Abstract
Purpose of Review
Massive blood loss secondary to major trauma is a leading cause of death worldwide. In recent years, multiple different strategies have evolved to counteract this life-threatening condition. In this review, we will review our understanding of trauma-induced coagulopathy and summarize current clinical transfusion regimes utilized in military and civilian settings. We will review currently available blood products used to rectify the coherent disturbances of haemostasis by outlining the characteristics of the different products.
Recent Findings
Current evidence suggests that fresh frozen plasma and fibrinogen components play a fundamental role in trauma resuscitation with recent studies suggesting pre-hospital plasma and fibrinogen administration might also be beneficial in counteracting trauma-induced coagulopathy. Based on experience out of combat zones, whole blood transfusion might experience a renaissance in the future.
Summary
Multiple different plasma-based products are available to treat and prevent trauma-induced coagulation disturbances. As randomized controlled trials in trauma population are difficult to conduct, most of the evidence is currently based on relatively small studies. While the overarching result of our review suggests the early use of plasma and fibrinogen products in combination with packed red blood cells will prevent trauma-induced coagulopathy, large, multi-centre studies are warranted to evaluate the long-term effects on patients’ outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Massive haemorrhage secondary to trauma is a leading cause of death worldwide necessitating blood component resuscitation. Military and civilian studies have shown an associated survival and morbidity benefit in trauma patients resuscitated with a high ratio of fresh frozen plasma (FFP) to packed red blood cell (PRBC) [1,2,3,4,5] and both European [6•] and North American [7] guidelines recommend an equal ratio of FFP to PRBC (and platelets). Some European authors have strongly recommended the initial use of factor concentrates for resuscitation in these casualties, although study results have not been conclusive [7, 8]. The presence of early hypofibrinogenemia in trauma patients is associated with an increased mortality [9], and the administration of fibrinogen concentrate (FC) has been shown to address this aspect of coagulopathy in trauma patients [10, 11]. Its use has subsequently gained favour in mainland European practice.
The initial acute traumatic coagulopathy (ATC) is a pathophysiological process that has been investigated extensively over the past two decades and is commonly described to include predominantly low fibrinogen and hyperfibrinolysis [12, 13]. The role of activated protein C (aPC) has been found to be a key element in this process [14], the activation of which lies at the site of the vascular endothelium [15] secondary to endothelial glycocalyx destruction [16]. FFP has been shown to have the ability to preserve the endothelial glycocalyx and consequently potentially improve survival. Despite evidence of the benefit of other components [17] and pharmacological adjuncts [18] in the resuscitation of bleeding trauma casualties, there is a strong argument that the continued use of FFP is a crucial element to a comprehensive resuscitation regime and remains a necessity in the management of coagulopathy in these patients.
Acute Traumatic Coagulopathy
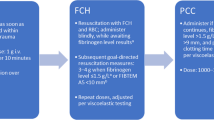
The presence of reduced coagulation in trauma casualties or ATC has been known since before the Vietnam War [19]. One aspect of trauma-induced coagulopathy (TIC), defined by the “lethal triad” of hypothermia, acidosis and dilution, is understood to be potentially present later in a patient’s pathway, and is generally understood to be a predominantly iatrogenic phenomenon, termed by some as resuscitation-associated coagulopathy [20] (Fig. 1). A separate pathological coagulation abnormality, ATC, has been identified that is found much earlier in the trauma process and temporally seems to be important on admission or in the pre-hospital environment.
In the last two decades, studies in Europe and the United States of America (USA) have found that some trauma casualties on admission to hospital are coagulopathic despite little or no haemodilution, no excessive acidosis and minimal reduction in temperature [21,22,23]. In civilian trauma casualties, the incidence of ATC is 24 to 34%, while in military ballistic casualties, ATC is present in up to 50% of patients [24,25,26] and is strongly correlated with mortality [27]. Other characteristics of ATC include low platelet counts, low clotting factors (factor V predominantly), low fibrinogen, fibrinolysis and reduced protein C levels [10, 28,29,30].
Activated Protein C
Hypovolaemia in casualties leads to tissue hypoperfusion and a hypoxic microcirculation, the main driver for ATC [31,32,33]. Tissue hypoperfusion produces a pathological amplification of protein C activation [34] which in turn has a negative feedback on thrombin production in addition to increasing fibrinolysis through removal of tissue plasminogen activator (tPA) inhibition [21, 34,35,36,37,38]. Increased injury severity and hypoperfusion increase the level of coagulopathy. An elevated level of APC is associated with an increased mortality [34, 39], and if inhibited experimentally in mice, coagulopathy is prevented [40].
Endothelial Glycocalyx
The endothelium has a vital role in the control and initiation of clotting. The surface of the endothelium is lined by a group of proteins linked with glycosaminoglycan chains termed the glycocalyx [41]. The glycocalyx, or rather its destruction, is an important intercessor for ATC development, and hypoperfusion is a crucial initiator of glycocalyx destruction. Syndecan-1 has been used in several studies as marker of glycocalyx destruction and damage. The glycocalyx has a key role in the pathophysiology of ATC with a number of theories linking the importance of the endothelium and coagulopathy. This is sometimes referred to as the “endotheliopathy of trauma” [42,43,44] or “shock-induced endotheliopathy” [45]. Trauma is not the only cause of endothelial pathology, however, with a number of factors being shown to produce evidence of glycocalyx disruption including hypoxia [46], sepsis [47] and traumatic sympathoadrenal activation [48]. Elements of all these are seen in hypo-perfused trauma patients so this response is not entirely unexpected.
More recently, four different types of shock-induced endotheliopathy phenotypes have been identified with very different responses to trauma-induced endothelial damage [49••]. These data suggest an important role of individual genetic background in contributing to the endothelial response to trauma.
This review will elucidate our current understanding on trauma resuscitation regarding transfusion of plasma and plasma products (Fig. 1). We will also discuss the differences and advantages of component and whole blood transfusion regimes in trauma patients.
Component Therapy—Plasma and Other Therapeutics
Fresh Frozen Plasma
Fresh frozen plasma (FFP) is prepared from a single unit of whole blood or plasma collected by apheresis into a citrate-containing anticoagulant solution. It needs to be ABO compatible with AB being the universal donor type.
Once thawed, it requires transfusion within 4 h, or if that is to be delayed, it can be kept at 4 °C for up to 24 h; however, factor VIII (FVIII) activity will decline at 24 h by up to 28% [50]. Use can be extended up to 72 h with a decline in FVIII activity of 40%, although the activities of all other factors (including factors II (FII) and V (FV)) remain almost normal [51]. After 5 days, FVIII has lost 60% activity, FV 34% activity and the remainder less than 30% of activity [50, 52].
Risks of transfusion are similar to PRBC and include infectious disease transmission (ranging from 1:7.8 million for HIV to 1:153,000 for hepatitis B [53]), transfusion-associated cardiac overload, transfusion-related acute lung injury, acute haemolytic reactions and anaphylaxis [54,55,56].
FFP is a blood component that has been available since World War II [57]. It was initially used as a volume expander but is now primarily used in the management of haemorrhage and prevention of haemostatic abnormalities in bleeding and coagulopathic patients. The proof of its efficacy in the management of massive haemorrhage in a trauma casualty is disappointingly lacking [58]. Although it has been used to treat trauma haemorrhage for many years, there is surprisingly limited knowledge of its utility and application in this role. Inadequate transfusion is potentially associated with poor outcomes and undoubtedly blind over-transfusion can result in volume overload as well as additional donor exposure with increased rates of sepsis and multi-organ failure [59, 60].
Despite this lack of data, FFP has been widely recommended for use in major haemorrhage simultaneously with PRBC [1] at either specific doses of 10–15 mL/kg [61] or to achieve a lab coagulation level of no more than 1.5 times normal prothrombin time (PT) and activated partial thromboplastin time (aPTT) [62]. Other guidelines and recommendations counsel that it should be transfused in a specified ratio to PRBC. These vary according to continent as well as military or civilian use. However, some key guidelines from noteworthy international bodies do not specify a particular ratio. [63,64,65,66,67].
Lyophilised Plasma
Freeze-dried human plasma (FDP), otherwise known as lyophilised plasma (LP), was first introduced in World War II for use in resuscitation. Plasma was converted into a fine, lightweight powder in significant quantities to answer the high demand under difficult logistical. Disappointingly, there were high rates of viral disease transmission secondary to pooling of plasma units and inadequate screening so the concept was abandoned [68]. Modern screening methods have significantly reduced the risk of virus transmission and the concept of lyophilised plasma has re-emerged as a logistically superior alternative to FFP.
How does LP coagulation capacity compare to FFP? Investigations after World War II demonstrated its haemostatic function was similar as measured by PT [69]. In vitro assays of dried porcine plasma and FFP show similar coagulation profiles (FII, FVII and FIX, PT, aPTT and fibrinogen in addition to thromboelastographic assessments) [70, 71]. In vivo studies using swine models of polytrauma and haemorrhage demonstrate that FDP clotting factor levels are comparable with FFP with only a 14% drop in coagulation factor activity [70, 72]. Subsequent animal trauma models show LP is equally effective as FFP in reversing coagulopathy and improving physiological markers as well as survival [71]. In addition to coagulation benefits, Spoerke and colleagues [70] suggested LP might lower the inflammatory response as indicated by reduced IL-6 levels suggesting this secondary effect (to the use of ascorbic acid in its manufacturing process) was contributing to the advantage offered by LP during trauma resuscitation [73].
Although there is very little evidence of the efficacy of LP or FDP in humans, the military has been using LP and FDP justified by preclinical animal studies to meet the logistical demands of treating remote combat casualties. Currently, Dutch, French, Israeli, German and the United Kingdom (UK) armed forces use LP or FDP with US forces having just received FDA approval (personal communications).
The French military has the most experience and regularly advocate for LP based on its significant shelf life (2 years), speed of reconstitution (3 min to rehydrate), and similar clotting factor and fibrinogen activity compared with FFP [74, 75]. Two recently published studies by the French military report their experience at a Role 3 hospital in Kabul, Afghanistan: In the first study, they used LP in 87 military and civilian casualties and observed an overall mortality of 10% among patients receiving LP despite two-thirds of these patients being in haemorrhagic shock at treatment initiation [76]. The second study looked at 72 transfusion episodes of which 63 received LP (average of 3 units) [77]. Like the first study, the authors noted a significant decrease in PT after LP administration. Though these studies are small and many patients were lost to follow-up, there were no reported complications attributable to LP administration.
Based on the combat data, several retrospective studies have examined the use of LP in the civilian pre-hospital environment. Data from Israel on 109 casualties over a 3-year period (83% penetrating, 50% multiple severe injuries) receiving FDP showed that it was both easy and feasible to use [78]. A French study demonstrated quicker delivery of blood products to trauma patients in a 1:1 ratio if LP is used instead of FFP [79] while a small dataset from Norway emphasizes the safety of use by pre-hospital helicopter emergency medical services (HEMS) [80].
The Freeze-dried Plasma in the Initial Management of Coagulopathy in Trauma Patients (TrauCC) trial was a prospective, randomized trial comparing the incidence of coagulopathy and fibrinogen levels in trauma patients receiving either French lyophilised plasma (FLyP) to FFP in a French hospital [81•]. The investigators found in the 48 patients enrolled that those in receipt of FLyP had higher fibrinogen concentrations and a more rapid improvement in their coagulopathy compared with FFP. Like previous studies, they also noted that FLyP patients received plasma quicker (15 min compared with more than 90 min) resulting in more rapid achievement of the target 1:1 FFP to PRBC ratio.
Despite the logistic advantages (i.e. lightweight, easily transportable, long shelf life), its limited availability and cost currently restrict ready access. These latter reasons are the principle rationalisation for its use only in austere situations in the British military—in the pre-hospital environment or with units who provide small surgical teams to areas that are more difficult to reach and support. Nevertheless, the small number of patients and the retrospective nature of most of these studies warrant future prospective randomized trials to evaluate clinical effectiveness and outcomes following LP transfusion.
Fibrinogen Concentrate
Fibrinogen depletion is considered a major challenge in trauma patients. Schlimp and colleagues found that patients with major trauma and an admission haemoglobin concentration lower than 100 g/L and base excess lower than − 6 commonly present with fibrinogen levels lower than 1.5 g/L [82]. Similarly, Rourke and colleagues found low fibrinogen levels in 41% of the patients with hypotension on admission, increased shock severity and high degree of injury (injury severity score, ISS ≥ 25) [10]. Specific fibrinogen replacement is arguably a key factor in trauma casualty resuscitation.
Fibrinogen concentrate (FC) is produced from pooled human plasma and stored as a lyophilised powder at room temperature [83]. It can be reconstituted rapidly with sterile water for immediate administration [84]. Viral infection risk is minimal as viral inactivation by exposure to solvent or pasteurisation occurs in the manufacturing process [85]. Unlike cryoprecipitate, the concentration of fibrinogen is standardized and there is no requirement for cross matching [84]. One study showed that 2 g of FC would increase plasma fibrinogen by 0.44 g/L, compared with only 0.26 g/L after 10 units of cryoprecipitate infusion (~ 1.8–2.2 g of fibrinogen), suggesting a superiority over cryoprecipitate; some have even reported a reduction of fibrinogen after infusion of cryoprecipitate [86].
Fibrinogen supplementation in cases of severe bleeding demonstrate an improvement in coagulation parameters [87,87,89], increased plasma fibrinogen levels and survival [87, 88, 90], and reduced transfusion requirements [88, 89]. However, a recent meta-analysis found no improvement of mortality in trauma patients by administration of FC, although recognizing the poor quality of included studies [91].
Subsequent trials aimed to overcome this limitation: In the Fibrinogen in the Initial Resuscitation of Severe Trauma (FiiRST) trial, FC was given within an hour of hospital arrival [92]. Despite fibrinogen levels being higher (up to 12 h), mortality did not differ between the groups. This may have been due to a lack of difference in transfused blood products with both groups receiving similar amounts of cryoprecipitate. The Reversal of Trauma Induced Coagulopathy Using Coagulation Factor Concentrates or Fresh Frozen Plasma (RETIC) trial [8] investigated the use of FFP versus coagulation factor concentrates. They used fibrinogen concentrate predominantly but the trial was abandoned due to the need for significant rescue therapy in the FFP group. Just over half of the FFP group needed rescue compared with 4% receiving FC with the number needing massive transfusion also greater in the FFP group (30% vs. 12%). Despite this study being stopped early, their findings suggest that FC should be looked at in a favourable light.
One more recent trial undertook a retrospective look at giving FC pre-emptively in trauma patients with higher ISS rather than waiting for threshold results [93]. Nearly 60% of the patients with an ISS of greater than 26 received 10 units of PRBC and found to have low serum fibrinogen. The 48-h mortality rate of those with ISS greater than 26 was 8.6% in the pre-emptive FC group compared with 22.9% in the standard treatment group. When ranking patients according to the ISS, the authors found that pre-emptive administration reduced mortality from 50 to 20% in patients with an ISS of greater than 41.
Similar results were presented by Itagaki and colleagues [94] in a recent study. Although limited by its retrospective nature, this study illustrates a mortality benefit when FC is given early and most definitely leads to the question of whether it should be taken into the pre-hospital environment (both civilian and military).
Prothrombin Complex Concentrate
Prothrombin complex concentrates (PCC) are intermediate purity pooled plasma products containing a mixture of vitamin K-dependent coagulation factors [95, 96] produced by ion-exchange chromatography of either three- (II, IX and X) or four-factors (addition of VII). The concentration of coagulation factors results in a 25 times higher clotting potential than normal plasma [97]. Although developed for the treatment of haemophilia B, PCC are now frequently used to treat congenital and acquired deficiency of vitamin K-dependent clotting factors [95].
The use of PCC in the treatment of trauma casualties has gained popularity in Europe with several studies comparing it with FFP in casualty resuscitation. In a porcine trauma model, the use of PCC leads to reduced time to haemostasis and number of blood products transfused [98]. Translational human studies have had similar findings with the use of PCC reducing time to correction of coagulopathy [99], reduced blood use [99] and reduced mortality. Unfortunately, the majority of these studies compare PCC and FFP with FFP alone rather than PCC alone [99, 100]. There remains no evidence of mortality benefit with PCC use. The reported morbidity benefit of normalization of haemostasis was measured in conventional laboratory coagulation measures and remained prolonged in all groups, although a difference between them was evident.
Whole Blood Transfusion
Although used by the military since World War I [101], both stored and fresh whole blood (FWB) are seeing increased usage in some trauma settings because these products may be logistically easier and less wasteful compared with classical multi-component transfusion strategies. Thereby, low-titre group O whole blood (LTOWB) has to be distinguished from leukoreduced, and leukoreduced whole blood using a platelet-sparing filter products provided by the American Red Cross (ARC) [102, 103]. Evaluated in regard to clotting factor activity in the presence of PRBCs, Huish and colleagues demonstrated that the factor activity remained above 50% despite prolonged storage (i.e. up to 35 days) and the presence of platelets [104]. Leukoreduced blood transfusions have become standard in most trauma centres, and therefore, transfusion of LTOWB through leukoreduction filters have been evaluated in regard to its haemostatic properties. Importantly, despite the fact that platelet count decreased during cold storage, haemostasis as assessed by thrombelastography and PFA-100 tests was not diminished over a 2-week storage period [102]. Praised by the military [105], whole blood products remain to be evaluated for its suitability during civilian trauma resuscitation.
Resuscitation in Trauma
The arguably pivotal study and a critical report for military resuscitation was from Borgman et al. based in Iraq [1] who looked at massive transfusions in 246 military combatants. All casualties had received more than 10 units of components in 24 h with FFP to PRBC ratios divided into 1:8, 1:2.5, and 1:1.4, respectively. The highest ratio resulted in both a 55% absolute reduction in mortality and increased survival time compared with the lowest FFP to PRBC ratio. Despite these dramatic results, concern about survivor bias tempered these findings and has led to further work.
The most recognized study investigating blood compound therapy is the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial [5], studying 680 trauma casualties suffering (or suspected to be suffering) from massive haemorrhage. The authors compared transfusion of FFP, platelets and PRBC in a 1:1:1 ratio compared with a 1:1:2 ratio. Although no long-term survival benefits were found between the two groups, the 1:1:1 group achieved haemostasis and had fewer deaths due to exsanguination at the 24-h mark. It should be noted that deaths primarily due to haemorrhage most commonly occur in the first 24 h. After 24 h, the proportion dying due to other causes—multi-organ failure, head injury and others—becomes more prevalent.
A number of the authors have investigated civilian trauma in their home trauma centres on return from their military deployment [3]. They found a similar survival advantage in the high ratio group, but a markedly dissimilar time to death (35 h compared with 4 h). Although the work by Borgman et al. was used as support for the fixed ratio resuscitation of military ballistic casualties, the authors disclosed that there was a survivorship bias. The patients, who died early, did so before they were able to get more FFP, and hence, their ratios were high. In contrast, a patient with less shock and less physiologically challenged by their injuries survived long enough to get more FFP, and as a result of having lesser injuries, their FFP to PRBC ratio was higher [106], suggesting injury severity was the cause of mortality difference, not the specific ratio of transfused blood products.
Interestingly, while initial studies suggested that prolonged storage time of PRBC may negatively impact patients outcome [107], more recent data suggested that storage time does not impact patients’ outcome in severely injured patients [108, 109]. In summary, a wide range of both military and civilian retrospective studies on early empirical ratio haemostatic resuscitation are available [2, 110,110,111,112,113,114,115,116,117,118,120], with the majority suggesting that higher ratios of FFP to PRBC will significantly reduce the mortality of bleeding trauma casualties. These mortality reductions ranged from 15 to 62% and originated mainly from civilian trauma centres in the USA and Europe.
PRO Plasma—Resuscitation with Plasma Products
As the glycocalyx is particularly sensitive to injury during ATC, the administration of FFP has been proved to be beneficial for its function. A prospective, observational study in severely injured patients with haemorrhagic shock demonstrated that resuscitation with FFP resulted in a 3-fold decrease of circulating syndecan-1 [121]. Although the levels were higher than normal healthy patients, this significant decrease illustrates the potential of FFP to protect and even restore the glycocalyx.
Plasma-based resuscitation of trauma patients in haemorrhagic shock certainly reduces mortality [1, 3, 120]. Recently, these data have been extended to the preclinical application of FFP. The Prehospital Air Medical Plasma (PAMPer) trial demonstrated that patients receiving FFP-only resuscitation in the pre-hospital environment had a significantly lower mortality with minimal adverse effects compared with conventional resuscitation regimes [122, 123]. By administering 2 units of FFP before any resuscitation fluid, 30-day mortality decreased by 10%. Importantly, transfusion of FFP did not delay the transport time to the trauma centre (42 vs. 40 min).
A paucity of evidence of the detrimental effect of plasma in conjunction with good evidence that it is beneficial for treating the pathophysiological origin of ATC intimates that FFP is crucial in managing and resuscitating trauma casualties with ATC. Considering the impaired coagulation cascade, FFP and plasma products constitute one of the main components of a multiple-component transfusion strategy.
CONTRA Plasma—Whole Blood Transfusion or NO Plasma
In a recent review, Spinella suggested that transfusion of FWB in haemorrhage may result in favourable outcome [17] according to recent studies on combat casualties in Iraq [124] and Afghanistan [125]. The advantage of refrigerated storage for stored FWB compared with multiple storage options for components, including agitation requirements for stand-alone platelets, means FWB has a significant logistical advantage [126]. Concerns over infection and grouping mismatch are valid, with 2 infections and one transfusion-associated graft versus host fatality in 10,000 FWB transfusions on US personnel [127]. Nevertheless, FWB has been more frequently used with at least 5 trauma centres in the USA and Norway studying LTOWB for trauma resuscitation [128]. Simultaneously, a number of larger studies (LITES Network, NCT03402035) are beginning to investigate the feasibility and potential advantages of LTOWB in trauma resuscitation (Table 1).
The UK military experience so far has been sporadic and has centred on emergency donor panel provision in response to patient extremis or when platelet provision was inadequate or non-existent. FWB has considerable potential, particularly in the pre-hospital environment and in austere military environments. Unfortunately, within the UK at present, National Health Service (NHS) Blood and Transplant does not supply FWB as standard. Despite this, requests from the UK military and other agencies have had recent impact, and the London helicopter emergency medical service are currently undertaking a study on its use for pre-hospital trauma resuscitation (RABBIT trial, NCT03522636).
A recent survey by the ARC demonstrated an increased acceptance of FWB transfusion in trauma patients [129••]. Although 80% of responding trauma centres reported using component therapy without laboratory guidance for the management of massive blood transfusion, 10% of the respondents mainly from hospitals of less than 550 beds confirmed using FWB as part of their transfusion regime [129••]. Furthermore, most responders preferred low-titre WB over leukoreduced FWB using platelet-sparing filters.
As the fibrinolysis and lack of fibrinogen appear to be major features of ATC [31], balancing these deficits by fibrinogen replacement appears clearly beneficial for mortality [10, 93]. This strategy would not be sufficient alone since successful resuscitation requires replacement of volume and therefore alternatives to excessive crystalloid [130] or colloid solutions [131] might only be available through transfusion of FWB or single components.
Therefore, the first report of using FWB for civilian trauma resuscitation might be considered as guidance for future patient care in this particular setting [132].
Conclusion
A number of potential alternatives to plasma have been investigated but none have yet proven to be a realistic option. PCC and FC have proven benefits and are key additions in trauma resuscitation of patients with ATC. FWB is probably one of the more realistic alternatives; however, the universal lack of availability and clinical evidence limits its current use despite the excitement of some institutions (i.e. ARC. For the time being, plasma resuscitation at ratios approaching 1:1:1 with PRBC and platelets appears to be the most appropriate resuscitation regime in treatment of ATC (Table 1).
Abbreviations
- aPC:
-
Activated protein C
- aPTT:
-
Activated partial thromboplastin time
- ARC:
-
American Red Cross
- ATC:
-
Acute traumatic coagulopathy
- FC:
-
Fibrinogen concentrate
- FDA:
-
US Food and Drug Administration
- FDP:
-
Freeze-dried human plasma
- FFP:
-
Fresh frozen plasma
- FLyP:
-
French lyophilised plasma
- FVIII:
-
Coagulation factor VIII
- FWB:
-
Fresh whole blood
- ISS:
-
Injury severity score
- LP:
-
Lyophilised plasma
- LTOWB:
-
Low-titre group O whole blood
- PCC:
-
Prothrombin complex concentrate
- PRBC:
-
Packed red blood cell
- PT:
-
Prothrombin time
- PT:
-
prothrombin time
- tPA:
-
Tissue plasminogen activator
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13.
Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Niles SE, McLaughlin DF, et al. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008;64:S69–77 discussion S77.
Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–58.
Shackelford SA, Del Junco DJ, Powell-Dunford N, Mazuchowski EL, Howard JT, Kotwal RS, et al. Association of pPrehospital bBlood pProduct tTransfusion dDuring mMedical eEvacuation of cCombat cCasualties in Afghanistan wWith aAcute and 30-dDay sSurvival. JAMA. 2017;318:1581–91.
Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82.
• Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23:98 This manuscript summarized the current guidelines for resuscitation and provides a clear overview of the state-of-the-art treatment strategies.
Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: aA practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82:605–17.
Innerhofer P, Fries D, Mittermayr M, Innerhofer N, von Langen D, Hell T, et al. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017;4:e258–71.
McQuilten ZK, Wood EM, Bailey M, Cameron PA, Cooper DJ. Fibrinogen is an independent predictor of mortality in major trauma patients: aA five-year statewide cohort study. Injury. 2017;48:1074–81.
Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10:1342–51.
Nardi G, Agostini V, Rondinelli B, Russo E, Bastianini B, Bini G, et al. Trauma induced coagulopathy: impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit Care. 2015;19:817.
Leeper CM, Strotmeyer SJ, Neal MD, Gaines BA. Window of oOpportunity to mMitigate tTrauma-induced cCoagulopathy: fFibrinolysis sShutdown not pPrevalent uUntil 1 hHour pPost-injury. Ann Surg. 2019;270:528–34.
Gall LS, Davenport RA. Fibrinolysis and antifibrinolytic treatment in the trauma patient. Curr Opin Anaesthesiol. 2018;31:227–33.
Gando S, Mayumi T, Ukai T. The roles of activated protein C in experimental trauma models. Chin J Traumatol. 2018;21:311–5.
Davenport R. Pathogenesis of acute traumatic coagulopathy. Transfusion. 2013;53(Suppl 1):23S–7S.
van Zyl N, Milford EM, Diab S, Dunster K, McGiffin P, Rayner SG, et al. Activation of the protein C pathway and endothelial glycocalyx shedding is associated with coagulopathy in an ovine model of trauma and hemorrhage. J Trauma Acute Care Surg. 2016;81:674–84.
Spinella PC, Cap AP. Whole blood: back to the future. Curr Opin Hematol. 2016;23:536–42.
Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1–79.
Simmons RL, Collins JA, Heisterkamp CA, Mills DE, Andren R, Phillips LL. Coagulation disorders in combat casualties. I. Acute changes after wounding. II. Effects of massive transfusion. 3. Post-resuscitative changes. Ann. Surg. 1969;169:455–82.
Kushimoto S, Kudo D, Kawazoe Y. Acute traumatic coagulopathy and trauma-induced coagulopathy: an overview. J Intensive Care. 2017;5:6.
Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–30.
MacLeod JBA, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44.
Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304.
Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64:S64–8.
Carroll RC, Craft RM, Langdon RJ, Clanton CR, Snider CC, Wellons DD, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res. 2009;154:34–9.
Doran CM, Woolley T, Midwinter MJ. Feasibility of using rotational thromboelastometry to assess coagulation status of combat casualties in a deployed setting. J Trauma. 2010;69(Suppl 1):S40–8.
Thorn S, Lefering R, Maegele M, Gruen RL, Mitra B. Early prediction of acute traumatic coagulopathy: a validation of the COAST score using the German Trauma Registry. Eur J Trauma Emerg Surg. 2019.
Curry NS, Davenport RA, Hunt BJ, Stanworth SJ. Transfusion strategies for traumatic coagulopathy. Blood Rev. 2012;26:223–32.
Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, et al. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma. 2011;71:S427–34.
Floccard B, Rugeri L, Faure A, Saint Denis M, Boyle EM, Peguet O, et al. Early coagulopathy in trauma patients: an on-scene and hospital admission study. Injury. 2012;43:26–32.
Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–7 discussion 1217.
Gruen RL, Brohi K, Schreiber M, Balogh ZJ, Pitt V, Narayan M, et al. Haemorrhage control in severely injured patients. Lancet. 2012;380:1099–108.
Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200.
Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet J-F. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–8.
Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–63 discussion 1463.
Frith D, Cohen MJ, Brohi K. Animal models of trauma-induced coagulopathy. Thromb Res. 2012;129:551–6.
Frith D, Brohi K. The pathophysiology of trauma-induced coagulopathy. Curr Opin Crit Care. 2012;18:631–6.
Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;75:S40–7.
Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255:379–85.
Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32:659–65.
Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ. oude Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–59.
Pati S, Potter DR, Baimukanova G, Farrel DH, Holcomb JB, Schreiber MA. Modulating the endotheliopathy of trauma: fFactor concentrate versus fresh frozen plasma. J Trauma Acute Care Surg. 2016;80:576–84 discussion 584.
Naumann DN, Hazeldine J, Dinsdale RJ, Bishop JR, Midwinter MJ, Harrison P, et al. Endotheliopathy is associated with higher levels of cell-free DNA following major trauma: aA prospective observational study. PLoS One. 2017;12:e0189870.
Naumann DN, Hazeldine J, Davies DJ, Bishop J, Midwinter MJ, Belli A, et al. Endotheliopathy of tTrauma is an on-sScene pPhenomenon, and is aAssociated with mMultiple oOrgan dDysfunction sSyndrome: aA pProspective oObservational sStudy. Shock. 2018;49:420–8.
Johansson PI, Stensballe J, Ostrowski SR. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. 2017;21:25.
Annecke T, Fischer J, Hartmann H, Tschoep J, Rehm M, Conzen P, et al. Shedding of the coronary endothelial glycocalyx: effects of hypoxia/reoxygenation vs ischaemia/reperfusion. Br J Anaesth. 2011;107:679–86.
Nieuwdorp M, Meuwese MC, Mooij HL, van Lieshout MHP, Hayden A, Levi M, et al. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202:296–303.
Ostrowski SR, Henriksen HH, Stensballe J, Gybel-Brask M, Cardenas JC, Baer LA, et al. Sympathoadrenal activation and endotheliopathy are drivers of hypocoagulability and hyperfibrinolysis in trauma: aA prospective observational study of 404 severely injured patients. J Trauma Acute Care Surg. 2017;82:293–301.
•• Henriksen HH, McGarrity S, SigurÐardóttir RS, Nemkov T, D’Alessandro A, Palsson BO, et al. Metabolic sSystems aAnalysis of sShock-iInduced eEndotheliopathy (SHINE) in tTrauma: aA nNew rResearch pParadigm. Ann Surg. 2019; In this manuscript, the authors clearly describe 4 different, trauma- induced endotheliopathy phenotypes. This manuscript clearly suggests that trauma response is most likely driven by genetic components.
O’Shaughnessy DF, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, Yates S, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28.
Tholpady A, Monson J, Radovancevic R, Klein K, Bracey A. Analysis of prolonged storage on coagulation fFactor (F)V, FVII, and FVIII in thawed plasma: is it time to extend the expiration date beyond 5 days? Transfusion. 2013;53:645–50.
Chapman JF, Elliott C, Knowles SM, Milkins CE, Poole GD, Working Party of the British Committee for Standards in Haematology Blood Transfusion Task Force. Guidelines for compatibility procedures in blood transfusion laboratories. Transfus Med. 2004;14:59–73.
O’Brien SF, Yi Q-L, Fan W, Scalia V, Kleinman SH, Vamvakas EC. Current incidence and estimated residual risk of transfusion-transmitted infections in donations made to Canadian Blood Services. Transfusion. 2007;47:316–25.
Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019;133:1840–53.
Maskens C, Downie H, Wendt A, Lima A, Merkley L, Lin Y, et al. Hospital-based transfusion error tracking from 2005 to 2010: identifying the key errors threatening patient transfusion safety. Transfusion. 2014;54:66–73 quiz 65.
Popovsky MA. Pulmonary consequences of transfusion: TRALI and TACO. Transfus Apher Sci. 2006;34:243–4.
Emerson CP, Ebert RV. A study of shock in battle casualties: measurements of the blood volume changes occurring in response to therapy. Ann Surg. 1945;122:745–72.
Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DBL, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–52.
Watson GA, Sperry JL, Rosengart MR, Minei JP, Harbrecht BG, Moore EE, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:221–7 discussion 228.
Norda R, Knutson F, Berseus O, Akerblom O, Nilsson-Ekdahl K, Stegmayr B, et al. Unexpected effects of donor gender on the storage of liquid plasma. Vox Sang. 2007;93:223–8.
Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52.
British Committee for Standards in Haematology, Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–41.
Padhi S, Kemmis-Betty S, Rajesh S, Hill J, Murphy MF. Guideline Development Group. Blood Transfus: summary of NICE guidance BMJ. 2015;351:h5832.
Kanani AN, Hartshorn S. NICE clinical guideline NG39: Major trauma: assessment and initial management. Arch Dis Child Educ Pract Ed. 2017;102:20–3.
Iorio A, Basileo M, Marchesini E, Materazzi M, Marchesi M, Esposito A, et al. The good use of plasma. A critical analysis of five international guidelines. Blood Transfus. 2008;6:18–24.
American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208.
Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100.
Inaba K. Freeze-dried plasma. J Trauma. 2011;70:S57–8.
Oktavec WA, Smetana EJ. Lyophilized normal human plasma control in the prothrombin-time clotting test. Am J Clin Pathol. 1954;24:250.
Spoerke N, Zink K, Cho SD, Differding J, Muller P, Karahan A, et al. Lyophilized plasma for resuscitation in a swine model of severe injury. Arch Surg. 2009;144:829–34.
Shuja F, Shults C, Duggan M, Tabbara M, Butt MU, Fischer TH, et al. Development and testing of freeze-dried plasma for the treatment of trauma-associated coagulopathy. J Trauma. 2008;65:975–85.
Lee TH, Van PY, Spoerke NJ, Hamilton GJ, Cho SD, Watson K, et al. The use of lyophilized plasma in a severe multi-injury pig model. Transfusion. 2013;53(Suppl 1):72S–9S.
Hamilton GJ, Van PY, Differding JA, Kremenevskiy IV, Spoerke NJ, Sambasivan C, et al. Lyophilized plasma with ascorbic acid decreases inflammation in hemorrhagic shock. J Trauma. 2011;71:292–7 discussion 297.
Bux J, Dickhörner D, Scheel E. Quality of freeze-dried (lyophilized) quarantined single-donor plasma. Transfusion. 2013;53:3203–9.
Daban JL, Clapson P, Ausset S, Deshayes AV, Sailliol A. Freeze dried plasma: a French army specialty. Crit Care. 2010;14:412.
Martinaud C, Ausset S, Deshayes AV, Cauet A, Demazeau N, Sailliol A. Use of freeze-dried plasma in French intensive care unit in Afghanistan. J Trauma. 2011;71:1761–4 discussion 1764.
Patrick J, Cauet A, Deshayes AV, Demazeau N, Sailliol A, Ausset S. Clinical use of freeze dried plasma (FDP) in deployed operations: aAnalysis of 72 episodes of transfusion (ET): 6AP5-2. Eur J Anaesthesiol (EJA). 2011;28.
Shlaifer A, Siman-Tov M, Radomislensky I, Peleg K, Shina A, Baruch EN, et al. Prehospital administration of freeze-dried plasma, is it the solution for trauma casualties? J Trauma Acute Care Surg. 2017;83:675–82.
Nguyen C, Bordes J, Cungi P-J, Esnault P, Cardinale M, Mathais Q, et al. Use of French lyophilized plasma transfusion in severe trauma patients is associated with an early plasma transfusion and early transfusion ratio improvement. J Trauma Acute Care Surg. 2018;84:780–5.
Sunde GA, Vikenes B, Strandenes G, Flo K-C, Hervig TA, Kristoffersen EK, et al. Freeze dried plasma and fresh red blood cells for civilian prehospital hemorrhagic shock resuscitation. J Trauma Acute Care Surg. 2015;78:S26–30.
• Garrigue D, Godier A, Glacet A, Labreuche J, Kipnis E, Paris C, et al. French lyophilized plasma versus fresh frozen plasma for the initial management of trauma-induced coagulopathy: a randomized open-label trial. J Thromb Haemost. 2018;16:481–9 This study describes a comparison of the military- manufactured French lyophilized plasma (FLyP) with conventional fresh frozen plasma during early trauma resuscitation in a civilian patient population. Although only 47 patients were enrolled in this single-centre, open-label trial, the data clearly suggest that early transfusion of FLyP was more effective in correcting trauma- induced coagulopathy.
Schlimp CJ, Voelckel W, Inaba K, Maegele M, Ponschab M, Schöchl H. Estimation of plasma fibrinogen levels based on hemoglobin, base excess and Injury Severity Score upon emergency room admission. Crit Care. 2013;17:R137.
Rahe-Meyer N, Sørensen B. For: Fibrinogen concentrate for management of bleeding. J Thromb Haemost. 2011;9:1–5.
Fenger-Eriksen C, Jensen TM, Kristensen BS, Jensen KM, Tønnesen E, Ingerslev J, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost. 2009;7:795–802.
Pereira A. Cryoprecipitate versus commercial fibrinogen concentrate in patients who occasionally require a therapeutic supply of fibrinogen: risk comparison in the case of an emerging transfusion-transmitted infection. Haematologica. 2007;92:846–9.
Theodoulou A, Berryman J, Nathwani A, Scully M. Comparison of cryoprecipitate with fibrinogen concentrate for acquired hypofibrinogenaemia. Transfus Apher Sci. 2012;46:159–62.
Danés AF, Cuenca LG, Bueno SR, Mendarte Barrenechea L, Ronsano JBM. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox Sang. 2008;94:221–6.
Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, Ingerslev J, Sørensen B. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth. 2008;101:769–73.
Thorarinsdottir HR, Sigurbjornsson FT, Hreinsson K, Onundarson PT, Gudbjartsson T, Sigurdsson GH. Effects of fibrinogen concentrate administration during severe hemorrhage. Acta Anaesthesiol Scand. 2010;54:1077–82.
Schöchl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55.
Mengoli C, Franchini M, Marano G, Pupella S, Vaglio S, Marietta M, et al. The use of fibrinogen concentrate for the management of trauma-related bleeding: a systematic review and meta-analysis. Blood Transfus. 2017;15:318–24.
Nascimento B, Callum J, Tien H, Peng H, Rizoli S, Karanicolas P, et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): a randomized feasibility trial. Br J Anaesth. 2016;117:775–82.
Yamamoto K, Yamaguchi A, Sawano M, Matsuda M, Anan M, Inokuchi K, et al. Pre-emptive administration of fibrinogen concentrate contributes to improved prognosis in patients with severe trauma. Trauma Surg Acute Care Open. 2016;1:e000037.
Itagaki Y, Hayakawa M, Maekawa K, Saito T, Kodate A, Honma Y, et al. Early administration of fibrinogen concentrate is associated with improved survival among severe trauma patients: a single-centre propensity score-matched analysis. World J Emerg Surg. 2020;15:7.
Hellstern P, Halbmayer WM, Köhler M, Seitz R, Müller-Berghaus G. Prothrombin complex concentrates: indications, contraindications, and risks: a task force summary. Thromb Res. 1999;95:S3–6.
Hellstern P. Production and composition of prothrombin complex concentrates: correlation between composition and therapeutic efficiency. Thromb Res. 1999;95:S7–12.
Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfus Med Rev. 2007;21:37–48.
Dickneite G, Pragst I. Prothrombin complex concentrate vs fresh frozen plasma for reversal of dilutional coagulopathy in a porcine trauma model. Br J Anaesth. 2009;102:345–54.
Joseph B, Aziz H, Pandit V, Hays D, Kulvatunyou N, Yousuf Z, et al. Prothrombin complex concentrate versus fresh-frozen plasma for reversal of coagulopathy of trauma: is there a difference? World J Surg. 2014;38:1875–81.
Jehan F, Aziz H, Oʼ Keeffe T, Khan M, Zakaria ER, Hamidi M, et al. The role of four-factor prothrombin complex concentrate in coagulopathy of trauma: aA propensity matched analysis. J Trauma Acute Care Surg. 2018;85:18–24.
Stansbury LG, Hess JR. The 100th anniversary of the first blood bank. Transfusion. 2017;57:2562–3.
Haddaway K, Bloch EM, Tobian AAR, Frank SM, Sikorski R, Cho BC, et al. Hemostatic properties of cold-stored whole blood leukoreduced using a platelet-sparing versus a non-platelet-sparing filter. Transfusion. 2019;59:1809–17.
Sivertsen J, Braathen H, Lunde THF, Kristoffersen EK, Hervig T, Strandenes G, et al. Cold-stored leukoreduced CPDA-1 whole blood: in vitro quality and hemostatic properties. Transfusion. 2020;
Huish S, Green L, Curnow E, Wiltshire M, Cardigan R. Effect of storage of plasma in the presence of red blood cells and platelets: re-evaluating the shelf life of whole blood. Transfusion. 2019;59:3468–77.
Meledeo MA, Peltier GC, McIntosh CS, Bynum JA, Cap AP. Optimizing whole blood storage: hemostatic function of 35-day stored product in CPD, CP2D, and CPDA-1 anticoagulants. Transfusion. 2019;59:1549–59.
Magnotti LJ, Zarzaur BL, Fischer PE, Williams RF, Myers AL, Bradburn EH, et al. Improved survival after hemostatic resuscitation: does the emperor have no clothes? J Trauma. 2011;70:97–102.
Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39.
Cartotto R, Taylor SL, Holmes JH, Peck M, Cochran A, King BT, et al. The effects of storage age of blood in massively transfused burn patients: a secondary analysis of the randomized transfusion requirement in burn care evaluation study. Crit Care Med. 2018;46:e1097–104.
• Bautista A, Wright TB, Meany J, Kandadai SK, Brown B, Khalafalla K, et al. Red cell storage duration does not affect outcome after massive blood transfusion in trauma and nontrauma patients: a retrospective analysis of 305 patients. Biomed Res Int. 2017;2017:3718615 This single-centre, retrospective study analysed the impact of storage time on trauma resuscitation outcome in severe injured patients. Despite limitations based on the design, the trial clearly demonstrates that the so- called “storage” effect, does not apply in patients undergoing massive blood transfusion in the trauma setting.
Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–8 discussion 48.
Gunter OL, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65:527–34.
Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B, et al. Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft für Unfallchirurgie. Vox Sang. 2008;95:112–9.
Savage SA, Zarzaur BL, Croce MA, Fabian TC. Time matters in 1: 1 resuscitations: concurrent administration of blood: plasma and risk of death. J Trauma Acute Care Surg. 2014;77:833–7 discussion 837.
Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66:1616–24.
Teixeira PGR, Inaba K, Shulman I, Salim A, Demetriades D, Brown C, et al. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66:693–7.
Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197:565–70 discussion 570.
Snyder CW, Weinberg JA, McGwin G, Melton SM, George RL, Reiff DA, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66:358–62 discussion 362.
Sim ES, Guyette FX, Brown JB, Daley BJ, Miller RS, Harbrecht BG, et al. Massive transfusion and the response to prehospital plasma: it is all in how you define it. J Trauma Acute Care Surg. 2020.
•• Nederpelt CJ, El Hechi MW, Kongkaewpaisan N, Kokoroskos N, Mendoza AE, Saillant NN, et al. Fresh frozen plasma-to-packed red blood cell ratio and mortality in traumatic hemorrhage: nationwide analysis of 4,427 patients. J Am Coll Surg. 2019;230(6):893–901 This large retrospective nationwide cohort study analysed the effect of different FFP to PRBC ratios in trauma patients. The trial confirmed in a much larger setting previous findings, which suggested that a 1:1 FFP to PRBC ration is associated with the lowest mortality in haemorrhaging trauma patients.
Roquet F, Neuschwander A, Hamada S, Favé G, Follin A, Marrache D, et al. Association of eEarly, hHigh pPlasma-to-rRed bBlood cCell tTransfusion rRatio wWith mMortality in aAdults wWith sSevere bBleeding aAfter tTrauma. JAMA Netw Open. 2019;2:e1912076.
Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6:e23530.
•• Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–26 The “PAMPER” trial is a multi-centre, cluster-randomized, phase 3 superiority study comparing pre-hospital administration of thawed plasma with standard-care resuscitation during air medical transport. With a total of 501 patients, this trail clearly demonstrates a 9.8% reduction in mortality in patients who received pre-hospital FFP. Interestingly, the authors did not find any difference in respect to multi-organ failure, acute lung injury, nosocomial infections, or allergic or transfusion-related reactions. The trial opens the discussion whether pre-hospital FFP should be limited to air -ambulance services only.
Guyette FX, Sperry JL, Peitzman AB, Billiar TR, Daley BJ, Miller RS, et al. Prehospital blood product and crystalloid resuscitation in the severely injured patient: aA secondary analysis of the prehospital air medical plasma trial. Ann Surg. 2019.
Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66:S69–76.
Nessen SC, Eastridge BJ, Cronk D, Craig RM, Berséus O, Ellison R, et al. Fresh whole blood use by forward surgical teams in Afghanistan is associated with improved survival compared to component therapy without platelets. Transfusion. 2013;53(Suppl 1):107S–13S.
Cap AP, Beckett A, Benov A, Borgman M, Chen J, Corley JB, et al. Whole bBlood tTransfusion. Mil Med. 2018;183:44–51.
Gilstad C, Roschewski M, Wells J, Delmas A, Lackey J, Uribe P, et al. Fatal transfusion-associated graft-versus-host disease with concomitant immune hemolysis in a group A combat trauma patient resuscitated with group O fresh whole blood. Transfusion. 2012;52:930–5.
Yazer MH, Spinella PC. The use of low-titer group O whole blood for the resuscitation of civilian trauma patients in 2018. Transfusion. 2018;58:2744–6.
•• Young PP, Borge PD. Making whole blood for trauma available (again): the AMERICAN Red Cross experience. Transfusion. 2019;59:1439–45 This review by the American Red Cross emphasis their efforts to implement the usage of whole blood for trauma resuscitation. According to their survey, 93% of trauma surgeons would prefer whole blood transfusion instead of component therapy; despite the lack of clinical evidence. While the article points out some of the challenges related to preparing whole blood to immediate use for hospitals, the authors point out that the simplicity of transfusion might reign its limitations.
Ley EJ, Clond MA, Srour MK, Barnajian M, Mirocha J, Margulies DR, et al. Emergency department crystalloid resuscitation of 1.5 L or more is associated with increased mortality in elderly and nonelderly trauma patients. J Trauma. 2011;70:398–400.
Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst. Rev. 2013;CD000567.
Condron M, Scanlan M, Schreiber M. Massive transfusion of low-titer cold-stored O-positive whole blood in a civilian trauma setting. Transfusion. 2019;59:927–30.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Anesthesia for Trauma
Rights and permissions
About this article
Cite this article
Nordmann, G.R., Obal, D. Is Fresh Frozen Plasma Still Necessary for Management of Acute Traumatic Coagulopathy?. Curr Anesthesiol Rep 10, 297–307 (2020). https://doi.org/10.1007/s40140-020-00397-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-020-00397-3