or grip strength). Based on the FRAX studies suggesting that the questions without bone mineral density can be used to screen for osteoporosis, there is now a valid simple questionnaire to screen for sarcopenia, i.e., the SARC-F. Numerous factors have been implicated in the pathophysiology of sarcopenia. These include genetic factors, mitochondrial defects, decreased anabolic hormones (e.g., testosterone, vitamin D, growth hormone and insulin growth hormone-1), inflammatory cytokine excess, insulin resistance, decreased protein intake and activity, poor blood flow to muscle and deficiency of growth derived factor-11. Over the last decade, there has been a remarkable increase in our understanding of the molecular biology of muscle, resulting in a marked increase in potential future targets for the treatment of sarcopenia. At present, resistance exercise, protein supplementation, and vitamin D have been established as the basic treatment of sarcopenia. High-dose testosterone increases muscle power and function, but has a number of potentially limiting side effects. Other drugs in clinical development include selective androgen receptor molecules, ghrelin agonists, myostatin antibodies, activin IIR antagonists, angiotensin converting enzyme inhibitors, beta antagonists, and fast skeletal muscle troponin activators. As sarcopenia is a major predictor of frailty, hip fracture, disability, and mortality in older persons, the development of drugs to treat it is eagerly awaited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia was originally defined as the age-related loss of muscle mass [1]. Subsequently, it became obvious to clinicians that it was muscle quality, rather than muscle mass that determined the function of muscle [2, 3]. This led to the suggestion that it was muscle power (force x velocity) which should be utilized to determine the role of muscle in determining outcomes. It was suggested that this should be termed dynapenia [4]. From this developed the concept of a sarcopenia-disability cascade (Table 1). Each component of this cascade can be separately measured and theoretically would lead to worse outcomes.
However, in 2010, Cruz-Jentoft et al. [5] published the “European Consensus on Definition and Diagnosis of Sarcopenia.” They redefined sarcopenia as being muscle loss coupled with a decline in function (either walking speed or grip strength). This definition was validated as having a strong predictive ability of poor outcomes [6–8]. Subsequently, 4 other definitions of sarcopenia, all using gait speed and grip strength, as well as some measurement of low muscle mass have been published [9–12]. Each uses slightly different cut off points and 2 recognized the importance of having different cut offs for different ethnic groups. Woo et al. [13] compared each of these definitions and found that they had slightly different predictive abilities. Of the definitions, the Foundation of NIH (FNIH) sarcopenia criteria using gait speed, but not grip strength, had slightly better predictive value for poor outcomes.
Based on the parallels between osteoporosis and sarcopenia and the finding that the 6 FRAX questions without Bone Mineral Density are highly predictive of fracture risk [14], we developed a simple sarcopenic questionnaire to predict poor muscle function (Table 2) [15]. This questionnaire has been shown to be a valid predictor of poor outcomes similar to that of the FNIH (walking speed) definition in both the United States and Asia [13, 16–18].
Sarcopenia has multiple causes and, as older persons develop a variety of diseases with increased production of cytokines, it may overlap with cachexia [19]. In this review, we will first explore the physiological causes of sarcopenia with a special emphasis on potential pharmaceutical targets. We will then review the available and developing treatments for sarcopenia.
The Pathophysiology of Sarcopenia
When muscle contracts this activates mechanoreceptors, i.e., titin and dystroglycan, and causes muscle injury. The mechanoreceptors increase the activity of muscle growth factors (IGF1-Ea and muscle growth factor) which increase muscle protein synthesis and recruit satellite cells and motor units. This leads to muscle regeneration and increased muscle function (Fig. 1). With aging, there is increased muscle injury with a decrease in muscle regeneration and function. This is due to a decrease in muscle growth factors leading to a reduction in the protein synthesis/degradation cycle and the activation of satellite cells and motor units. Anatomically, with aging there is Type II fiber atrophy resulting in decreased muscle mass, strength, and power [20].
Old muscle shows fiber size heterogeneity and fiber grouping with an increase in myosin heavy chain [21]. This differs from cachexia where fiber size variability is not seen. This is similar to the histological changes seen with Amyotrophic Lateral Sclerosis. Sarcopenic patients have a reduction in the motor unit number index (MUNIX) which is intermediate between that seen in healthy older persons and in patients with Amyotropic Lateral Sclerosis [22]. Further evidence of motor neuron degeneration is the increase in C-terminal agrin fragments in about a third of sarcopenic patients [23]. With aging, there is a 25 % loss of motoneurons leading to sprouting of small motor neurons that innervate Type II fibers leading to an eventual loss of type II fibers [24]. Circulating levels of ciliary neurotopic factor (CNTF), which stimulate motor unit formation, decline with aging [25, 26]. Older persons who have the null allele rs1800169 for CNTF have lower grip strength [27]. Axokine, a modified version of CNTF, was tried for weight loss due to its anorectic properties. The trials were suspended when subjects developed antibodies to CNTF.
Myokines
Besides CNTF, skeletal muscle produces a variety of myokines that can modulate muscle growth and repair (Fig. 2) [28–30]. Some of these, such as interleukin-6, may be predominantly produced by adipose tissue infiltrating muscle [31]. A number of these myokines have direct effects on muscle such as IGF-1, IGF binding proteins, myostatin, musclin, leukemia inhibitory factor and CXCL-1. Others such as VEGF-B, IL-8 and Follistatin-like 1 increase angiogenesis in muscles. Proteomics of muscle secretions should lead to the discovery of many more myokines that modulate muscle growth [30].
Myokines and their target sites. SPARC secreted protein acidic and rich in cysteine, IL interleukin, GDF growth differentiation factor, FGF fibroblast growth factor, BMP bone morphogenetic protein, LIF leukemia inhibitory factor, CXCL-1 chemokine ligand 1, VEGF-B Vascular endothelial growth factor, CNTF ciliary neurotrophic-derived factor
IL-15 is an inflammatory cytokine produced by muscle that increases contractile protein accumulation and causes myotube hypertrophy [32]. In vivo IL-15 reduces fast muscle fatigue and enhanced oxidative metabolism. Fatigue is an important component of the frailty phenotype that is separate from sarcopenia [33, 34]. IL-15 agonists or stimulants could be useful for the treatment of fatigue in older persons. Fatigue is, in part, related to a loss of muscle. A number of studies are examining the role of IL-15 agonists in advanced cancer (www.clinicaltrials.gov).
Genetics
Genes play a role in 65 % of the muscle mass and 50–80 % of muscle strength in older persons [35]. Studies on genes and muscle mass and strength have been rudimentary, with a number of results being controversial [36]. Angiotensin converting enzyme alleles have been shown to play a role in efficiency of muscle contraction. The alpha-actin 3 is responsible for anchoring the actin filaments to the 2-disk in fast twitch fibers. An ACTN3 gene deficiency is associated with reduced power and with endurance activity in men [37, 38]. Bradykinin increases muscle blood flow and the B2R gene associated with high function is more frequent in endurance athletes. Other genes associated with muscle strength include CNTF, IL-15, collagen type, insulin growth factor II, myostatin, the vitamin D receptor, and the androgen CAG receptor. In older persons, expression of genes for insulin growth factor-1, myostatin, matrix metalloproteinase-2, ciliary neurotrophic factor, and myostatin correlated most optimally with training-induced strength gains [39]. Variants in the activin receptor 1B play an important role in human muscle strength [40]. Perilipin 2 is a protein associated with lipid droplets. Perilipin 2 is higher in older persons and is related to a decline in muscle strength and proteins associated with muscle atrophy, viz MURF1, and atrogin [41]. This suggests that expression of perilipin 2 may play a role in the development of obese sarcopenia [42, 43].
Mitochondria
The peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) regulates mitochondrial biogenesis and function and regulates muscle fiber adaptation to exercise [44]. There is a reduction of PGC-1α gene expression in old animals and older persons [45]. This reduction results in a low-grade inflammatory reaction with increased levels of IL-6 and TNFα. PGC-1α activity decreases functional loss of mitochondrial enzymes in old animals and protects muscle from damage [46]. Biochemically, PGC-1α inhibits FOXO and NFkB and thus decreases autophagy and the ubiquitin–proteasome systems. PGC-1α promotes mitochondrial biogenesis and fusion, thus maintaining ATP levels and reducing AMPK. Excess expression of PGC-1α damages heart and muscle. It has been suggested that increasing PGC-1α levels in sarcopenic tissue to physiological levels may be a key therapeutic approach to treating muscle wasting.
Mitochondrial dysfunction plays a major role in the pathogenesis of aging [47, 48]. Mitochondria control the production of cellular energy, free radical signaling, and can activate apoptotic pathways. The importance of bioenergetics in the development of sarcopenia is demonstrated by the correlation of ATP synthesis/oxygen consumption and walking speed in older persons [49]. Walking speed is a key component of the modern definitions of sarcopenia. In addition, with aging, there is often increased fusion leading to giant mitochondria which are difficult to remove from cells and function poorly. Older mitochondria tend to lose their outer membrane increasing their propensity to apoptosis [50]. This is related to a decrease in CisD2 gene expression in older humans. Transgenic mice with CisD2 have enhanced longevity and improved muscle quality [51]. Finally, the decline in PGC1α levels with aging leads to translocation of BAX to the mitochondrial membrane with activation of the mitochondrial membrane pore and loss of cytochrome C. This results in mitochondrial apoptosis.
For all of the above reasons, targeting muscle mitochondria appears to be a reasonable approach to the therapeutics of sarcopenia. However, this is not that simple. Antioxidants tend to over reduce free radicals leading to loss of their necessary functions, e.g., nitric oxide effects on blood flow. Coenzyme Q10, a lipid soluble benzoquinone with a side chain of 10 isoprenoid units, freely diffuses across the inner mitochondrial membrane and couples electron flow to proton movement. It is also a membrane stabilizer but is a potent free radical scavenger. Mitoquinones are antioxidants targeted to accumulate in mitochondria. To date they have not been shown to be clinically useful. Another approach would be to develop substances that could replace the loss of CisD2. Substances that theoretically enhance nuclear/mitochondrial protein interactions include sirtuins (e.g., reservatol) and polyphenols. These have not yet been shown to have major effects on muscle function. Metformin enhances nitric oxide function and may prevent BAX translocation to the mitochondrial membrane. All of these approaches need to be further explored as possible therapeutic approaches to sarcopenia.
Vascular
Another component of the development of sarcopenia with aging is the reduction in blood flow to muscles [52, 53]. With aging, there is a decrease in endothelium-dependent vasodilation, due in part to decreased nitric oxide bioavailability [54]. These changes together with reduced lineal density of the perfused capillaries [55] lead to decreased microvascular oxygenation of muscles [55].
Protein Synthesis
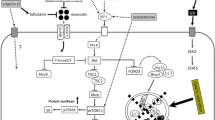
At a basic level, protein synthesis and/or degradation are controlled by activation of the insulin or IGF-1 receptor (Fig. 3). This activates the phosphoinositide 3-kinase (PI3K)-AKT—mammalian target of rapamycin (mTOR) signaling pathway [56]. Increased mTOR, which is also stimulated by essential aminoacids, leads to increased protein synthesis. Both AKT and PGC1α block FOXO activity, thus decreasing the transcription of atrogenes. These include the muscle-specific ligases viz. muscle-specific RING-finger 1 (MURF1 or TRIM63) and atrogin 1. Atrogin 1 degrades proteins that enhance protein synthesis. MURF-1 and ubiquitin tripartite motif containing protein 32 (TRIM32) directly control myofibril breakdown. MURF1 attacks the myosin binding protein and the myosin light chain eventually leading to destruction of the thick myosin filament. TRIM32 destroys desmin and then the Z-band and eventually the thin actin filament. At the same time TRIM32 directly inhibits PI3 K-AKT activity resulting in increased proteolysis. Myofibrils constitute the vast majority of muscle protein and their destruction leads to loss of muscle function [57, 58]. There is an increase in protein destruction and ubiquination in sarcopenia. Obviously, inhibitors of TRIM32 and/or MURF1 represent attractive therapeutic targets for treating sarcopenia.
MicroRNAs
MicroRNAs (miRNA) are small molecules which regulate posttranscriptional gene function by silencing RNA. They are cleaved in the nucleus by Drosha and then exported into the cytoplasm, where they are processed by DICER and combined with AGO to form RNA-induced silencing complexes. These then repress translation of mRNAs. Satellite cells decline with aging [59]. miRNAs play a central role in satellite cell quiescence [60]. The miRNAs (miR-1, miR-208, and MiR486) regulate satellite cell renewal by modulating Pax7. Decreased skeletal muscle miRNA expression in older persons is associated with a decrease in the function of the IGF-1/PIsK/AKT pathway [61]. Exercise modulates the response of a number of muscle-specific miRNAs. There is evidence that miRNAs are sensitive to a variety of drugs such as mu opioids [62] and drugs for Parkinson’s disease [63]. There appears to be tremendous potential to treat sarcopenia by modulating miRNAs. As more is known about the role of miRNAs in modulating muscle growth, it will also become possible to modulate specific RNAs by mimicking the positive patterns with phosphorothiolated antisenses.
Electrical stimulation of both thighs for 9 weeks improved timed up and go test, walking speed and 5 time chair rise [64]. This training led to an increase in diameter and percent of fast fibers. There was a stimulation of IGF-1 isoforms with a reduction of MuRF-1 and atrogin-1 leading to a reduction in proteolysis. Electrical stimulation also produces an increase of satellite cells. Electrical stimulation increased miR-29 which would decrease fibrotic infiltration of muscle. Overall, this study strongly supports the concept of electrical muscle stimulation for treatment of sarcopenia.
Parabiosis
Studies with parabiotic mice have shown that the combination of a young and an old mouse leads to rejuvenation in the muscle of the older mouse [65]. This was due to an increase in Notch signaling leading to an increase in satellite cells. The humoral agent responsible for this appears, in a large part, to be growth differentiation factor 11 (GDF11) [66]. Sinha et al. [67] have demonstrated that testosterone plays a permissive role in muscle mass and fiber cross-sectional area in parabiotic mice. These data suggest a role for GDF11 in treatment of sarcopenia.
Hibernating animals maintain their muscle structure during winter, raising the question of whether a circulating factor, in addition to shivering thermogenesis, is responsible for the protection of muscle during hibernation. The extensor digitorum longus muscle of the rat, when incubated with serum obtained from hibernating bears had a 40 % decrease in proteolysis, associated with a decline in cathepsin B and ubiquitin [68]. During hibernation, there is an increase in PGC-1α, which is associated with a decrease in FOXO and MURF-1 [69]. Serum- and glucocorticoid-inducible kinase 1 (SGK1) has been shown to downregulate proteolysis, autophagy and increase protein synthesis in hibernating animals [70]. This bypasses the classical AKT-FOXO pathway. SGK-1 may represent an important therapeutic target to prevent atrophy-induced muscle loss.
Table 3 provides a list of potential targets for future drug development. Figure 4 gives an overview of the major factors so far demonstrated to be a component of the pathophysiology of sarcopenia. Many of these already have drugs available or under development. These and their physiological rationale will be discussed in the next section.
Management of Sarcopenia
The primary treatment of sarcopenia is resistance exercise [71–73]. As was shown by the LIFE study, aerobic exercise can also decrease functional decline in lower limb muscles [74]. Exercise has also been shown to be an important therapeutic approach to reversing frailty [75]. There is evidence to support that excess protein [1–1.2 g (kj day)] may also enhance muscle mass and, to a lesser extent, function [76–80]. This is particularly true for leucine enriched essential amino acids (whey protein) [81]. Essential amino acid supplementation prevents muscle mass loss due to bed rest [82]. A recent multicenter study has shown that whey protein together with vitamin D increased both muscle mass and stair climb [83]. There is some evidence for synergistic effects of exercise and protein to enhance muscle function [84–86]. Vitamin D supplementation increases muscle strength without increasing muscle mass or power [87]. Vitamin D is more effective in older persons and those with low vitamin D levels. It also decreases falls in persons who are vitamin D deficient [88]. At present, no drugs have been shown to be clinically more therapeutically effective.
Testosterone
Testosterone levels decline at the rate of 1 % per year from 30 years of age [89, 90]. This decline in testosterone is associated with a decline in muscle mass and strength [91]. Since the original studies showing that testosterone increased muscle strength in older persons [92–94], numerous studies have shown that in low doses testosterone increased muscle mass and decreases fat mass [95] and in higher doses increased both muscle and power [96]. In frail older persons [97–99] and in persons with heart failure [100–103] testosterone increased both strength and walking distance. Testosterone improves muscle strength in women as well as in men [104]. In frail older persons, testosterone in combination with a protein supplement decreased hospitalization [105].
In lower doses, testosterone increases protein synthesis resulting in an increase in muscle mass [106, 107]. In high doses, testosterone activates satellite cell recruitment and reduces adipose stem cells [108]. Testosterone effects on muscle cells bypass the WNT system and activate β-catenin [109]. This leads to increased myogenesis and cell cycling and decreased adipogenesis.
While testosterone as a therapeutic agent has been utilized since the 1940s, there is a fear that it will produce excessive side effects [110]. A meta-analysis of the controlled studies of testosterone in older males found no increase in mortality [111]. Whether or not it increases cardiovascular events, and particularly in the first 3 months after administration, remains controversial [112, 113]. Persons with diabetes mellitus have accelerated sarcopenia [114–116], and a recent study in diabetics found a decrease in mortality in diabetics receiving testosterone [117]. Nevertheless, this fear of negative effects from testostosterone has driven the exploration for selective androgen receptor modulators (SARMs) which may be, theoretically, safer. At present, of the drugs developed and being developed for sarcopenia, testosterone remains the most efficacious and safest. In view of the fact that testosterone also increases bone mineral density and bone strength [118, 119] and osteoporosis often co-exists with sarcopenia (osteosarcopenia), it would seem that more clinical attention should be paid to the potential role of testosterone for treating sarcopenia. Two major trials are presently underway and these may help determine the place of testosterone in the management of sarcopenia, osteoporosis, and frailty (The Testosterone Trial in Older Men—www.clinicaltrials.gov and the T4DM trial—www.t4dm.org.au).
Anabolic Steroids/Selective Androgen Receptor Modulators (SARMs)
Nandrolone is an injectable anabolic steroid. It increased fiber area and muscle mass, but there is no evidence that it increased strength [120–122]. In three studies of persons with hip fracture, it had a nonstatistical improvement in functional status [123].
MK0773 (TFM-4AS-1) is a 4-aza steroidal drug that has androgen gene selectivity. In females, it increased IGF-1 as well as stair climbing power and gait speed [124]. This study was terminated because of an increased signal for cardiac failure. In women with sarcopenia, it increased muscle mass, bilateral leg press, and stair climbing power but not gait speed (www.clinicaltrials.gov). The study in males was reported at the 90th Endocrine Society in 2008. It showed anabolic effects of MK0773.
SARMs are androgen receptor ligands that band to the androgen receptor with differing sensitivity compared to testosterone [125]. Steroidal SARMs were first developed in the 1940s. More recently, a number of nonsteroidal SARMs have been developed [126].
LGD-4033 is a nonsteroidal, orally active SARM. The phase I trial showed an increase in muscle mass, but no effect on fat mass in a 21-day trial [127]. BMS-564929 is also in phase I trials.
In a 12-week study, enobosarm increased total lean mass and stair climb [128]. In female patients with cancer, enobosarm increased lean mass compared to baseline, but not significantly compared to placebo [129]. In 2 phase 3 trials, it maintained body mass and improved stair climb in one of the 2 trials in patients with cancer [130].
Overall, these studies of SARMs have shown no advantage over testosterone.
Growth Hormone/Insulin Growth Factor-1
Rudman et al. [131] originally showed that growth hormone increased lean body mass in older men. The excitement created by their original data was dampened by finding that a growth hormone treatment for a year led to a variety of side effects such as carpal tunnel syndrome and gynecomastia [132]. Subsequently, growth hormone has been shown to increase muscle mass but not muscle strength in older persons [133]. A combination of growth hormone and testosterone increased muscle mass at 8 weeks and 1 repetition maximum strength only by week 17 [134]. Growth hormone, which produces its effects through the release of liver-derived IGF-1, also increased nitrogen retention [135]. Adverse effects include joint and muscle pain, edema, carpal tunnel syndrome, and hyperglycemia [136].
There are marked reductions of circulating IGF-1 with aging [137]. Both low and high levels of IGF-1 are associated with increased risk of cardiovascular disease. Similarly, there is limited evidence that circulating IGF-1 is associated with muscle power. A single small study of IGF-1 in older persons found an increase in side effects viz. orthostatic hypotension, gynecomastia, myositis, and edema [138].
Ghrelin
Ghrelin is produced from the fundus of the stomach. It increases food intake and growth hormone. These effects of ghrelin are due to the hypothalamic release of nitric oxide [139]. Ghrelin increased food intake and produced muscle mass gain in persons with cancer [140, 141]. The ghrelin agonist, anamorelin, increased food intake, and muscle mass, but not strength in persons with cancer cachexia [142, 143]. Macimorelin is another ghrelin agonist under development.
Capromorelin, a ghrelin receptor agonist, was tested in older sarcopenic individuals [144]. It increased lean mass, tandem walk, and stair climb at the end of treatment for a year. MK-0677, which also activates the ghrelin receptor to increase growth hormone, was studied for 24 weeks in persons with hip fracture [145]. Over this period, it increased ability to stair climb and also decreased falls. The treatment was associated with an increase in heart failure.
Overall, while ghrelin agonists will increase food intake and muscle mass, it is unlikely that they will produce a significant effect on function in persons with sarcopenia.
Myostatin and Activin II Receptor Inhibitors
Myostatin or growth differentiation factor-8 is produced in skeletal muscle and prevents muscle growth and satellite cell production [146]. Myostatin activates the Activin IIR receptor to increase SMAD (Fig. 3). Lack of myostatin in animals leads to “double muscled” cows (Belgian Blue and Piedmontese). Heterozygote deletion of myostatin in whippets leads to an increase in running ability, while homozygotes are more muscular but not as good runners. A homozygote muscle deletion of myostatin in a young boy resulted in an increase in muscle mass [147].
In humans, creatine, together with resistance exercise, results in an amplification of the normal decrease in myostatin with resistance training alone [148]. Creatine produces a small increase in strength in persons with muscular dystrophy [149]. Myostatin monoclonal antibodies increase muscle mass in mice [150]. In humans with muscular dystrophy, a myostatin antibody (MYO-029) enhanced muscle mass [151]. Muscle fiber diameter increased in the 10 mg/kg dose. Side effects included urticaria and aseptic meningitis at high doses. Another myostatin antibody (AMG 745) increased lean body mass and decreased fat after 28 days in persons on androgen deprivation therapy for prostate cancer [152]. Diarrhea, confusion, and fatigue were more common in the persons receiving active drug. LY2495655 increased muscle volume and handgrip strength in persons with advanced cancer (www.clinicaltrials.gov). REGN1033 (GDF8 antibody) has reported promising effects on muscle at the sarcopenia meeting in Barcelona.
An activin II receptor ligand trap (ACE-011) increased bone mass and strength in monkeys [153]. ACE-031 in 48 postmenopausal women increased lean mass and thigh muscle volume after a single dose [154]. Another ligand trap ACE-083 is also under development. Side effects including telangiectasia, epistaxis, and changes in gonadotrophin levels resulted in the company stopping the development of these compounds.
Inclusion body myositis is a rare autoimmune disorder. It occurs in persons 50 years and older. Its hallmark is amyloid inclusion bodies. Bimagramab is an activin receptor inhibitor. In persons with inclusion body myositis, bimagramab increased thigh muscle volume, lean muscle mass, and 6-min walking distance [155].
Espindolol (Mixed Agonist/Antagonist B1,B2, B3 Activity)
Espindolol is the S-enantiomer of pindolol. It increases muscle mass and decreases fat mass in older animals [156]. A phase II trial showed an increase in muscle mass and a decrease in fat mass [157]: It also increased handrip strength.
Angiotensin Converting Enzyme Inhibitor (Perindopril)
Perindopril has been shown to increase distance walked in older persons with left ventricular systolic dysfunction [158]. It also improved 6-min walking distance in older persons with functional impairment [159]. There was, however, no enhancement in persons undergoing exercise training [160]. In addition, in the HYVET study perindopril decreased hip fracture [161].
Fast Skeletal Troponin Activitors (Terasemtiv)
There are drugs which amplify motor neuron input, resulting in improved muscle power and muscle fatigability. Terasemtiv slowed the rate of decline in muscle strength [162]. In persons with peripheral vascular disease, terasemtiv increased work done in a bilateral heel raising test (www.cytokinetics.com/ck2017357).
Conclusion
Sarcopenia (loss of muscle mass and muscle function) is a strong predictor of frailty, disability, and mortality in older persons. At present resistance exercise is the primary treatment for sarcopenia. Supplementation with essential amino acids, creatine, and vitamin D may enhance the effect of resistance exercise. The effects of testosterone on muscle include increased muscle power and function. At present, the side effects of testosterone, though minimal in placebo controlled trials, remain a possible limitation to its use. No other drugs under development have been shown to be more potent than testosterone. All the drugs under development have their own set of side-effects and will clearly be more expensive than resistance exercise or injectable testosterone (Table 4). There are numerous potential targets for enhancing muscle function, and the development of new drugs for sarcopenia represents a potentially exciting clinical area.
References
Rosenberg IH, Roubenoff R (1995) Stalking sarcopenia. Ann Intern Med 123:727–728
Clark BC, Manini TM (2008) Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci 63:829–834
Barbat-Artigas S, Rolland Y, Vellas B, Aubertin-Leheudre M (2013) Muscle quantity is not synonymous with muscle quality. J Am Med Dir Assoc 14:852.e1-7. doi:10.1016/j.jamda.2013.06.003
Manini TM, Clark BC (2012) Dynapenia and aging: an update. J Gerontol A 67:28–40
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing 39:412–423
Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G (2013) Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 42:203–209
Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, Tosato M, Bernabei R, Onder G (2012) Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 13:121–126
Lee WJ, Liu LK, Peng LN, Lin MH, Chen LK, ILAS Research Group (2013) Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: results from the I-Lan longitudinal aging study. J Am Med Dir Assoc 14:528.e1-7
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G et al (2011) Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 12:249–256
Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop et al (2011) Sarcopenia with limited mobility: An international consensus. J Am Med Dir Assoc 12:403–409
Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, Shardell M, Alley DE, Kenny A, Ferrucci L, Guralnik J, Kiel DP, Kritchevsky S, Vassileva MT, Studenski S (2014) An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A 69:584–590
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Ck Liang, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101
Woo J, Leung J, Morley JE (2014) Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir. doi:10.1016/jamda.2014.11.013
Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD (2012) FRAX® with and without bone mineral density. Calcif Tissue Int 90:1–13
Malmstrom TK, Morley JE (2013) SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 14:531–532
Woo J, Leung J, Morley JE (2014) Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc 15:630–634
Cao L, Chen S, Zou C, Ding X, Gao L, Liao Z, Liu G, Malmstrom TK, Morley JE, Flaherty JH, An Y, Dong B (2014) A pilot study of the SARC-F scale on screening sarcopenia and physical disability in the Chinese older people. J Nutr Health Aging 18:277–283
Malmstrom TK, Simonsick EM, Ferrucci L, Miller DK, Morley JE (2015) SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarc Muscle. doi:10.1002/jcsm.12048
Argiles JM, Anker SD, Evans WJ, Morley JE, Fearon KC, Strasser F, Muscaritoli M, Baracos VE (2010) Consensus on cachexia definitions. J Am Med Dir Assoc 11:229–230
Purves-Smith FM, Sgarioto N, Hepple RT (2014) Fiber typing in aging muscle. Exerc Sport Sci Rev 42:45–52
Hepple RT (2003) Sarcopenia: a critical perspective. Sci Aging Knowl Environ 45:31
Drey M, Grosch C, Neuwirth C, Bauer JM, Sieber CC (2013) The motor unit number index (MUNIX) in sarcopenic patients. Exp Gerontol 48:381–384
Hettwer S, Dahinden P, Kucsera S, Farina C, Ahmed S, Fariello R et al (2013) Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol 48:69–75
Drey M, Krieger B, Sieber CC, Bauer JM, Hettwer S, Bertsch T, DISARCO Group (2014) Motoneuron loss is associated with sarcopenia. J Am Med Dir Assoc 15:435–439
McNeil CJ, Doherty TJ, Stashuk DW, Rice CL (2005) Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31:461–467
Guillet C, Auguste P, Mayo W, Kreher P, Gascan H (1999) Ciliary neurotrophic factor is a regulator of muscular strength in aging. J Neurosci 19:1257–1262
Arking DE, Fallin DM, Fried LP, Li T, Beamer BA, Xue QL, Chakravarti A, Walston J (2006) Variation in the ciliary neurotrophic factor gene and muscle strength in older Caucasian women. J Am Geriatr Soc 54:823–826
Demontis F, Piccirillo R, Goldberg AL, Perrimon N (2013) The influence of skeletal muscle on systemic aging and lifespan. Aging Cell 12:943–949
Iizuka K, Machida T, Hirafuji M (2014) Skeletal muscle is an endocrine organ. J Pharmacol Sci 125:125–131
Yoon JH, Kim J, Song P, Lee TG, Suh PG, Ryu SH (2012) Secretomics for skeletal muscle cells: a discovery of novel regulators? Adv Biol Regul 52:340–350
Pal M, Febbraio MA, Whitham M (2014) From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol Cell Biol 92:331–339
Pistilli EE, Quinn LS (2013) From anabolic to oxidative: reconsidering the roles of Il-15 and IL-15Rα in skeletal muscle. Exerc Sport Sci Rev 41:100–106
Morley JE, von Haehling S, Anker SD, Vellas B (2014) From sarcopenia to frailty: a road less travelled. J Cachexia Sarcopenia Muscle 5:5–8
Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J et al (2013) Frailty consensus: a call to action. J Am Med Dir Assoc 14:392–397
Garatachea N, Lucia A (2013) Genes and the ageing muscle: a review on genetic association studies. Age 35:207–233
Stewart CEH, Rittweger J (2006) Adaptive processes in skeletal muscle: molecular regulators and genetic influences. J Musculoskelet Neuronal Interact 6:783–786
Seto JT, Quinlan KG, Lek M, Zheng XF, Garton F, MacArthur DG et al (2013) ACTN3 genotype influences muscle performance through the regulation of calcineurin signaling. J Clin Invest 123:4255–4263
Seto JT, Chan S, Turner N, MacArthur DG, Raftery JM, Berman YD et al (2011) The effect of α-actinin-3 deficiency on muscle aging. Exp Gerontol 46:292–302
Dennis RA, Zhu H, Kortebein PM, Bush HM, Harvey JF, Sullivan DH, Peterson CA (2009) Muscle expression of genes associated with inflammation, growth, and remodeling is strongly correlated in older adults with resistance training outcomes. Physiol Genomics 38:169–175
Windelinckx A, De Mars G, Huygens W, Peeters MW, Vincent B, Wijmenga C, Lambrechts D et al (2011) Comprehensive fine mapping of chr12q12-14 and follow-up replication identify activin receptor 1B (ACVR1B) as a muscle strength gene. Eur J Hum Genet 19:208–215
Conte M, Vasuri F, Trisolino G, Bellavista E, Santoro A, Degiovanni A et al (2013) Increased Plin2 expression in human skeletal muscle is associated with sarcopenia and muscle weakness. PLoS ONE 8(8):e73709
Rolland Y, Lauwers-Cances V, Crostini C, Abellan van Kan G, Janssen I, Morley JE, Vellas B (2009) Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women. EPIDOS Study 89:1895–1900
Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE (2004) Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 12:1995–2004
Amold A-S, Egger A, Handschin C (2011) PGC-1α and myokines in the aging muscle—a mini-review. Gerontology 57:37–43
Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A (2004) Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest 114:1518–1526
Sandri M, Lin J, Handschin C, Yang W, Zp Arany, Lecker SH, Goldberg AL, Spiegelman BM (2006) PGC-1α protects skeletal muscle from atrophy by suppressing fox03 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103:16260–16265
Hepple RT (2014) Mitochondrial involvement and impact in aging skeletal muscle. Front Aging Neurosci 6:211. doi:10.3389/fnagi.2014.00211
Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C (2013) Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int J Biochem Cell Biol 45:2288–2301
Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW et al (2013) Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A 68:447–455
Wang CH, Kao CH, Chen YF, Wei YH, Tsai TF (2014) Cisd2 mediates lifespan: Is there an interconnection among Ca2+ homeostasis, autophagy, and lifespan? Free Radic Res 48:1109–1114
Wu CY, Chen YF, Wang CH, Kao CH, Zhuang HW, Chen CC, Chen LK et al (2012) A persistent level of Cisd2 extends healthy lifespan and delays aging in mice. Hum Mol Genet 21:3956–3968
Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS (2003) Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285:H1023–H1031. doi:10.1152/ajpheart.00135.2003
Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS (2006) Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290:H272–H278
Seals DR, Jablonski KL, Donato AJ (2011) Aging and vascular endothelial function in humans. Clin Sci 120:357–375
Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC (2005) Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol 146:259–268
Cohen S, Nathan JA, Goldberg AL (2015) Muscle wasting in disease: molecular mechanisms and promising therapies. Nature 14:58–74
Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Geratner C, Latres E, Goldberg AL (2009) During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185:1083–1095
Cohen S, Zhai B, Gygi SP, Goldberg AL (2012) Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy. J Cell Biol 198:575–589
Verdijk LB, Koopmans R, Schaart G, Meijer K, Savelberg HHCM, van Loon LJC (2007) Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292:E151–E157
McGregoe RA, Poppitt SD, Cameron-Smith D (2014) Role of microRNAs in the age-related changes in skeletal muscle and diet or exercise interventions to promote healthy aging in humans. Ageing Res Rev 17:25–33
Rivas DA, Lessard SJ, Rice NP, Lustgarten MS, So K, Goodyear LJ, Parnell LD, Fielding RA (2014) Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J 28:4133–4147
Barbierato M, Zusso M, Skaper SD, Giusti P (2015) MicroRNAs: emerging role in the endogenous mu opioid system. CNS Neurol Disord Drug Targets 14:239–250
Alieva AKH, Filatova EV, Karabonov AV, Illarioshkin SN, Limborska SA, Shadrina MI, Slominsky PA (2015) miRNA expression is highly sensitive to a drug therapy in Parkinson’s disease. Parkinsonism Relat Disord 21:72–74
Kern H, Barberi L, Löfler S, Sbardella S, Burggraf S, Fruhmann H et al (2014) Electrical stimulation counteracts muscle decline in seniors. Front Aging Neurosci. doi:10.3389/fnagi.2014.00189
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433(7027):760–764
Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C et al (2014) Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344(6184):649–652
Sinha I, Sinha-Hikim AP, Wagers AJ, Sinha-Hikim I (2014) Testosterone is essential for skeletal muscle growth in aged mice in a heterochronic parabiosis model. Cell Tissue Res 357(3):815–821
Fuster G, Busquets S, Almendro V, Lopez-Soriano FJ, Argiles JM (2007) Antiproteolytic effects of plasma from hibernating bears: a new approach for muscle wasting therapy? Clin Nutr 26:658–661
Bodine SC (2013) Hibernation: The search for treatments to prevent disuse-induced skeletal muscle atrophy. Exper Neurol 248:129–135
Andres-Mateos E, Brinkmeier H, Burks TN, Mejias R, Files DC, Steinberger M, Soleimani A et al (2013) Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Mol Med 5:80–91
Valenzuela T (2012) Efficacy of progressive resistance training interventions in older adults in nursing homes: a systematic review. J Am Med Dir Assoc 13:418–428
Cadone Cadore EL, Izquierdo M (2013) New strategies for the concurrent strength-, power-, and endurance-training prescription in elderly individuals. J Am Med Dir Assoc 14:623–624
Singh NA, Quine S, Clemson LM, Williams EJ, Williamson DA, Stavrinos TM et al (2012) Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc 13:24–30
Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS et al (2014) Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 311(23):2387–2396
Yamada M, Arai H, Sonoda T, Aoyama T (2012) Community-based exercise program is cost-effective by preventing care and disability in Japanese frail older adults. J Am Med Dir Assoc 13:507–511
Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE et al (2013) Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc 14:542–559
Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L (2013) Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Dir Assoc 14:10–17
Boirie Y (2013) Fighting sarcopenia in older frail subjects: protein fuel for strength, exercise for mass. J Am Med Dir Assoc 14:140–143
Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ et al (2012) Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 13:720–726
Cermak NM, de Groot LC, van Loon LJ (2013) Perspective: Protein supplementation during prolonged resistance type exercise training augments skeletal muscle mass and strength gains. J Am Med Dir Assoc 14:71–72
Gryson C, Ratel S, Rance M, Penando S, Bonhomme C, Le Ruyet P, Duclos M, Boirie Y, Walrand S (2014) Four-month course of soluble mil proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants. J Am Med Dir Assoc 15:958.e1-9
Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W (2010) EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr 29:18–23
Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. (2015) Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults. The PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir (in press)
Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE et al (2010) Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc 11:391–396
Van Wetering C, Hoogendoorn M, Broekhuizen R, Geraerts-Keeris GJ, de Munck DR, Rutten-van Molken MP et al (2010) Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the INTERCOM trial. J Am Med Dir Assoc 11:179–187
Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, van Loon LJ (2012) Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 13:713–719
Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J et al (2014) The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 99:4336–4345
Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM et al (2011) Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011(96):2997–3006
Morley JE (2011) Should frailty be treated with testosterone? Aging Male 14:1–3
Morley JE, Fe Kaiser, Perry HM 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ (1997) Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 46:410–413
Baumgartner RN, Dl Waters, Gallagher D, Morley JE, Garry PJ (1999) Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev 107:123–136
Morley JE, Perry HM 3rd, Kaiser FE, Kraenzle D, Jensen J, Houston K, Mattammal M, Perry HM Jr (1993) Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc 41:149–152
Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ et al (2005) Exogenous testosterone (T) alone or with finasteride increses physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90:1502–1510
Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C (1997) Testosterone replacement in older hypogonadal men: A 12-month randomized controlled trial. J Clin Endocrinol Metab 82:1661–1667
Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE (2003) Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A 58:618–625
Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE et al (2005) Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrniol Metab 90:678–688
Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA et al (2010) Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 95:639–650
Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO et al (2010) Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc 58:1134–1143
Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, Farwell WR et al (2011) Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci 66:1090–1099
Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, Miceli M et al (2009) Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure: a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol 54:919–927
Iellamo F, Volterrano M, Caminiti G, Karam R, Massaro R, Fini M et al (2010) Testosterone therapy in women with chronic heart failure: a pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol 56:1310–1316
Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, Channer KS (2006) Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J 27:57–64
Stout M, Tew GA, Doll H, Zwierska I, Woodroofe N, Channer KS, Saxton JM (2012) Testosterone therapy during exercise rehabilitation in male patients with chronic heart failure who have low testosterone status: a double-blind randomized controlled feasibility study. Am Heart J 164:893–901
Morley JE, Perry HM 3rd (2003) Androgens and women at the menopause and beyond. J Gerontol A Biol Sci Med Sci 58:M409–M416
Chapman IM, Visvanathan R, Hammond AJ, Morley JE, Field JB, Tai K et al (2009) Effect of testosterone and a nutritional supplement, alone and in combination, on hospital admissions in undernourished older men and women. Am J Clin Nutr 89:880–889
Wolfe R, Ferrando A, Sheffield-Moore M, Urban R (2000) Testosterone and muscle protein metabolism. Mayo Clin Proc 75(Suppl):S55–S59
Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ (2003) Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88:358–362
Kovacheva EL, Hikim AP, Shen R, Sinha I, Sinha-Hikim I (2010) Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrniology 151:628–638
Harin MT, Siddiqui AM, Armbrecht HJ, Kevorkian RT, Kim MJ, Haas MJ, Mazza A, Kumar VB, Green M, Banks WA, Morley JE (2011) Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. Int J Androl 34:55–68
Morley JE (2013) Scientific overview of hormone treatment used for rejuvenation. Fertil Steril 99:1807–1813
Carona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, Maggi M (2014) Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf 13:1327–1351
Mogentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM (2015) Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc 90:224–251
Borst SE, Shuster JJ, Zou B, Ye F, Jia H, Wokhlu A, Yarrow JF (2014) Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med 12:211–215
Morley JE, Malmstrom TK, Rodriguez-Manas L, Sinclair AJ (2014) Frailty, sarcopenia and disabetes. J Am Med Dir Assoc 15:853–859
Landi F, Onder G, Bernabei R (2013) Sarcopenia and diabetes: two sides of the same coin. J Am Med Dir Assoc 14:540–541
Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, van Loon LJ (2013) Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc 14:585–592
Muraleedharan V, March H, Kapoor D, Channer KS, Jones TH (2013) Tesosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol 169:725–733
Nieschlag E (2015) Current topics in testosterone replacement of hypogonadal men. Best Pract Res Clin Endocrinol Metab 29:77–90
Irwig MS (2014) Bone health in hypogonadal men. Curr Opin Urol 24:608–613
Sharma S, Arneja A, McLean L, Duerksen D, Leslie W, Sciberras D, Lertzman M (2008) Anabolic steroids in COPD: a review and preliminary results of a randomized trial. Chron Respir Dis 5:169–176
Frisoli A Jr, Chaves PH, Pinheiro MM, Vl Szejnfeld (2005) The effect of nandrolone decanoate on bone mineral density, muscle mass, and hemoglobin levels in elderly women with osteoporosis: a double-blind, randomized, placebo-controlled clinical trial. J Gerontol A 60:648–653
Macdonald JH, Marcora SM, Jibani MM, Kumwenda MJ, Ahmed W, Lemmey AB (2007) Nandrolone decanoate as anabolic therapy in chronic kidney disease: a randomized phase II dose-finding study. Nephron Clin Pract 106:c125–c135
Farooqi V, van den Berg ME, Cameron ID, Crotty M (2014) Anabolic steroids for rehabilitation after hip fracture in older people. Cochrane Database Syst Rev 10:CD008887
Papanicolaou DA, Ather SN, Zhu H, Zhou Y, Lutkiewicz J, Scott BB, Chandler J (2013) A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging 17:533–543
Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y et al (2009) Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J Med Chem 52:3598–3617
Bhasin S, Jasuja R (2009) Selective androgen receptor modulators as function promoting therapies. Curr Opin Clin Nutr Metab Care 12:232–240
Basario S, Collins L, Dillon EL, Orwoll K, Storer TW, Miciek R et al (2013) The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A 68:87–95
Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST et al (2011) The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle 2:153–161
Dobs AS, Boccia RV, Croot CC, Gabrial NY, Dalton JT, Hancock ML et al (2013) Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomized controlled phase 2 trial. Lancet Oncol 14:335–345
Steiner MS (2013) Enobosarm, a selective androgen receptor modulator, increases lean body mass in advanced non-small cell lung cancer patients in two pivotal, international phase 3 trials. J Cachexia Sarcopenia Muscle 4:69. doi:10.1007/s13539-013-0123-9
Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF et al (1990) Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6
Cohn L, Feller AG, Draper MW, Rudman IW, Rudman D (1993) Carpal tunnel syndrome and gynaecomastia druing growth hormone treatment of elderly men with low circulating IGF-1 concentrations. Clin Endocrniol (Oxf) 39:417–425
Kim MJ, Morley JE (2005) The hormonal fountains of youth: myth or reality? J Endocrinol Invest 28(11):5–14
Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE et al (2002) Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288:2282–2292
Kaiser FE, Silver AJ, Morley JE (1991) The effect of recombinant human growth hormone on malnourished older individuals. J Am Geriatr Soc 39:235–240
Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Am Garber, Hoffman AR (2007) Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med 146:104–115
Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P (2012) Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr Rev 33:314–377
Sullivan DH, Carter WJ, Warr WR, Williams LH (1998) Side effects resulting from the use of growth hormone and insulin-like growth factor-1 as combined therapy to frail elderly patients. J Gerontol A 53:M183–M187
Gaskin FS, Farr SA, Banks WA, Kumar VB, Morley JE (2008) Ghrelin-induced feeding is dependent on nitric oxide. Peptides 24:913–918
Argiles JM, Stemmler B (2013) The potential of ghrelin in the treatment of cancer cachexia. Expert Opin Biol Ther 13:67–76
Miyazaki T, Tanaka N, Hirai H, Yokobori T, Sano A, Sakai M et al (2012) Ghrelin level and body weight loss after esophagectomy for esophageal cancer. J Surg Res 176:74–78
Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, Friend J (2015) Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomized, placebo-controlled, double-blind trials. Lancet Oncol 16:108–116
Temel J, Bondarde S, Jain M, Yun Y, Duus E, Allen S et al (2013) Efficacy and safety results from a phase II study of anamorelin HC21, a ghrelin receptor agonist, in NSCLC patients. J Cachexia Sarcopenia Muscle 4:334
White HK, Petrie CD, Landschulz W, MacLean D, Taylor A, Lyles K et al (2009) Effects of an oral growth hormone scretagogue in older adults. J Clin Endocrinol Metab 94:1198–1206
Adunsky A, Chandler J, Heyden N, Lutkiewicz J, Scott BB, Berd Y et al (2011) MK-0677 (ibutamoren mesylate) for the treatment of patients recovering from hip fractures: a multicenter, randomized, placebo-controlled phase IIb study. Arch Gerontol Geriatr 53:183–189
Elkina Y, vono Haehling S, Anker SD, Springer J (2011) The role of muyostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle 2:143–151
Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350:2682–2688
Saremi A, Gharakhanloo R, Sharghi S, Gharaati MR, Larijani B, Omidfar K (2010) Effects of oral cretine and resistance training on serum myostatin and GASP-1. Mol Cell Endocrniol 317:25–30
Walter MC, Reilich P, Lochmuller H, Kohnen R, Schlotter B, Hautmann H, Dunkl E et al (2002) Creatine monohydrate in myotonic dystrophy: a double-blind, placebo-controlled clinical study. J Neurol 249:1717–1722
Tsuchida K (2008) Targeting myostatin for therapies against muscle-wasting disorders. Curr Opin Drug Discov Devel 11:487–494
Wagner KR, Fleckenstein JL, Amato AA, Barhn RJ, Bushby K, Eagle M, Florence JM, King WM et al (2008) A phase I/II Trial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol 63:561–571
Padhi D, Higano CS, Shore ND, Sieber P, Rasmussen E, Smith MR (2014) Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 99:E1967–E1975
Fajardo RJ, Manoharan RK, Pearsall RS, Davies MV, Marvell T, Monnell TE et al (2010) Treatment with a soluble receptor for activin improves bone mass and structure in the axial and appendicular skeleton of female cynomolgus macaques (Macaca fascicularis). Bone 46:64–71
Attie KM, Brogstein NG, Yang Y, Condon CH, Wilson DM, Pearsall AE et al (2013) A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve 47:416–423
Amato AA, Sivakumar K, Goyal N, David WS, Salajegheh M, Praestgaard J, Lach-Trifilieff E, Trendelenburg AU, Laurent D, Glass DJ, Roubenoff R, Tseng BS, Greenberg SA (2014) Treatment of Sporadic inclusion body myositis with bimagrumab. Neurology 83:2239–2246
Potsch MS, Tschirner A, Palus S, von Haehling S, Doehner W, Beadle J, Coats AJ, Anker SD, Springer J (2014) The anabolic catabolic transforming agenda (ACTA) espindolol increases muscle mass and decreases fat mass in old rats. J Cachexia Sarcopenia Muscle 5:149–158
Steward Coats AJ, Srinivasan Surendran J, Chicarmana H, Vangipuram SR, Bhatt NN, Jain M et al (2011) The ACT-ONE trial, a multicenter, randomized, double-blind, placebo-controlled dose-finding study of the anabolic/catabolic transforming agent, MT-102 in subjects with cachexia related to stage III and IV non-small cell lung cancer and colorectal cancer: study design. J Cachexia Sarcopenia Muscle 2:201–207
Hutcheon SD, Gillespie ND, Crombie IK, Struthers AD, McMurdo ME (2002) Perindopril improves six minute walking distance in older patients with left ventricular systolic dysfunction: a randomized double blind placebo controlled trial. Heart 88:373–377
Sumukadas D, Witham MD, Struthers AD, McMurdo ME (2007) Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ 177:867–874
Sumukadas D, Band M, Miller S, Cvoro V, Witham M, Struthers A, McConnachie A, Lloud SM, McMurdo M (2014) Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J Gerontol A 69:736–743
Peters R, Beckett N, Burch L, de Vernejoul MC, Liu L, Duggan J et al (2010) The effect of treatment based on diuretic (indapamide) ± ACE inhibitor (perindopril) on fractures in the hypertension in the very elderly trial (HYVET). Age Ageing 39:609–616
Malik F (2013) Fast skeletal muscle troponin activators and their application to disease-preclinical characterization. In: Abstracts, 7th Cachexia Conference, Kobe. p 50
Conflict of interest
The author declares that there were no funds received from any entity for the writing of this article. The author has received grants from Nestle (Purina) and Kemin that are not related to this work. The author is on the speaker’s bureau and has been a consultant for Nutricia and received consultant fees from Nutricia, Sanofi-Aventis, Astellas, Boehringer-Ingelheim, Merck, and Akros, none of which have any influence on the writing of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morley, J.E. Pharmacologic Options for the Treatment of Sarcopenia. Calcif Tissue Int 98, 319–333 (2016). https://doi.org/10.1007/s00223-015-0022-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0022-5