Abstract
Breast MRI is a highly sensitive imaging tool for breast cancer detection. In general clinical practice, breast MRI utilizes a limited dynamic contrast-enhanced (DCE) acquisition that provides morphologic and semi-quantitative kinetic information to allow characterization of breast findings. This approach provides sensitivities approaching 100 % for breast cancer detection; however, its specificity remains moderate due to limited ability to differentiate benign pathologic processes that enhance from malignancies. Several advanced MRI techniques, such as high-temporal resolution DCE that allows robust quantitative pharmacokinetic analysis, diffusion-weighted imaging that allows microstructural characterization, and MR spectroscopy that reflects chemical composition, hold promise to improve standard breast MRI specificity and to serve as imaging biomarkers that can guide treatment decisions. In this review article, we review recent updates in advanced breast MRI applications, including their potential clinical uses and challenges to implementation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite advances in early detection and treatment, breast cancer remains the second leading cause of cancer death in US women [1]. As a result, there is a strong clinical need to develop advanced imaging techniques that can improve detection and characterization of breast cancer and thereby further decrease associated mortality and morbidity. Due to its excellent sensitivity for breast cancer detection, breast MRI has gained clinical acceptance as an imaging tool useful for a range of clinical scenarios, including supplemental screening for women at high risk of developing breast cancer, pre-operative evaluation of extent of newly diagnosed breast cancer, evaluation and management of indeterminate findings identified on standard imaging and/or clinical exam, and assessment of breast cancer response to neoadjuvant chemotherapy. However, standard contrast-enhanced MRI provides only modest specificity, therefore exposing many patients to unnecessary biopsies and limiting its clinical use and acceptance across institutions. In this review article, we review the current evidence for advanced breast MRI applications, including dynamic contrast-enhanced (DCE) pharmacokinetic (PK) modeling, diffusion-weighted imaging (DWI), and MR spectroscopy (MRS), to improve standard contrast-enhanced breast MRI performance and remove barriers to more widespread use.
Current Clinical Indications for Clinical Breast MRI
The clinical indication for breast MRI based on the highest level of evidence is for supplemental screening (in addition to mammography) of asymptomatic women at elevated risk of developing breast cancer. Multiple single and multi-institution trials have found that breast MRI provides superior sensitivity for breast cancer detection when compared to mammographic and sonographic imaging in high-risk women [2–5]. Although more controversial [6], MRI also is commonly utilized to pre-operatively assess women newly diagnosed with breast cancer for the presence of additional breast cancers that are mammographically and clinically occult in both the ipsilateral [7] and contralateral breasts [3].
Other diagnostic uses of breast MRI include assessment of the effectiveness of neoadjuvant therapy and as a “problem solving” tool for further evaluation of equivocal imaging or clinical exam findings. Multiple studies have shown MRI to be a valuable tool to assess response to therapy, including prediction of pathologic complete response (pCR) [8–12]. However, its clinical impact for guiding chemotherapy regimens remains limited by high cost and modest overall performance in predicting meaningful clinical outcomes. Similarly, the practice to utilize MRI to evaluate equivocal findings in order to avoid unnecessary biopsies has not yet been proven to be cost-effective [13]. Additionally, current practice guidelines based on the American College of Radiology Breast Imaging Reporting and Data System require a negative predictive value of ~98 % [14], which to date has not been shown to be achievable with standard contrast-enhanced breast MRI.
Advanced Breast MRI Techniques
A multiparametric approach to breast MRI (Fig. 1) incorporating advanced techniques, such as high-temporal resolution DCE-MRI with quantitative PK modeling, DWI, and MRS, have the potential to provide imaging assays of specific biological features such as abnormal vessel permeability, cellularity, and chemical composition, which may address some of the shortcomings of routine clinical breast MRI.
Multiparametric MRI examination in a 51-year-old woman with invasive ductal carcinoma (Grade 3, ER+/PR+/HER2+) prior to therapy. a Post-contrast T1-weighted image from the DCE-MRI sequence. b K trans map for tumor region. c Single-voxel 1H-MRS tumor spectrum showing elevated choline at 3.2 ppm. d ADC map from DWI showing restricted diffusion (low ADC) in the tumor region
Dynamic Contrast-Enhanced (DCE) MRI
DCE-MRI approaches with high-temporal resolution can allow for more sophisticated contrast kinetic assessments through PK modeling versus the semi-quantitative measurements typical of routine clinical MRI. Such PK modeling assesses gadolinium contrast exchange between the intravascular and the interstitial spaces, and the model parameters can provide surrogate measurements of capillary permeability and tumor blood flow. Currently, the most common approach to PK modeling for breast MRI is the two-compartment (Tofts) model, which measures the exchange of contrast between breast tissue and the plasma space [15]. Using such PK models, physiologic metrics can be derived such as the volume transfer constant, K trans, which reflects the rate of transfer of gadolinium from plasma to the tissue, the transfer rate constant, k ep, which reflects the reflux of contrast agent from the extravascular extracellular space to the plasma compartment, and the fractional volume of the extravascular extracellular space, v e. An example of PK modeling and resulting K trans map for a breast cancer is provided in Fig. 2.
Dynamic contrast-enhanced (DCE) MRI in a subject with invasive ductal carcinoma. a Representative post-contrast image from a high-temporal resolution DCE-MR scan using a dynamic 4D THRIVE (Philips Healthcare, Best, The Netherlands) acquisition (15 s scan; 1 × 1 × 2 mm resolution). b Percent enhancement curve over time shows contrast dynamics for a region of interest in the invasive tumor. c Two-compartment model used for pharmacokinetic analysis, where K trans reflects the rate contrast leaves the plasma space and K trans/V e = k ep reflects the rate contrast returns to the plasma space. d Resulting color encoded K trans map from high-temporal resolution scan showing higher levels of capillary permeability in lesion
Multiple single-institution studies have shown promise in applying these PK parameters, particularly K trans, to improve the positive predictive value of standard breast MRI and as a biomarker of disease subtypes [16–22]. Huang et al. demonstrated that in lesions found to be suspicious on standard clinical breast MRI, a K trans “cutoff” value could be used such that lesions with lower K trans values could avoid biopsy and thereby decrease false positive MR examinations [17]. More recently, Li and colleagues demonstrated that K trans and k ep values progressively increased when measuring normal glands, benign lesions, and malignant lesions, respectively; also noting that invasive ductal carcinomas and ductal carcinoma in situ lesions exhibited significantly higher K trans and k ep values than ductal dysplasias [18]. Their study also found that these parameter values were higher in malignancies with elevated expression of CD105, a marker of angiogenesis, suggesting that PK measurements have potential to serve as an in vivo biomarker of breast cancer biology. Table 1 summarizes DCE-MRI K trans measures of benign and malignant lesions reported in several recent studies.
Beyond PK measures, other advanced metrics increasingly being explored in DCE-MRI are those related to texture and heterogeneity (e.g., energy, entropy, correlation, difference in variance) and shape and morphology (size, circularity, irregularity, margin sharpness, compactness, spiculation). Mahrooghy et al. found that breast cancer heterogeneity measures correlated with a multigene assay (Oncotype DX Score, Genomic Health) that has been validated to predict recurrence rates after treatment [23]. Also, Wang et al. demonstrated that the use of a computer-aided evaluation software to extract advanced imaging phenotypes along with incorporating PK measurements (k ep and V p), textural and shape analyses (energy and entropy), and automated 3D morphological assessments could differentiate malignant and benign breast lesions on MRI with 91 % accuracy (sensitivity 91 %, specificity 92 %) [22].
Early work assessing the ability of advanced DCE-MRI to determine response to neoadjuvant therapy established the potential of PK measurements to assess the efficacy of pre-operative therapies to achieve pCR. Specifically, K trans values were shown in a meta-analysis to be among the most promising MRI parameters for prediction of near pCR to neoadjuvant chemotherapy, outperforming standard tumor size measurements [24]. Moreover, recent work from Drisis et al. found that for triple-negative breast cancers, which can be more challenging to treat than other breast cancer subtypes due to limited therapeutic options, K trans levels measured at the pre-treatment timepoint provided the most accurate prediction of response to therapy, suggesting that DCE-MRI may play a role in improving treatment strategies for this tumor subtype [25].
One drawback of PK measures is the considerable variability in parameters between centers, which in part results from methodological differences in the PK models used to calculate these features. As a result, some have sought to identify quantitative methods that are less dependent on modeling methodology to predict treatment response [12, 26, 27]. Cho et al. recently reported that a parametric response map analysis based on voxelwise enhancement differences between pre-treatment and post-treatment DCE images has potential for predicting pCR [28]. However, Huang et al. showed that although significant parameter variations are seen when shared DCE-MRI datasets are analyzed using various PK models, each model independently showed excellent performance for predicting breast cancer response, suggesting that these systemic variations may not impact clinical utility [29•].
Nonetheless, consistency among different centers on how to best approach PK modeling remains a significant barrier to widespread implementation of advanced DCE parameters for clinical use. PK modeling requires measurement of both the pre-contrast T1 relaxation time of the tumor or tissue being imaged and the arterial input function (AIF), or the concentration of contrast agent as it changes over time within the arterial blood, which introduces unique challenges and potential for error. T1 mapping requires the acquisition of an additional series of images prior to DCE-MRI, most commonly using varying flip angle or inversion recovery approaches, which is time consuming and can be prone to inaccuracies due to B1 inhomogeneities and/or patient motion. Furthermore, many PK models require that the AIF be measured directly for each subject [30], which is challenging to perform routinely due to the very high temporal resolution required to accurately sample the rapidly changing signal that represents the AIF. An alternate approach that avoids the need for direct calculation of AIF for each subject is to use a population-based mean AIF determined from data where the injection site, dose, and rate were constant [17].
Diffusion-Weighted Imaging
Over the past decade, there has been increasing interest in the use of DWI as a non-contrast MRI technique for oncology applications. DWI measures the ability of water molecules to freely diffuse in tissue, which is impacted by biophysical characteristics such as cell density, membrane integrity, and microstructure. DWI is particularly appealing as an advanced breast MRI application due to its short acquisition time, its wide availability on most commercial MR scanners, and the lack of exogenous contrast agent needed. DWI is increasingly being incorporated into breast MRI protocols due to promising data from multiple single-center studies demonstrating value for the detection and characterization of breast cancer [31].
To date, the greatest evidence for the use of DWI as an advanced MRI application is as an adjunct sequence that can reduce false positives prompted by standard contrast-enhanced breast MRIs and thereby reduce the number of unnecessary biopsies performed. This has been the most widely explored application of DWI for breast imaging, and numerous groups have demonstrated significant differences in DWI apparent diffusion coefficient (ADC) values between benign and malignant lesions [32–35], Fig. 3. A recent meta-analysis of 13 individual studies on DWI diagnostic performance demonstrated pooled sensitivity of 84 % (95 % confidence interval [CI]: 82, 87 %) and specificity of 79 % (95 % CI: 75, 82 %) in discriminating malignant from benign lesions (964 breast lesions total, 615 malignant and 349 benign) [36]. Table 2 summarizes ADC measures reported for benign and malignant breast lesions in several recent studies. Further, multiple studies across a variety of field strengths have found that ADC measures are complementary to DCE-MRI parameters for discriminating benign and malignant breast lesions and can increase the accuracy of conventional breast MRI assessment [37–40]. More recently, Bickelhaupt et al. reported that DWI may also be useful as a fast and non-invasive approach to assess the likelihood of malignancy for suspicious lesions detected on screening X-ray mammograms and reduce unnecessary biopsies [41]
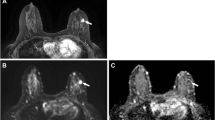
Diffusion-weighted MRI (DWI) for characterization of suspicious lesions found on dynamic contrast-enhanced (DCE) MRI. (Top) Malignant invasive ductal carcinoma in a 46-year-old woman. The lesion brightly enhanced on T1w DCE-MRI and exhibited restricted diffusion on DWI with low apparent diffusion coefficient (ADC) of 0.92 × 10−3 mm/s2. (Bottom) Benign fibroadenoma in a 51-year-old woman. The lesion brightly enhanced on T1w DCE-MRI but did not exhibit restricted diffusion on DWI, with ADC of 1.91 × 10−3 mm/s2
Others have explored DWI as a way to improve conventional MRI’s ability to assess response to neoadjuvant chemotherapy. Considering that the cytotoxic effects of chemotherapy can lead to a less restrictive environment for water to freely diffuse, it has been proposed that an increase in ADC values may be highly predictive of favorable treatment response. Indeed, several preliminary clinical studies have shown changes in tumor ADC due to neoadjuvant treatments may provide valuable early indication of treatment efficacy [42, 43]. Furthermore, it has been proposed that ADC values on pre-treatment MRIs can be used to identify patients who will best respond to neoadjuvant treatments. In a recent study of 118 women undergoing neoadjuvant chemotherapy for locally advanced breast cancer, Richard et al. found that lower pre-treatment tumor ADC values were predictive of pathological response, particularly for triple-negative tumors [44].
Finally, DWI has shown promise to serve as a non-contrast screening tool that is supplemental to mammography. Prior studies have shown that many mammographically and clinically occult breast cancers are visible on DWI with corresponding low ADC values [45]. Yabuuchi et al. demonstrated that using a non-contrast MRI approach with DWI can achieve a higher accuracy for breast cancer detection than mammography [46]. To date, there are limited studies exploring the potential role of DWI as a non-contrast alternative for breast MR screening, but promising preliminary data warrant further investigation [46, 47, 48••].
Although widespread clinical implementation of DWI for breast imaging applications has been limited in part by technical issues inherent to echo planar imaging technique (e.g., low spatial resolution, spatial distortions, and detrimental artifacts due to suboptimal fat suppression and susceptibility effects at air–tissue interfaces), a number of compelling advancements in DWI acquisition strategies are under development to overcome these technical challenges [49–53].
In addition, while data from early DWI trials are encouraging for improving the performance of conventional breast MRI, several advanced DWI approaches are also being investigated to extract additional biological information from breast DWI scans. These include intravoxel incoherent motion (IVIM) modeling, which provides tissue perfusion characterization (evident at low b-values < 200 s/mm2) [54–57]; diffusion kurtosis modeling, which describes tissue complexity and physical barriers to diffusion (evident at high b-values > 1500 s/mm2) [58••, 59, 60]; and diffusion tensor imaging, which assesses water diffusion directionality to probe glandular organization (ducts, lobules) and microarchitecture [61–63]. Iima et al. recently demonstrated in a pilot study using a novel DWI approach, incorporating a 16 b-value acquisition and combined IVIM and kurtosis modeling, that the perfusion fraction [f] and kurtosis [K] measures obtained were significantly higher in malignant breast lesions than in benign lesions, providing additional parameters that could be used to improve the positive predictive value of MRI [58••]. Bokacheva et al. also showed that IVIM parameters of increased f and decreased pseudodiffusion [D] are useful for discriminating malignancy from benign or normal breast tissue [54]. Further, Sun et al. recently reported that diffusion kurtosis measures can increase diagnostic accuracy over conventional ADC measures for assessing breast lesions and are significantly associated with histologic grade and proliferative activity (Ki-67 expression) in invasive tumors [60].
Magnetic Resonance Spectroscopy
Another advanced MR technique actively investigated for breast cancer detection and characterization is magnetic resonance spectroscopy (MRS). MRS provides spatially localized spectra representing the structure and concentration of different chemical compounds in that region. Proton MRS (1H MRS) studies of the breast have shown the metabolite choline to be highly elevated in malignant lesions compared with benign lesions and normal breast tissue [64–67]. Choline is known to be involved in cell membrane turnover (phospholipid synthesis and degradation) and is therefore generally considered a marker of cell proliferation.
Most breast MRS studies to date have utilized a single-voxel acquisition approach, which produces a single spectrum representing the average signal from a 3-dimensional voxel. Alternative approaches of chemical-shift imaging or MR spectroscopic imaging (MRSI) produce a spatially resolved grid of spectra for a larger volume of tissue. A variety of analysis approaches have been used to characterize the total choline (tCho) signal in breast MRS spectra, including qualitative assessment of the presence or absence of a tCho peak, semi-quantitative measurement of tCho signal-to-noise ratio (SNR), peak height or peak integral, and absolute quantification of tCho concentration (using internal water referencing or external phantom referencing techniques) [68].
MRS has demonstrated potential for both improving diagnostic accuracy of breast MRI and for providing prognostic biomarkers. 1H-MRS measures of choline levels in suspicious breast lesions have distinguished benign from malignant lesions with high specificity [69–71]. In a recent meta-analysis of 19 breast MRS studies, Baltzer and Dietzel found a pooled sensitivity of 73 % (95 % CI: 85, 91 %) and specificity of 88 % (95 % CI: 64, 82 %) for diagnosis of a total of 1198 lesions (773 malignant, 452 benign) [72•]. Table 3 summarizes absolute choline measures for benign and malignant lesions reported in several breast MRS studies evaluating suspicious breast lesions. Dorrius et al. showed that a quantitative MRSI approach could differentiate benign and malignant lesions and improve diagnostic accuracy of conventional contrast-enhanced MRI in the assessment of breast lesions ≥1 cm, Fig. 4. Their findings suggested that implementing a maximum lesion tCho concentration threshold of >1.5 mM to indicate malignancy could avoid biopsy of benign lesions while not missing any cancers [73, 74].
MR spectroscopic imaging in a 53-year-old patient with adenocarcinoma in the left breast. a Volume of interest (36 voxels of 0.25 cm3 each) centered on the lesion. b Unsuppressed spectral map showing water and fat peaks in the lesion and surrounding tissue. c After application of water and fat suppression, an intense Cho peak is seen on the summed tumor spectra, where the fit for choline is shown in red (range 2–4.5 ppm). d The choline map shows heterogeneity of choline concentration throughout the tumor with highest choline levels shown in red. Adapted from Sijens PE, Dorrius MD, Kappert P, Baron P, Pijnappel RM, Oudkerk M. Quantitative multivoxel proton chemical shift imaging of the breast. Magn Reson Imaging. 2010 Apr;28(3):314–9; with permission (Color figure online)
Breast MRS measures may also help distinguish disease subtypes based on metabolic differences. Evaluation of 184 breast cancer patients using single-voxel 1H-MRS at 1.5T, Shin et al. found that tumor tCho measures were higher in invasive versus in situ cancers, and correlated with several prognostic factors including nuclear grade, histologic grade, and estrogen receptor (ER) status [75].
Beyond lesion characterization, breast MRS may also provide an early predictive marker of treatment response. Treatment-induced reductions in cell proliferation may be reflected by alterations in tumor choline levels on MRS before any detectable changes in tumor size. Compelling results from Meisamy and colleagues [76, 77] using a 4T scanner showed that acute decreases in tumor tCho concentration levels were measurable within 24 to 48 h after the first dose of chemotherapy and correlated with final post-treatment changes in tumor size. Numerous other groups have investigated MRS for monitoring neoadjuvant therapy and many have also reported significant associations between tumor tCho levels and response to therapy, with early tCho decreases generally predictive of better pathologic and/or clinical response [78–81]. However, in a recent review of 15 single-center breast MRS studies, Leong et al. found that study designs and approaches for MRS acquisition, choline quantitation, and response determination varied widely, making comparisons of the findings across studies difficult [82].
Despite the valuable metabolic information that can be obtained, there are both logistical and technical challenges that limit routine clinical use of MRS of the breast. Breast MRS data quality is very susceptible to B0 inhomogeneities, which reduce SNR and ability to separate different spectral peaks and also negatively impact the performance of chemically selective fat and water suppression. Inadequate fat suppression is also a common issue in breast imaging that results in lipid contamination obscuring the choline signal. [68] While most prior studies have been performed on 1.5T MR scanners, increases in SNR and spectral resolution at higher field strengths may overcome some of the current clinical limitations of breast MRS by improving choline detectability, decreasing measurement errors, and enabling the assessment of smaller lesions [76].
Multivoxel MRS approaches, such as that shown in Fig. 3, also hold strong advantages over single-voxel MRS for improving the clinical utility of breast MRS. Perhaps most importantly, MRSI provides wider coverage and thus reduces the need for a priori knowledge of lesion location and real-time expertise for voxel placement during the MR scan. MRSI further provides the ability to spatially map tCho distributions, enabling assessment of multiple lesions simultaneously, characterization of tumor heterogeneity, and assessment of the extent of disease infiltration into surrounding tissue [83]. However, longer scan time requirements and greater technical challenges with regard to shimming and fat suppression versus single-voxel MRS have impeded widespread implementation of MRSI. Advancements in MRI hardware and software may facilitate expanded the use of this approach in clinical and research settings [73, 81, 84–87].
31-Phosphorus MRS (31P MRS) also holds promise as an alternative approach to overcome some of the current challenges of breast MRS. 31P MRS enables direct measurement of phosphocholine and avoids issues of lipid contamination commonly present in breast 1H MRS signal. Due to the low abundance of phosphorus in the body, 31P MRS becomes more feasible at high field strengths, as recently demonstrated in two breast studies performed at 7T [88, 89•] .
Conclusion
Advanced applications of breast MRI, including DCE with PK modeling, DWI, and MRS, continue to show potential to improve the specificity and positive predictive value of breast MRI and to expand its clinical use in both the screening and diagnostic setting. To date, several centers have demonstrated that advanced MRI applications can be implemented into their routine clinical protocol to improve characterization of breast lesions, and thereby to improve the accuracy of conventional breast MRI [90], to predict and monitor response to medical therapies [79, 91, 92], and to discriminate biological subtypes of cancer [89•]. While promising, these advanced applications continue to require multi-center validation, effort to address persistent technical challenges, and standardization of imaging technique and measurement parameters before they can be implemented broadly in clinical practice.
References
Recently published papers that are of particular interest have been highlighted as: • Of importance •• Of major importance
American Cancer Society. Breast cancer facts and figures. Atlanta, GA 2007–2008.
Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. New Engl J Med. 2004;351(5):427–37. doi:10.1056/NEJMoa031759.
Lehman CD, Isaacs C, Schnall MD, Pisano ED, Ascher SM, Weatherall PT, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244(2):381–8.
Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
Warner E, Plewes DB, Hill KA, Causer PA, Zubovits JT, Jong RA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317–25.
Rahbar H, Lehman CD. Rethinking preoperative breast magnetic resonance imaging. JAMA Oncol. 2015;1(9):1226–7. doi:10.1001/jamaoncol.2015.3029.
Schnall MD, Blume J, Bluemke DA, Deangelis GA, Debruhl N, Harms S, et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. J Surg Oncol. 2005;92(1):32–8.
Martincich L, Montemurro F, De Rosa G, Marra V, Ponzone R, Cirillo S, et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83(1):67–76. doi:10.1023/B:BREA.0000010700.11092.f4.
Pickles MD, Lowry M, Manton DJ, Gibbs P, Turnbull LW. Role of dynamic contrast enhanced MRI in monitoring early response of locally advanced breast cancer to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2005;91(1):1–10. doi:10.1007/s10549-004-5819-2.
Rosen EL, Blackwell KL, Baker JA, Soo MS, Bentley RC, Yu D, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181(5):1275–82. doi:10.2214/ajr.181.5.1811275.
Weatherall PT, Evans GF, Metzger GJ, Saborrian MH, Leitch AM. MRI vs. histologic measurement of breast cancer following chemotherapy: comparison with X-ray mammography and palpation. J Magn Reson Imaging. 2001;13(6):868–75.
Hylton NM, Blume JD, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy–results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263(3):663–72. doi:10.1148/radiol.12110748.
Lee CI, Bensink ME, Berry K, Musa Z, Bodnar C, Dann R, et al. Performance goals for an adjunct diagnostic test to reduce unnecessary biopsies after screening mammography: analysis of costs, benefits, and consequences. J Am Coll Radiol. 2013;10(12):924–30. doi:10.1016/j.jacr.2013.09.009.
DeMartini W, Lehman C. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Topics Magn Reson Imaging. 2008;19(3):143–50. doi:10.1097/RMR.0b013e31818a40a5.
Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17(2):357–67.
El Khouli RH, Macura KJ, Kamel IR, Jacobs MA, Bluemke DA. 3-T dynamic contrast-enhanced MRI of the breast: pharmacokinetic parameters versus conventional kinetic curve analysis. AJR Am J Roentgenol. 2011;197(6):1498–505. doi:10.2214/AJR.10.4665.
Huang W, Tudorica LA, Li X, Thakur SB, Chen Y, Morris EA, et al. Discrimination of benign and malignant breast lesions by using shutter-speed dynamic contrast-enhanced MR imaging. Radiology. 2011;261(2):394–403. doi:10.1148/radiol.11102413.
Li L, Wang K, Sun X, Wang K, Sun Y, Zhang G, et al. Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit. 2015;21:376–82. doi:10.12659/MSM.892534.
Ma ZS, Wang DW, Sun XB, Shi H, Pang T, Dong GQ, et al. Quantitative analysis of 3-Tesla magnetic resonance imaging in the differential diagnosis of breast lesions. Exp Ther Med. 2015;9(3):913–8. doi:10.3892/etm.2014.2154.
Schabel MC, Morrell GR, Oh KY, Walczak CA, Barlow RB, Neumayer LA. Pharmacokinetic mapping for lesion classification in dynamic breast MRI. J Magn Reson Imaging. 2010;31(6):1371–8. doi:10.1002/jmri.22179.
Veltman J, Stoutjesdijk M, Mann R, Huisman HJ, Barentsz JO, Blickman JG, et al. Contrast-enhanced magnetic resonance imaging of the breast: the value of pharmacokinetic parameters derived from fast dynamic imaging during initial enhancement in classifying lesions. Eur Radiol. 2008;18(6):1123–33. doi:10.1007/s00330-008-0870-8.
Wang TC, Huang YH, Huang CS, Chen JH, Huang GY, Chang YC, et al. Computer-aided diagnosis of breast DCE-MRI using pharmacokinetic model and 3-D morphology analysis. Magn Reson Imaging. 2014;32(3):197–205. doi:10.1016/j.mri.2013.12.002.
Mahrooghy M, Ashraf AB, Daye D, McDonald ES, Rosen M, Mies C, et al. Pharmacokinetic tumor heterogeneity as a prognostic biomarker for classifying breast cancer recurrence risk. IEEE Trans Bio-Med Eng. 2015;62(6):1585–94. doi:10.1109/TBME.2015.2395812.
Marinovich ML, Sardanelli F, Ciatto S, Mamounas E, Brennan M, Macaskill P, et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast. 2012;21(5):669–77. doi:10.1016/j.breast.2012.07.006.
Drisis S, Metens T, Ignatiadis M, Stathopoulos K, Chao SL, Lemort M. Quantitative DCE-MRI for prediction of pathological complete response following neoadjuvant treatment for locally advanced breast cancer: the impact of breast cancer subtypes on the diagnostic accuracy. Eur Radiol. 2015. doi:10.1007/s00330-015-3948-0.
Li KL, Partridge SC, Joe BN, Gibbs JE, Lu Y, Esserman LJ, et al. Invasive breast cancer: predicting disease recurrence by using high-spatial-resolution signal enhancement ratio imaging. Radiology. 2008;248(1):79–87. doi:10.1148/radiol.2481070846.
Li X, Arlinghaus LR, Ayers GD, Chakravarthy AB, Abramson RG, Abramson VG, et al. DCE-MRI analysis methods for predicting the response of breast cancer to neoadjuvant chemotherapy: pilot study findings. Magn Reson Med. 2014;71(4):1592–602. doi:10.1002/mrm.24782.
Cho N, Im SA, Park IA, Lee KH, Li M, Han W, et al. Breast cancer: early prediction of response to neoadjuvant chemotherapy using parametric response maps for MR imaging. Radiology. 2014;272(2):385–96. doi:10.1148/radiol.14131332.
• Huang W, Li X, Chen Y, Li X, Chang MC, Oborski MJ, et al. Variations of dynamic contrast-enhanced magnetic resonance imaging in evaluation of breast cancer therapy response: a multicenter data analysis challenge. Transl Oncol. 2014;7(1):153–66. This study demonstrates that substantial variations exist in measured pharmacokinetic parameters between models utilized, highlighting the importance for transparency in methodology.
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–32.
Partridge SC, McDonald ES. Diffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applications. Magn Reson Imaging Clin N Am. 2013;21(3):601–24. doi:10.1016/j.mric.2013.04.007.
Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16(2):172–8.
Rubesova E, Grell AS, De Maertelaer V, Metens T, Chao SL, Lemort M. Quantitative diffusion imaging in breast cancer: a clinical prospective study. J Magn Reson Imaging. 2006;24(2):319–24.
Sinha S, Lucas-Quesada FA, Sinha U, DeBruhl N, Bassett LW. In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Magn Reson Imaging. 2002;15(6):693–704.
Woodhams R, Matsunaga K, Kan S, Hata H, Ozaki M, Iwabuchi K, et al. ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci. 2005;4(1):35–42.
Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693. doi:10.1186/1471-2407-10-693.
Ei Khouli RH, Jacobs MA, Mezban SD, Huang P, Kamel IR, Macura KJ, et al. Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging. Radiology. 2010;256(1):64–73. doi:10.1148/radiol.10091367.
Partridge SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol. 2009;193(6):1716–22. doi:10.2214/AJR.08.2139.
Pinker K, Baltzer P, Bogner W, Leithner D, Trattnig S, Zaric O, et al. Multiparametric MR imaging with high-resolution dynamic contrast-enhanced and diffusion-weighted imaging at 7 T improves the assessment of breast tumors: a feasibility study. Radiology. 2015;276(2):360–70. doi:10.1148/radiol.15141905.
Rahbar H, Partridge SC, Demartini WB, Gutierrez RL, Allison KH, Peacock S, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology. 2012;263(2):374–82. doi:10.1148/radiol.12111368.
Bickelhaupt S, Laun FB, Tesdorff J, Lederer W, Daniel H, Stieber A, et al. Fast and noninvasive characterization of suspicious lesions detected at breast cancer X-ray screening: capability of diffusion-weighted MR imaging with MIPs. Radiology. 2015. doi:10.1148/radiol.2015150425.
Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24(7):843–7. doi:10.1016/j.mri.2005.11.005.
Sharma U, Danishad KK, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22(1):104–13. doi:10.1002/nbm.1245.
Richard R, Thomassin I, Chapellier M, Scemama A, de Cremoux P, Varna M, et al. Diffusion-weighted MRI in pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol. 2013;23(9):2420–31. doi:10.1007/s00330-013-2850-x.
Partridge SC, Demartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Differential diagnosis of mammographically and clinically occult breast lesions on diffusion-weighted MRI. J Magn Reson Imaging. 2010;31(3):562–70. doi:10.1002/jmri.22078.
Yabuuchi H, Matsuo Y, Sunami S, Kamitani T, Kawanami S, Setoguchi T, et al. Detection of non-palpable breast cancer in asymptomatic women by using unenhanced diffusion-weighted and T2-weighted MR imaging: comparison with mammography and dynamic contrast-enhanced MR imaging. Eur Radiol. 2011;21(1):11–7. doi:10.1007/s00330-010-1890-8.
Kazama T, Kuroki Y, Kikuchi M, Sato Y, Nagashima T, Miyazawa Y, et al. Diffusion-weighted MRI as an adjunct to mammography in women under 50 years of age: an initial study. J Magn Reson Imaging. 2012;36(1):139–44. doi:10.1002/jmri.23626.
•• Trimboli RM, Verardi N, Cartia F, Carbonaro LA, Sardanelli F. Breast cancer detection using double reading of unenhanced MRI including T1-weighted, T2-weighted STIR, and diffusion-weighted imaging: a proof of concept study. AJR Am J Roentgenol. 2014;203(3):674–81. doi: 10.2214/AJR.13.11816. This study demonstrates promise for use of a non-contrast diffusion weighted imaging (DWI) approach for breast cancer screening.
Bogner W, Pinker K, Zaric O, Baltzer P, Minarikova L, Porter D, et al. Bilateral diffusion-weighted MR imaging of breast tumors with submillimeter resolution using readout-segmented echo-planar imaging at 7 T. Radiology. 2015;274(1):74–84. doi:10.1148/radiol.14132340.
Lee SK, Tan ET, Govenkar A, Hancu I. Dynamic slice-dependent shim and center frequency update in 3 T breast diffusion weighted imaging. Magn Reson Med. 2014;71(5):1813–8. doi:10.1002/mrm.24824.
Singer L, Wilmes LJ, Saritas EU, Shankaranarayanan A, Proctor E, Wisner DJ, et al. High-resolution diffusion-weighted magnetic resonance imaging in patients with locally advanced breast cancer. Acad Radiol. 2012;19(5):526–34. doi:10.1016/j.acra.2011.11.003.
Teruel JR, Fjosne HE, Ostlie A, Holland D, Dale AM, Bathen TF, et al. Inhomogeneous static magnetic field-induced distortion correction applied to diffusion weighted MRI of the breast at 3T. Magn Reson Med. 2014. doi:10.1002/mrm.25489.
Bogner W, Pinker-Domenig K, Bickel H, Chmelik M, Weber M, Helbich TH, et al. Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology. 2012;263(1):64–76. doi:10.1148/radiol.12111494.
Bokacheva L, Kaplan JB, Giri DD, Patil S, Gnanasigamani M, Nyman CG, et al. Intravoxel incoherent motion diffusion-weighted MRI at 3.0 T differentiates malignant breast lesions from benign lesions and breast parenchyma. J Magn Reson Imaging. 2014;40(4):813–23. doi:10.1002/jmri.24462.
Iima M, Yano K, Kataoka M, Umehana M, Murata K, Kanao S, et al. Quantitative non-gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest Radiol. 2014. doi:10.1097/RLI.0000000000000094.
Liu C, Liang C, Liu Z, Zhang S, Huang B. Intravoxel incoherent motion (IVIM) in evaluation of breast lesions: comparison with conventional DWI. Eur J Radiol. 2013;82(12):e782–9. doi:10.1016/j.ejrad.2013.08.006.
Sigmund EE, Cho GY, Kim S, Finn M, Moccaldi M, Jensen JH, et al. Intravoxel incoherent motion imaging of tumor microenvironment in locally advanced breast cancer. Magn Reson Med. 2011;65(5):1437–47. doi:10.1002/mrm.22740.
•• Iima M, Yano K, Kataoka M, Umehana M, Murata K, Kanao S, et al. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Investig Radiol. 2015;50(4):205–11. doi:10.1097/RLI.0000000000000094. This study provides strong early pilot data demonstrating the potential added benefit of utilizing intravoxel incoherent motion (IVIM) and kurtosis diffusion modeling in order to better discriminate malignant and benign lesions.
Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23(7):698–710. doi:10.1002/nbm.1518.
Sun K, Chen X, Chai W, Fei X, Fu C, Yan X, et al. Breast Cancer: Diffusion kurtosis MR imaging-diagnostic accuracy and correlation with clinical-pathologic factors. Radiology. 2015;277(1):46–55. doi:10.1148/radiol.15141625.
Partridge SC, Ziadloo A, Murthy R, White SW, Peacock S, Eby PR, et al. Diffusion tensor MRI: preliminary anisotropy measures and mapping of breast tumors. J Magn Reson Imaging. 2010;31(2):339–47. doi:10.1002/jmri.22045.
Baltzer PA, Schafer A, Dietzel M, Grassel D, Gajda M, Camara O, et al. Diffusion tensor magnetic resonance imaging of the breast: a pilot study. Eur Radiol. 2011;21(1):1–10. doi:10.1007/s00330-010-1901-9.
Eyal E, Shapiro-Feinberg M, Furman-Haran E, Grobgeld D, Golan T, Itzchak Y, et al. Parametric diffusion tensor imaging of the breast. Invest Radiol. 2012;47(5):284–91. doi:10.1097/RLI.0b013e3182438e5d.
Roebuck JR, Cecil KM, Schnall MD, Lenkinski RE. Human breast lesions: characterization with proton MR spectroscopy. Radiology. 1998;209(1):269–75. doi:10.1148/radiology.209.1.9769842.
Gribbestad IS, Singstad TE, Nilsen G, Fjosne HE, Engan T, Haugen OA, et al. In vivo 1H MRS of normal breast and breast tumors using a dedicated double breast coil. J Magn Reson Imaging. 1998;8(6):1191–7.
Cecil KM, Schnall MD, Siegelman ES, Lenkinski RE. The evaluation of human breast lesions with magnetic resonance imaging and proton magnetic resonance spectroscopy. Breast Cancer Res Treat. 2001;68(1):45–54.
Yeung DK, Cheung HS, Tse GM. Human breast lesions: characterization with contrast-enhanced in vivo proton MR spectroscopy—initial results. Radiology. 2001;220(1):40–6. doi:10.1148/radiology.220.1.r01jl0240.
Bolan PJ. Magnetic resonance spectroscopy of the breast: current status. Magn Reson Imaging Clin N Am. 2013;21(3):625–39. doi:10.1016/j.mric.2013.04.008.
Bartella L, Morris EA, Dershaw DD, Liberman L, Thakur SB, Moskowitz C, et al. Proton MR spectroscopy with choline peak as malignancy marker improves positive predictive value for breast cancer diagnosis: preliminary study. Radiology. 2006;239(3):686–92. doi:10.1148/radiol.2393051046.
Meisamy S, Bolan PJ, Baker EH, Pollema MG, Le CT, Kelcz F, et al. Adding in vivo quantitative 1H MR spectroscopy to improve diagnostic accuracy of breast MR imaging: preliminary results of observer performance study at 4.0 T. Radiology. 2005;236(2):465–75. doi:10.1148/radiol.2362040836.
Bartella L, Thakur SB, Morris EA, Dershaw DD, Huang W, Chough E, et al. Enhancing nonmass lesions in the breast: evaluation with proton (1H) MR spectroscopy. Radiology. 2007;245(1):80–7. doi:10.1148/radiol.2451061639.
• Baltzer PA, Dietzel M. Breast lesions: diagnosis by using proton MR spectroscopy at 1.5 and 3.0 T—systematic review and meta-analysis. Radiology. 2013;267(3):735–46. doi:10.1148/radiol.13121856. This recent meta-analysis provides a realistic assessment of the current status of MR spectroscopy performance for discriminating between benign and malignant breast lesions.
Dorrius MD, Pijnappel RM, van der Weide Jansen MC, Jansen L, Kappert P, Oudkerk M, et al. The added value of quantitative multi-voxel MR spectroscopy in breast magnetic resonance imaging. Eur Radiol. 2012;22(4):915–22. doi:10.1007/s00330-011-2322-0.
Dorrius MD, Pijnappel RM, Jansen-van der Weide MC, Jansen L, Kappert P, Oudkerk M, et al. Determination of choline concentration in breast lesions: quantitative multivoxel proton MR spectroscopy as a promising noninvasive assessment tool to exclude benign lesions. Radiology. 2011;259(3):695–703. doi:10.1148/radiol.11101855.
Shin HJ, Baek HM, Cha JH, Kim HH. Evaluation of breast cancer using proton MR spectroscopy: total choline peak integral and signal-to-noise ratio as prognostic indicators. AJR Am J Roentgenol. 2012;198(5):W488–97. doi:10.2214/AJR.11.7292.
Haddadin IS, McIntosh A, Meisamy S, Corum C, Styczynski Snyder AL, Powell NJ, et al. Metabolite quantification and high-field MRS in breast cancer. NMR Biomed. 2009;22(1):65–76. doi:10.1002/nbm.1217.
Meisamy S, Bolan PJ, Baker EH, Bliss RL, Gulbahce E, Everson LI, et al. Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo (1)H MR spectroscopy—a pilot study at 4 T. Radiology. 2004;233(2):424–31. doi:10.1148/radiol.2332031285.
Baek HM, Chen JH, Nie K, Yu HJ, Bahri S, Mehta RS, et al. Predicting pathologic response to neoadjuvant chemotherapy in breast cancer by using MR imaging and quantitative 1H MR spectroscopy. Radiology. 2009;251(3):653–62. doi:10.1148/radiol.2512080553.
Jacobs MA, Stearns V, Wolff AC, Macura K, Argani P, Khouri N, et al. Multiparametric magnetic resonance imaging, spectroscopy and multinuclear ((2)(3)Na) imaging monitoring of preoperative chemotherapy for locally advanced breast cancer. Acad Radiol. 2010;17(12):1477–85. doi:10.1016/j.acra.2010.07.009.
Baek HM, Chen JH, Nalcioglu O, Su MY. Proton MR spectroscopy for monitoring early treatment response of breast cancer to neo-adjuvant chemotherapy. Ann Oncol. 2008;19(5):1022–4. doi:10.1093/annonc/mdn121.
Danishad KK, Sharma U, Sah RG, Seenu V, Parshad R, Jagannathan NR. Assessment of therapeutic response of locally advanced breast cancer (LABC) patients undergoing neoadjuvant chemotherapy (NACT) monitored using sequential magnetic resonance spectroscopic imaging (MRSI). NMR Biomed. 2010;23(3):233–41. doi:10.1002/nbm.1436.
Leong KM, Lau P, Ramadan S. Utilisation of MR spectroscopy and diffusion weighted imaging in predicting and monitoring of breast cancer response to chemotherapy. J Med Imaging Radiat Oncol. 2015;59(3):268–77. doi:10.1111/1754-9485.12310.
Jacobs MA, Barker PB, Argani P, Ouwerkerk R, Bhujwalla ZM, Bluemke DA. Combined dynamic contrast enhanced breast MR and proton spectroscopic imaging: a feasibility study. J Magn Reson Imaging. 2005;21(1):23–8. doi:10.1002/jmri.20239.
Gruber S, Debski BK, Pinker K, Chmelik M, Grabner G, Helbich T, et al. Three-dimensional proton MR spectroscopic imaging at 3 T for the differentiation of benign and malignant breast lesions. Radiology. 2011;261(3):752–61. doi:10.1148/radiol.11102096.
Hu J, Yu Y, Kou Z, Huang W, Jiang Q, Xuan Y, et al. A high spatial resolution 1H magnetic resonance spectroscopic imaging technique for breast cancer with a short echo time. Magn Reson Imaging. 2008;26(3):360–6. doi:10.1016/j.mri.2007.07.004.
Jacobs MA, Barker PB, Bottomley PA, Bhujwalla Z, Bluemke DA. Proton magnetic resonance spectroscopic imaging of human breast cancer: a preliminary study. J Magn Reson Imaging. 2004;19(1):68–75. doi:10.1002/jmri.10427.
Zhao C, Bolan PJ, Royce M, Lakkadi N, Eberhardt S, Sillerud L, et al. Quantitative mapping of total choline in healthy human breast using proton echo planar spectroscopic imaging (PEPSI) at 3 Tesla. J Magn Reson Imaging. 2012;36(5):1113–23. doi:10.1002/jmri.23748.
Klomp DW, van de Bank BL, Raaijmakers A, Korteweg MA, Possanzini C, Boer VO, et al. 31P MRSI and 1H MRS at 7 T: initial results in human breast cancer. NMR Biomed. 2011;24(10):1337–42. doi:10.1002/nbm.1696.
• Schmitz AM, Veldhuis WB, Menke-Pluijmers MB, van der Kemp WJ, van der Velden TA, Kock MC, et al. Multiparametric MRI with dynamic contrast enhancement, diffusion-weighted imaging, and 31-phosphorus spectroscopy at 7 T for characterization of breast cancer. Investig Radiol. 2015. doi:10.1097/RLI.0000000000000183. This study demonstrates that a multi-parametric 7 tesla approach is feasible in the clinical setting and may be useful for identifying specific pathologic features of malignancy.
Pinker K, Bogner W, Baltzer P, Gruber S, Bickel H, Brueck B, et al. Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast-enhanced magnetic resonance imaging, diffusion-weighted imaging, and 3-dimensional proton magnetic resonance spectroscopic imaging. Invest Radiol. 2014;49(6):421–30. doi:10.1097/RLI.0000000000000029.
Jacobs MA, Ouwerkerk R, Wolff AC, Gabrielson E, Warzecha H, Jeter S, et al. Monitoring of neoadjuvant chemotherapy using multiparametric, (2)(3)Na sodium MR, and multimodality (PET/CT/MRI) imaging in locally advanced breast cancer. Breast Cancer Res Treat. 2011;128(1):119–26. doi:10.1007/s10549-011-1442-1.
Li X, Abramson RG, Arlinghaus LR, Kang H, Chakravarthy AB, Abramson VG, et al. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol. 2015;50(4):195–204. doi:10.1097/RLI.0000000000000100.
Bogner W, Gruber S, Pinker K, Grabner G, Stadlbauer A, Weber M, et al. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology. 2009;253(2):341–51. doi:10.1148/radiol.2532081718.
Spick C, Pinker-Domenig K, Rudas M, Helbich TH, Baltzer PA. MRI-only lesions: application of diffusion-weighted imaging obviates unnecessary MR-guided breast biopsies. Eur Radiol. 2014;24(6):1204–10. doi:10.1007/s00330-014-3153-6.
Min Q, Shao K, Zhai L, Liu W, Zhu C, Yuan L, et al. Differential diagnosis of benign and malignant breast masses using diffusion-weighted magnetic resonance imaging. World J Surg Oncol. 2015;13:32. doi:10.1186/s12957-014-0431-3.
Arponent O, Sudah M, Masarwah A, Taina M, Rautiainen S, Kononen M, et al. Diffusion-weighted imaging in 3.0 tesla breast MRI: diagnostic performance and tumor characterization using small subregions vs. whole tumor regions of interest. PLoS One. 2015;10(10):e0138702. doi:10.1371/journal.pone.0138702.
Nogueira L, Brandao S, Matos E, Gouveia Nunes R, Ferreira HA, Loureiro J, et al. Improving malignancy prediction in breast lesions with the combination of apparent diffusion coefficient and dynamic contrast-enhanced kinetic descriptors. Clin Radiol. 2015;70(9):1016–25. doi:10.1016/j.crad.2015.05.009.
Thakur SB, Brennan SB, Ishill NM, Morris EA, Liberman L, Dershaw DD, et al. Diagnostic usefulness of water-to-fat ratio and choline concentration in malignant and benign breast lesions and normal breast parenchyma: an in vivo (1) H MRS study. J Magn Reson Imaging. 2011;33(4):855–63. doi:10.1002/jmri.22493.
Sah RG, Sharma U, Parshad R, Seenu V, Mathur SR, Jagannathan NR. Association of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status with total choline concentration and tumor volume in breast cancer patients: an MRI and in vivo proton MRS study. Magn Reson Med. 2012;68(4):1039–47. doi:10.1002/mrm.24117.
Baek HM. Diagnostic value of breast proton magnetic resonance spectroscopy at 1.5T in different histopathological types. Sci World J. 2012;2012:508295. doi:10.1100/2012/508295.
Mizukoshi W, Kozawa E, Inoue K, Saito N, Nishi N, Saeki T, et al. (1)H MR spectroscopy with external reference solution at 1.5 T for differentiating malignant and benign breast lesions: comparison using qualitative and quantitative approaches. Eur Radiol. 2013;23(1):75–83. doi:10.1007/s00330-012-2555-6.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Breast Imaging.
Rights and permissions
About this article
Cite this article
Rahbar, H., Kitsch, A.E. & Partridge, S.C. Magnetic Resonance Imaging: Advanced Applications in Breast Cancer. Curr Radiol Rep 4, 14 (2016). https://doi.org/10.1007/s40134-016-0142-3
Published:
DOI: https://doi.org/10.1007/s40134-016-0142-3