Abstract
Purpose of the Review
Transesophageal echocardiography (TEE), a relatively non-invasive method, is an ideal intraoperative monitoring tool. In this review, we evaluated all potential applications of TEE in general anesthesia.
Recent Findings
The uses of intraoperative TEE have expanded over the years. TEE’s main application is in the intraoperative assessment and monitoring of the cardiac function. Its impact in clinical decision making has been demonstrated in non-cardiac surgery in the operating room and as a rescue in case of hemodynamic instability and cardiac arrest. TEE may also provide more complete quantitative hemodynamic monitoring of intracardiac pressures as the pulmonary artery catheter. Newer intraoperative TEE application includes imaging of extra-cardiac structures such as the kidney, the intrahepatic inferior vena cava, the lungs, the pleura space, and the epidural space. Several scanning protocols have been proposed ranging from a focused to a comprehensive cardiac assessment. Current guidelines for intraoperative use of TEE include basic and advanced TEE exam.
Summary
Intraoperative TEE is a very powerful tool in the hands of the TEE trained anesthesiologist. It has a positive safety profile but requires a significant initial manpower and financial investment. While less expensive equipment has become available, specific training is necessary and requires a significant time commitment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transesophageal echocardiography (TEE) is an ideal intraoperative monitoring tool. It does not interfere with most surgical procedures, and the probe can be easily inserted without the need for sterile technique in the great majority of anesthetized intubated patients.
Given the proximity of the esophagus to the heart and great vessels, TEE provides superior imaging of most cardiac structures when compared to transthoracic echocardiography (TTE) [1••, 2]. In the last 20 years, TEE has become a standard of care in the hands of cardiac anesthesiologists for the intraoperative management of cardiac surgical patients and to guide minimally invasive and percutaneous cardiac interventions [1••, 3, 4•].
In the operating room, TEE provides precise quantitative assessment of standard hemodynamic parameters such as cardiac output, intra cardiac, and pulmonary pressures, becoming a viable alternative to pulmonary artery catheters [5•]. The ability to assess cardiac and extra-cardiac flows, biventricular function, and structural anatomy makes TEE potentially useful in guiding intraoperative management in any major surgery [2, 5•].
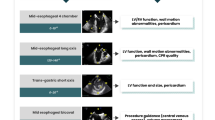
The ability to visualize and guide intravascular wires and catheters, as well as other extra-cardiac imaging applications further add to the potential applications of TEE at each stage of general anesthesia (Fig. 1).
Summary of intraoperative TEE applications. “Modified from www.pie.med.utoronto.ca/POCUS”
Although TEE is mainly used outside of the cardiac operating rooms as a monitoring tool, it becomes a powerful diagnostic tool in emergency situations in guiding treatment of undifferentiated shock and cardiac arrest in the perioperative setting [6••, 7•].
The clinical impact of intraoperative TEE on clinical decision making and management, in the assessment of myocardial function, volume status, and response to therapy, has led to its increasing use in the non-cardiac surgical population [4•, 7•, 8••].
The high cost of the equipment, need for specific training, and required probe maintenance and infection control remain the main limitations to the wider use of TEE outside the cardiac operating room. The introduction of single use, miniaturized probes, and availability of TEE probes for point of care ultrasound (POCUS) systems that have become more widely available for line insertion or regional anesthesia has offset some of the above limitations [2, 9].
General Indication for Intraoperative TEE
Basic and FOCUS TEE have mostly been used in non-cardiac surgery to identify hypovolemia, decreased left or right systolic function, wall motion abnormalities, and PE [4•, 6••, 10]. Severe unexplained hemodynamic instability and hypoxia are the two main indication for the use of TEE in non-cardiac surgery [11••]. Despite the recent increase in the use of TEE in non-cardiac surgery, current data on its impact is largely limited to small retrospective or observational trials, often more than a decade old [2].
The American Society of Anesthesiologist (ASA) and the American Society of Echocardiography (ASE) practice guidelines recommend the use of TEE in the event that unexplained life-threatening circulatory instability persists despite appropriate therapy, or based on the integration of the patient’s condition, the risk of the surgical procedure and specific circumstances that might result in severe hemodynamic, pulmonary, or neurologic compromise [6••]. The European guidelines are limited to TEE in intraoperative monitoring during cardiac surgery or peri-interventional imaging and do not specifically refer to the use of TEE in non-cardiac cases, deferring instead to the American Society of Echocardiography (ASE) guidelines [11••, 12••, 13, 14].
Sudden intraoperative hemodynamic instability is thus a category I indication; however, TEE may not be always readily available in all non-cardiac operating rooms due to lack of trained personnel and equipment [8••]. A risk score for more precise indications would be helpful to allocate resources to the patients who would likely benefit from it.
Indications for specific clinical scenarios in non-cardiac surgery are limited and are organized by classes of recommendation (A— supportive; B— suggestive; C— equivocal; D— insufficient) and level of evidence (1— multiple randomized controlled trials and meta-analysis; 2— multiple randomized controlled trials alone not supported by meta-analysis; 3— single randomized controlled trial). The ASE recommends TEE for trauma (D1); prosthetic valve thrombus (B2); kidney (B3), liver (B2), and lung (B2) transplantation; major vascular surgery (B2); orthopedic/spinal surgery (B2); and neurosurgery/sitting position (B2) [8••].
Scanning Protocols
Standard adult TEE probes are omni-plane and allow clockwise plane rotation of the ultrasound plane from 0 to 180° by 1° increments [13]. Standard TEE views are obtained at different depths: upper esophageal (UE), mid-esophageal (ME), and transgastric (TG) levels, and at different omni-plane angles. Anteflexion, retroflexion, sideward flexion, left-right rotation, and advancement and withdrawal of the probe are complementary maneuvers used to optimize the image [13].
The latest generation of TEE probes, known as matrix array, allows simultaneous imaging of multiple 2D planes (at different omni-plane angles) as well as real-time 3D imaging.
Disposable probes (hTEE) consist of a 16-French catheter that can be inserted orally or nasally and left in place for up to 72 h [9]. The transducer is mono-plane and does not allow Doppler assessment.
A complete TEE exams include a standard set of views (11 to 28) at different levels in the esophagus and stomach. A comprehensive TEE examination is aimed at a complete assessment of heart structures and function and requires acquisition of 28 views. They include 2D images of all four cardiac valves, chambers, and great vessels and assessment of all cardiac valves with color and spectral Doppler. Specific 3D views are also required [1••, 6••, 13].
A basic TEE examination is aimed at intraoperative monitoring in non-cardiac surgery and requires 11 of the standard 28 views with limited assessment of valvular function and pathology [6••].
“FOCUS” TEE exam has been described in emergency medicine as an alternative to focused cardiac ultrasound for the assessment of shock and includes four standard 2D views and no Doppler. [10, 15, 16] A limited four-view protocol without Doppler has also been used for hTEE [9].
Safety and Contraindications
Complications from TEE in intubated patients under general anesthesia are mostly related to traumatic mechanical damage at insertion and during probe manipulation. The overall safety profile is acceptable, but severe and life-threatening complications have been reported contributing to an overall morbidity ranging from 0.2 to 1.2% [17].
The most common complications are minor oropharyngeal injuries (incidence between 0.1 and 13%) such as lip trauma, hoarseness, sore throat, dysphagia (with no consistent independent correlation), and dental injury [17].
Esophageal perforation is the most feared and life-threatening complication. It occurs with an incidence between 0.03 and 0.09% [18]. Elderly female patients appear to be at increased risk.
Absolute contraindications are perforated viscus, esophageal stricture, esophageal tumor, esophageal perforation or laceration, esophageal diverticulum, and active upper GI bleed [1••].
Relative contraindications are history of radiation to neck and mediastinum, history of GI surgery, recent upper GI bleed, Barrett’s esophagus, history of dysphagia, restriction of neck mobility (severe cervical arthritis, atlantoaxial joint disease), symptomatic hiatal hernia, esophageal varices, coagulopathy, thrombocytopenia, active esophagitis, and active peptic ulcer disease [1••].
In order to guarantee infection prevention and control, a thorough cleaning of the probes is required and includes standard mechanical cleaning, 20 to 30 min of 2% glutaraldehyde solution bath, a final rinse, and thorough drying [19]. Given the toxicity of gluteraldehydesome, institutions have opted for more expensive closed, automated TEE probe-cleaning systems, or UV light disinfection.
Vascular Access Guidance
Surface ultrasound to help confirm proper positioning of a guidewire within the internal jugular vein does not allow complete visualization of the guidewire advancing into the superior vena cava (SVC) and cannot exclude the possibility of carotid artery puncture [20•]. TEE can be used to verify, in real time, correct guidewire positioning during central venous cannulation by using the mid-esophageal bicaval view, prior to introducing the dilator or catheter [20•].
Assessment of Fluid Status
Volume status can be evaluated by directly assessing the right and left chamber or vessel size or by dynamic evaluation of blood flow.
LV end-diastolic area in the TG mid-papillary SAX view is the single recommended measurement to assess volume responsiveness (increase in cardiac index of at least 11% induced by volume expansion) and appears to provide a better index of LV preload in patients with normal LV function, when compared to a pulmonary artery catheter [6••].
Using the ME bicaval view, both size and collapsibility of the inferior vena cava (IVC) can also be evaluated for estimation of CVP to predict response to fluid management [8••]. IVC inspiratory collapse occurs in hypovolemic spontaneous breathing patients, but may not occur in mechanically ventilated patients where we observe inspiratory IVC distension. Respiratory variation of the superior vena cava (distensibility >36%), accurately predicts fluid responsiveness in patients during controlled mechanical ventilation [8••, 21•]. Another way to predict fluid responsiveness during controlled mechanical ventilation is to measure the variation of the peak aortic blood flow velocity during the respiratory cycle in the deep TG five-chamber view with pulsed Doppler as it is directly related to stroke volume variation [21•]. Variation greater than 12% in the peak aortic outflow velocity accurately predicts fluid responsiveness [22]. This measurement is not reliable in patients with arrhythmia, right ventricular dilation or dysfunction, an open chest, or those ventilated with low tidal volumes (<8 ml/kg) [23, 24].
Global and Regional Ventricular Function
Basic echocardiography can assess LV and RV global systolic function by qualitative, visual estimation. This is an accepted quick method to estimate ventricular function in patients with severe hemodynamic disturbances and unknown ventricular function [6••].
The TG mid-papillary SAX view provides visualization of 6-mid-papillary segments of the LV and all three coronary territories. For this reason, it is the most commonly used view for rapid assessment of LV function. Current ASE guidelines suggest that even for basic TEE echocardiography, this view should be combined with the ME four-chamber, ME two-chamber, and ME LAX views for more comprehensive evaluation and monitoring of global and regional LV function [6••].
TEE allows direct estimation of RV size and function. RV size can be qualitatively assessed in comparison to that of the LV (normally 2/3 or less). Right ventricular hypertrophy and right atrial dilation will indicate chronic RV pressure or volume overload. Flattening of the interventricular septum in the TG mid-papillary SAX view indicates RV pressure (during systole) or volume (during diastole) overload. In surgical cases with high risk of RV dysfunction, in particular in non-thoracic cases where the RV cannot be inspected, the ASE recommends basic RV PTE monitoring [6••, 8••]. Right ventricular function can be quantified by fractional area change (FAC) of the RV cavity in the ME four-chamber view, or by measuring the distance traveled by the tricuspid valve annulus in systole (TAPSE), in the TG RV inflow view, or using anatomical M-Mode in the ME four-chamber view [25••].
Intraoperative Hemodynamic Monitoring
Pressures
Most left and right heart hemodynamic measurements (Table 1) derived from TEE imaging and Doppler assessment have been extensively studied [2, 26••].
Central Venous Pressure
Right ventricular (RV) preload (central venous pressure (CVP)) can be estimated by TEE with the analysis of the trans-tricuspid pulsed-wave Doppler inflow profile at the level of the ME four-chamber view, from the acceleration rate of RV filling. The early diastolic wave (E) acceleration rate, in centimeter per second squared, estimates CVP based on the formula CVP = −1.263 + (0.01116 × E acceleration rate) [5•].
Pulmonary Artery Pressures
Pulmonary artery pressures (PAP) can be estimated at the ME modified bicaval tricuspid (TV) view by continuous-wave Doppler assessment of the tricuspid regurgitant jet (TRV), using the following formula PAP = (TRV2 × 4) + CVP (mmHg), based on the modified Bernoulli equation, where TRV is the maximal velocity of a tricuspid regurgitation jet [5•].
Pulmonary Capillary Wedge Pressure
The pulmonary capillary wedge pressure (PCWP), as an indirect measure of LV preload can be estimated by TEE using the ME four-chamber view with pulsed-wave Doppler interrogation of the mitral inflow to assess the peak early velocity (E), and tissue Doppler interrogation of the lateral mitral annulus to assess the early diastolic annular wave (e′). PCWP can be estimated from the following formula PCWP = 1.24 × (E/e′) + 1.9 (mmHg) [5•].
Pulmonary Vascular Resistance
Pulmonary vascular resistance (PVR) is estimated based on TRV, and the right ventricular outflow tract velocity time integral (VTI RVOT) is measured in the TG RV basal view, using pulsed-wave Doppler at the proximal level of the pulmonary valve. PVR can then be estimated with the following formula PVR = [(5.19 × TRV2/VTIRVOT−0.4)] × 80 (dyn × s× cm−5) [27].
Cardiac Output
The best way to calculate the SV by TEE is multiplying the left or right ventricle outflow tract (LVOT or RVOT) VTI, which is the sum of instantaneous systolic velocities through the LVOT or RVOT over time in cm (stroke distance), by the cross-sectional area (CSA) of the LVOT or RVOT, which is Pi times radius squared (r 2) in square centimeter. Some authors have recommended the use the Simpson method of the disks instead, but this technique is more cumbersome, requires clear endocardial border delineation and also relies on geometric assumptions [5•, 8••].
LVOT VTI can be calculated by pulse-wave Doppler at the level of the LVOT, between 2 and 5 mm below the aortic valve, from the deep TG five-chamber view. In the same manner, RVOT VTI is calculated from the ME ascending aorta SAX view or the UE aortic arch SAX view, between 2 and 5 mm below the pulmonic valve.
CSA of the LVOT is calculated from the LVOT diameter (d), (r = d/2), from the ME AV LAX view. CSA calculation though is based on the geometrical assumption of the LVOT being cylindrical. CSA of the RVOT is calculated from the ME RV inflow-outflow view.
CO can be then calculated based on the following formula CO = SV × HR; SV = CSA (cm2) × VTI (cm); CSALVOT = π × r 2, where r = d/2.
For more experienced echocardiographers, real-time 3D images of the LV allow dynamic semi-automated reconstruction of the LV cavity, providing a faster automated measurement of the SV, independent of any geometrical assumptions [2]. This method is more reproducible and faster than the Simpson method of the disks. Newer technology allows detection of ventricular chambers form a 3D block of the entire heart and automatic measurement of 3D volumes throughout the cardiac chambers. Despite its potential applications, this is currently not available for TEE acquired 3D datasets [28].
Systemic Vascular Resistance
SVR can be calculated based on the following formula SVR = [(MAP−CVP)/CO] × 80 (dyn × s × cm−5), where MAP refers to mean arterial pressure and pressures are measured in millimeter of mercury, and CO in liter per min.
Diastolic Function
LV diastolic dysfunction is a sensitive sign of myocardial dysfunction and has been associated with poor outcome after non-cardiac and cardiac surgery [29, 30]. A simplified approach is based on the measurement of mitral annulus tissue Doppler e′ velocity and mitral valve inflow early velocity E over e′ ratio. A E/e’ > 8 has been associated with an increase in postoperative cardiovascular events, pulmonary congestion, arrhythmias, and longer ICU and hospital stays [29] (Table 2).
Heart Rhythm
When the electrocardiogram (ECG) tracing is not available due to significant artifacts or other technical problems, Doppler interrogation of the transmitral flow has been described as an alternative and reliable method to confirm or rule-out arrhythmias [31].
Rule-out Gross Valvular Lesions
Basic TEE provides the ability to perform a qualitative assessment of valvular regurgitation and/or stenosis of the aortic, mitral, tricuspid, and pulmonic valves based on valve morphology [6••]. Jet area or vena contracta width by Color Doppler allows differentiation of mild from moderate or severe regurgitation. The assessment of severe valvular lesions, eccentric jets, and prosthetic valves should be left to advanced TEE examination [1••, 6••].
Perioperative Pulmonary Embolism
TEE is not the gold standard for the diagnosis of pulmonary embolism (PE). In unstable patients, not suitable for contrast CT scan, TEE allows identification large clots in the proximal pulmonary arteries as well as indirect signs of PE such as RV dilatation. The identification of the thrombus is not always possible, as it might have migrated too distally or into the left pulmonary artery, which is not visible to TEE. Differentiation of acute vs. chronic RV dilatation requires advanced skills; however, a normal RV size and function is unlikely to be present with a hemodynamically significant PE [6••].
Assessment of Extra-cardiac Structures
Lung Ultrasonography
TEE has been used to image the lungs and pleura during surgical procedures and in the evaluation of acute respiratory deterioration in critically ill patients, where transthoracic ultrasonography is either suboptimal or not available [32••].
Just as in surface lung ultrasound, lung assessment with TEE is based on accurate images of true lung tissues and interpretation of artifacts; TEE may be used to assess four main lung diagnosis: pleural effusions, lung consolidation, alveolar-interstitial syndrome, and pneumothorax, as well as for monitoring of extravascular lung water (EVLW) to guide fluid/diuretic therapy [32••, 33•].
Pleural effusions can be detected with high sensitivity and specificity. Effusions as small as125 ml can be easily visualized, especially on the left side due to the aortic acoustic window (descending aorta SAX view). Right-sided effusions and pathologies can also be visualized but with less sensitivity due to the acoustic shadow created by the vertebral bodies [32••]. A semi-quantitative evaluation of pleural effusion can be done manually, tracing the cross-sectional area of the effusion (CSAmax) in the transverse plan. This quantifies the effusion as small (<400 mL, CSA <20 cm2), moderate (400–1200 mL; 20–40 cm2), or severe (>1200 mL; >40 cm2) [32••].
The lung consolidation due to pneumonia, atelectasis, pulmonary contusion, neoplasm, or pulmonary infarction are characterized on lung ultrasound by increased density of B-lines and by absence of alveolar air producing a tissue-like echotexture. B-lines are hyperechoic vertical linear artifacts generated in the pleural interface or subpleural tissue which move in tandem with the parenchymal pleura [32••]. In the most severe cases of consolidation and atelectasis, the lung can take on a sonographic texture closely resembling that of the liver.
Pulmonary edema or fibrosis resulting in interstitial thickening, increase in hydrostatic capillary pressure, (as in left ventricular failure), or in capillary permeability (as in ARDS) will all result into multiple vertical B-lines, whose number seems to be proportional to EVLW [32••]. The pattern and the location will suggest a global or localized process.
It is highly unlikely that TEE would be able to detect the typical ultrasonographic signs of a pneumothorax (absence of lung sliding, B-lines, and lung pulse); however, in case of tension pneumothorax collapse of the right atrium or diastolic obstruction of the right ventricular outflow tract, may be noticed [32••].
Renal Perfusion
Early acute kidney injury may be detected by ultrasound assessment of renal artery flow, with high sensitivity and specificity [34]. From the SAX view of the descending aorta, advancing the probe 4–6 cm with the aorta in view, the origin of the left renal artery can be found and followed. Right rotation of the probe by approximately 90° will move the posteriorly directed beam to the left, allowing visualization of the left kidney. Color-flow Doppler (CFD) at a Nyquist limit of 15–20 cm/s can then be used to interrogate blood flow in the left renal vessels. Two indices can be calculated on the main renal artery by pulsed-wave Doppler: resistive index (RI; [peak systolic velocity−minimum diastolic velocity]/peak systolic velocity; normal range 0.64–0.70) and the pulsatility index (PI; [peak systolic velocity−minimum diastolic velocity]/mean velocity; normal range 0.93–1.25). Increased resistance to flow distal to the point of measurement causes an increase in the pulse pressure relative to the peak and mean velocities, yielding higher PI and RI values. In one study, RI values >0.79 measured postoperatively predicted onset of AKI, with values >0.83 predicting the need for dialysis [34]. Other causes for elevated PI and RI include vasoconstriction, bradycardia, hypo- or hypertension, chronic renal failure, hydronephrosis, renal vein or artery thrombosis/stenosis and significant aortic regurgitation [34].
Imaging of Epidural Space
Transesophageal echocardiography has been used to identify spinal canal structures and epidural catheter position in adult patients [35].
To visualize the spinal canal structures, the probe is advanced to obtain a SAX view of the descending thoracic aorta, an additional rotation of 10–20° allows the visualization of the vertebral bodies. Gentle withdrawal of the probe allows scanning through the gelatinous intervertebral disks to obtain a window to the spinal canal [35].
This technique has been used to locate the position of epidural catheters.
Rescue TEE in Pre or Cardiac Arrest
TEE can be performed in course of CPR in intubated patients, without interfering with chest compressions and defibrillation. The high feasibility (98%) combined with the ability to image continuously with quality that is superior to TTE and documented impact on diagnostic and therapeutic decisions (65–80% in published retrospective reviews) make TEE an essential tool in emergency situations [10]. The most common indications of TEE as a rescue tool among intubated patients are cardiac arrest, post arrest management, and undifferentiated hypotension [10]. Basic TEE has demonstrated the ability to identify common causes of hemodynamic instability and cardiac arrest such as massive pulmonary embolism, cardiac tamponade, severe hypovolemia, and ventricular failure, and to guide appropriate therapeutic interventions [2].
Specific Surgical Scenarios
Liver Transplant
During liver transplantation, patients invariably undergo major hemodynamic derangement possibly leading to tissue hypoperfusion. Despite the high incidence of esophageal varices in liver transplant patients, TEE has been found to be relatively safe. For patients at higher risk of hemodynamic compromise, TEE should not be viewed as absolutely contraindicated and risk-benefit balance should be carefully assessed [36, 37]. TEE has the ability to provide real-time information on cardiac function, contractility, and volume status at all stages of liver transplantation and to identify the etiology of hemodynamic compromise [8••, 36]. Common liver transplantation complications detected by TEE include development of pulmonary hypertension, intracardiac thrombosis, pulmonary embolism, myocardial ischemia, cardiac tamponade, IVC compression or obstruction, acute right heart failure, and systolic anterior motion of the anterior MV leaflet [8••]. For these reasons, TEE is a standard of care in many centers for intraoperative hemodynamic monitoring of liver transplant patients.
Kidney Transplant
The recommendations from the ASE for the use of TEE in patients undergoing kidney transplantation are limited to patients with coexisting cardiovascular disease [8••]. TEE is recommended to evaluate LV and RV systolic and diastolic function in these patients [8••].
Lung Transplant
TEE is recommended in lung transplantation to identify different causes of hypotension and hypoxia [2]. During off pump and after lung transplantation, TEE assessment of right ventricular function allows pre-emptive initiation of inotropic support and inhaled pulmonary vasodilators [8••, 38]. TEE also allows intraoperative assessment of surgical anastomoses [39].
Identification of intracardiac shunts is also critical in the differential diagnosis of hypoxia. TEE is also recommended for similar reasons for elective pulmonary endarterectomy [2, 40].
Vascular Surgery
Dynamic changes during clamping of major vessels such aorta or vena cava in major vascular surgery cases often lead to end-organ hypoperfusion, systolic and diastolic dysfunction, or myocardial ischemia. TEE is more sensitive than PA catheters in detecting changes in different hemodynamic parameters such as CO, LV ejection fraction, LV end-diastolic dimension, and regional wall motion abnormalities (in the transgastric SAX view) [8••, 40]. Resection of renal cell carcinomas (RCC) with tumor thrombus invasion into the inferior vena cava (IVC) is another case where TEE is of critical importance for the perioperative management, providing real-time surveillance of the proximal extension of the thrombus and surgical guidance in both the intra-abdominal and supra-diaphragmatic resection, guiding need for cardiopulmonary bypass, IVC snaring, and complete removal of the thrombus [41]. TEE provides high quality real-time imaging of the descending thoracic aorta. It is more accurate than angiography in detecting minor aortic injury and entry point of aortic dissection and can possibly detect end-organ flow [42,42,44]. TEE is used to guide endovascular stent placement and is more sensitive than angiography in detecting endo-leaks when contrast is used [45,45,47].
Orthopedic Surgery
Intraoperative TEE can be used to monitor cement and fat pulmonary microemboli and intracardiac shunting during hip arthroplasty, spinal surgery, and knee arthroplasty; however, its routine use is not supported by current guidelines given the relatively low incidence of these complications [11••]. TEE remains a rescue option in case of hemodynamic collapse [8••]. Right ventricle dysfunction due to an acute increase in pulmonary vascular resistance can be assessed by 2D TEE using TAPSE or FAC and by Doppler, using pulmonic valve VTI and peak tricuspid regurgitant jet velocity measurements. Intracardiac shunting through a patent foramen ovale can be detected by color-flow Doppler [8••].
Neurosurgery
TEE is recommended as a monitoring tool during craniotomies in the sitting position [11••]. Due to the common occurrence of venous air embolism during these procedures, basic TEE provides real-time visual surveillance and quantification of air embolism [2, 6••, 8••]. Screening for a patent foramen ovale (a relatively common finding), by evaluation of the interatrial septum using color-flow Doppler and agitated saline contrast, is instrumental in decreasing the risk of paradoxical air embolism [8••]. Changes secondary to venous air embolic load can also be monitored using Doppler to assess the right-sided pulmonary pressures and 2D to assess the right ventricular function [48]. Extreme precautions are needed to prevent the known complications of TEE during the sitting positions, such as posterior tongue edema or necrosis [17].
Extracorporeal Membrane Oxygenation
Although there are currently no guidelines with respect to the optimal monitoring of patients on extracorporeal membrane oxygenators (ECMO), TEE plays a crucial role in every step of ECMO support. It allows confirmation of the underlying diagnosis, supporting the choice between veno-venous (VV) and veno-arterial (VA) ECMO. It provides guidance at the time of cannulation and also detects cannula dislodgement or obstruction, provides guidance during the weaning phase in detecting and quantifying myocardial recovery [49].
Training and Certification
Competent performance of intraoperative TEE requires a combination of cognitive, perceptual, and motor skills including understanding of echocardiographic technology, knowledge of anatomy and pathophysiology, image acquisition and optimization, artifact recognition, and image interpretation [1••]. Several pathways for certification in intraoperative TEE have been suggested in North America and Europe [6••].
All require a component of supervised training, a portfolio of studies personally performed, a written exam, or in some cases, the completion of a full year of a specific anesthesia fellowship [2]. Given the time commitment required and the fact that certification is not required in all jurisdictions, formal training, maintenance of certification, and quality assurance for all anesthesiologists performing TEE remains a significant challenge in many centers.
Conclusion
TEE is a powerful intraoperative imaging modality with potentially critical clinical impact in the management of surgical patients. While a detailed TEE examination is required for comprehensive assessment of cardiac structure and function, basic exam is recommended for hemodynamic monitoring and is adequate for most non-cardiac surgical scenarios. TEE provides guidance during vascular cannulation, quantitative hemodynamic monitoring, as well as diagnosis in most acute cases of hemodynamic instability and hypoxemia. Furthermore, it is useful in the detection of intracardiac thrombi and air emboli and in the assessment of surgical anastomoses in lung transplantation.
Newer technologies may bring the use of 3D TEE to less expert users and allow accurate automated quantitative chamber quantification.
The decreasing cost of equipment, the introduction of simplified scanning protocols, and the introduction of mandatory training in point of care ultrasound in postgraduate curricula will likely contribute to spread the use of this tool for intraoperative monitoring during non-cardiac surgery.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26(9):921–64. Last recommended comprehensive transesophageal echocardiographic examination by the ASE and SCA
Meineri M. Transesophageal echocardiography: what the anesthesiologist has to know. Minerva Anestesiol. 2016;82(8):895–907.
Jha AK, Malik V, Hote M. Minimally invasive cardiac surgery and transesophageal echocardiography. Ann Card Anaesth. 2014;17(2):125–32.
• Jasudavisius A, Arellano R, Martin J, McConnell B, Bainbridge D. A systematic review of transthoracic and transesophageal echocardiography in non-cardiac surgery: implications for point-of-care ultrasound education in the operating room. Can J Anaesth. 2016;63(4):480–7. Systematic review examining the use of TTE or TEE in non-cardiac surgery
• Meersch M, Schmidt C, Zarbock A. Echophysiology: the transesophageal echo probe as a noninvasive Swan-Ganz catheter. Curr Opin Anaesthesiol. 2016;29(1):36–45. Transesophageal echocardiography as valid alternative to Swan–Ganz catheters in the hemodynamic assessment of patients in the perioperative period.
•• Reeves ST, Finley AC, Skubas NJ, Swaminathan M, Whitley WS, Glas KE, et al. Special article: basic perioperative transesophageal echocardiography examination: a consensus statement of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2013;117(3):543–58. Last basic Perioperative Transesophageal Echocardiography Examination consensus of the ASE and SCA.
• Mahmood F, Shernan SK. Perioperative transoesophageal echocardiography: current status and future directions. Heart. 2016;102(15):1159–67. Intraoperative guidance provided by TEE in minimally invasive structural heart disease interventions, as a critical component in cardiac anaesthesiologist’s skill sets.
•• Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ, et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28(1):40–56. Guidelines of the ASE of the role of echocardiographic monitoring in guiding management of pulmonary emboli, pericardial effusions, thrombosed prosthetic valves, and acute heart failure management.
Vieillard-Baron A, Slama M, Mayo P, Charron C, Amiel JB, Esterez C, et al. A pilot study on safety and clinical utility of a single-use 72-hour indwelling transesophageal echocardiography probe. Intensive Care Med. 2013;39(4):629–35.
Arntfield R, Pace J, Hewak M, Thompson D. Focused transesophageal echocardiography by emergency physicians is feasible and clinically influential: observational results from a novel ultrasound program. J Emerg Med. 2016;50(2):286–94.
•• Echocardiography ASoAaSoCATFoT. Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112(5):1084–96. Determination of the appropriate application of TEE and improvement of the outcomes of surgical patients by definition of the utility of perioperative TEE based on the strength of supporting evidence.
•• Rebel A, Klimkina O, Hassan ZU. Transesophageal echocardiography for the noncardiac surgical patient. Int Surg. 2012;97(1):43–55. Value of perioperative TEE for patients undergoing noncardiac surgery, providing life-saving information very accurately and quickly.
Flachskampf FA, Badano L, Daniel WG, Feneck RO, Fox KF, Fraser AG, et al. Recommendations for transoesophageal echocardiography: update 2010. Eur J Echocardiogr. 2010;11(7):557–76.
Flachskampf FA, Wouters PF, Edvardsen T, Evangelista A, Habib G, Hoffman P, et al. Recommendations for transoesophageal echocardiography: EACVI update 2014. Eur Heart J Cardiovasc Imaging. 2014;15(4):353–65.
Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):567–81.
Markin NW, Gmelch BS, Griffee MJ, Holmberg TJ, Morgan DE, Zimmerman JM. A review of 364 perioperative rescue echocardiograms: findings of an anesthesiologist-staffed perioperative echocardiography service. J Cardiothorac Vasc Anesth. 2015;29(1):82–8.
Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D'Ambra MN, Eltzschig HK. Safety of transesophageal echocardiography. J Am Soc Echocardiogr. 2010;23(11):1115–27. quiz 220-1
Sainathan S, Andaz S. A systematic review of transesophageal echocardiography-induced esophageal perforation. Echocardiography. 2013;30(8):977–83.
Nyhsen CM, Humphreys H, Nicolau C, Mostbeck G, Claudon M. Infection prevention and ultrasound probe decontamination practices in Europe: a survey of the European Society of Radiology. Insights Imaging. 2016;7(6):841–7.
Carrasco Del Castillo JL, Bussières J, Rochon AG, Denault AY. Guidewire localization with transesophageal echocardiography: do not forget the left side. Can J Anaesth. 2012;59(8):811–2.
• Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, et al. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30(9):1734–9. SVC collapsibility as a new index of fluid responsiveness obtainable during routine bedside echocardiography in mechanically ventilated patients.
Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119(3):867–73.
Slama M, Maizel J. Pulse pressure variations in acute respiratory distress syndrome: “Fifty Shades of Grey”. Crit Care Med. 2016;44(2):452–3.
Mahjoub Y, Pila C, Friggeri A, Zogheib E, Lobjoie E, Tinturier F, et al. Assessing fluid responsiveness in critically ill patients: false-positive pulse pressure variation is detected by Doppler echocardiographic evaluation of the right ventricle. Crit Care Med. 2009;37(9):2570–5.
•• Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. quiz 86–8. Echocardiographic assessment of the right ventricle guidelines as per the ASE.
•• Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–70. Summary of the cardiac chamber quantification by echocardiography in adults by the EACI.
Milan A, Magnino C, Veglio F. Echocardiographic indexes for the non-invasive evaluation of pulmonary hemodynamics. J Am Soc Echocardiogr. 2010;23(3):225–39. quiz 332-4
Tsang W, Salgo IS, Medvedofsky D, Takeuchi M, Prater D, Weinert L, et al. Transthoracic 3D echocardiographic left heart chamber quantification using an automated adaptive analytics algorithm. JACC Cardiovasc Imaging. 2016;9(7):769–82.
Cabrera Schulmeyer MC, Arriaza N. Good prognostic value of the intraoperative tissue Doppler-derived index E/e' after non-cardiac surgery. Minerva Anestesiol. 2012;78(9):1013–8.
Swaminathan M, Nicoara A, Phillips-Bute BG, Aeschlimann N, Milano CA, Mackensen GB, et al. Utility of a simple algorithm to grade diastolic dysfunction and predict outcome after coronary artery bypass graft surgery. Ann Thorac Surg. 2011;91(6):1844–50.
Alexander BL, Skubas NJ. Diagnosis of cardiac rhythm with transmitral flow. Anesth Analg. 2014;118(3):521–4.
•• Cavayas YA, Girard M, Desjardins G, Denault AY. Transesophageal lung ultrasonography: a novel technique for investigating hypoxemia. Can J Anaesth. 2016;63(11):1266–76. Novel use of TEE in lung assessment.
• Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–91. Summary of the indications and use of point-of-care lung ultrasound.
Bandyopadhyay S, Kumar Das R, Paul A, Sundar Bhunia K, Roy D. A transesophageal echocardiography technique to locate the kidney and monitor renal perfusion. Anesth Analg. 2013;116(3):549–54.
Goswami V, Kumar B, Puri GD, Singh H. Utility of transesophageal echocardiography in identifying spinal canal structures and epidural catheter position: a prospective observational study of intraoperative hemodynamics and postoperative analgesia. Can J Anaesth. 2016;63(8):911–9.
De Pietri L, Mocchegiani F, Leuzzi C, Montalti R, Vivarelli M, Agnoletti V. Transoesophageal echocardiography during liver transplantation. World J Hepatol. 2015;7(23):2432–48.
Meineri M, Jerath A, Karski J, Vegas A. Intraoperative use of 3D TEE: initial experience. Can J Anesth. 2009;56:S37.
Sullivan B, Puskas F, Fernandez-Bustamante A. Transesophageal echocardiography in noncardiac thoracic surgery. Anesthesiol Clin. 2012;30(4):657–69.
González-Fernández C, González-Castro A, Rodríguez-Borregán JC, López-Sánchez M, Suberviola B, Francisco Nistal J, et al. Pulmonary venous obstruction after lung transplantation. Diagnostic advantages of transesophageal echocardiography. Clin Transpl. 2009;23(6):975–80.
Ashes C, Roscoe A. Transesophageal echocardiography in thoracic anesthesia: pulmonary hypertension and right ventricular function. Curr Opin Anaesthesiol. 2015;28(1):38–44.
Fukazawa K, Gologorsky E, Naguit K, Pretto EA, Salerno TA, Arianayagam M, et al. Invasive renal cell carcinoma with inferior vena cava tumor thrombus: cardiac anesthesia in liver transplant settings. J Cardiothorac Vasc Anesth. 2014;28(3):640–6.
Goarin JP, Catoire P, Jacquens Y, Saada M, Riou B, Bonnet F, et al. Use of transesophageal echocardiography for diagnosis of traumatic aortic injury. Chest. 1997;112(1):71–80.
Taams MA, Gussenhoven WJ, Schippers LA, Roelandt J, van Herwerden LA, Bos E, et al. The value of transoesophageal echocardiography for diagnosis of thoracic aorta pathology. Eur Heart J. 1988;9(12):1308–16.
Orihashi K, Matsuura Y, Sueda T, Shikata H, Morita S, Hirai S, et al. Abdominal aorta and visceral arteries visualized with transesophageal echocardiography during operations on the aorta. J Thorac Cardiovasc Surg. 1998;115(4):945–7.
Rapezzi C, Rocchi G, Fattori R, Caldarera I, Ferlito M, Napoli G, et al. Usefulness of transesophageal echocardiographic monitoring to improve the outcome of stent-graft treatment of thoracic aortic aneurysms. Am J Cardiol. 2001;87(3):315–9.
Dobson G, Maher N, Ball M, Kryski A, Moore R. Images in anesthesia: echo contrast as an adjunct to intraoperative angiography in the detection of endoleaks. Can J Anaesth. 2006;53(5):516–7.
Fattori R, Caldarera I, Rapezzi C, Rocchi G, Napoli G, Parlapiano M, et al. Primary endoleakage in endovascular treatment of the thoracic aorta: importance of intraoperative transesophageal echocardiography. J Thorac Cardiovasc Surg. 2000;120(3):490–5.
Feigl GC, Decker K, Wurms M, Krischek B, Ritz R, Unertl K, et al. Neurosurgical procedures in the semisitting position: evaluation of the risk of paradoxical venous air embolism in patients with a patent foramen ovale. World Neurosurg. 2014;81(1):159–64.
Douflé G, Roscoe A, Billia F, Fan E. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care. 2015;19:326.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jacobo Moreno Garijo, Azad Mashari, and Massimiliano Meineri declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
The original version of this article was revised: The original version of this article unfortunately contained a mistake. The reference citation “[4•]” under the sections “Central Venous Pressure”, “Pulmonary Artery Pressures”, and “Pulmonary Capillary Wedge Pressure” should be reference number “[5•].”
This article is part of the Topical Collection on Cardiovascular Anesthesia
Rights and permissions
About this article
Cite this article
Garijo, J.M., Mashari, A. & Meineri, M. Role of Transesophageal Echocardiography in General Anesthesia. Curr Anesthesiol Rep 7, 273–282 (2017). https://doi.org/10.1007/s40140-017-0221-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-017-0221-x