Abstract
The spleen plays an instrumental role in the immunological homeostasis in the body, particularly in young patients. Infectious involvement of the spleen can be primary or secondary to a manifestation of systemic disease. Imaging plays a crucial role in evaluation of diseases of the spleen in children. In this review, we describe current imaging techniques and the multimodality imaging findings of common and atypical infections affecting the spleen with an emphasis on US and MRI. In addition, conditions that mimic infection will be discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dedicated imaging of the spleen is infrequent, and typically reserved for specific diagnostic situations. Two primary indications for splenic imaging include trauma and suspected infection. Although many publications have described imaging and management of splenic trauma in children, to date there are few dedicated publications addressing imaging of infections of the spleen, particularly in children [1]. In this article, we review imaging manifestations of common and uncommon splenic infections as well as mimickers of infection in a variety of imaging modalities, with emphasis on those without ionizing radiation.
Imaging Characteristics of Normal Spleen
On ultrasound (US), the normal spleen demonstrates homogenous echotexture, slightly more echogenic compared with the adjacent kidney and isoechoic to the liver. On non-enhanced computed tomography (CT), the normal spleen is higher in attenuation than the liver. The normal magnetic resonance imaging (MRI) signal characteristics of the spleen varies with age. The neonatal spleen demonstrates iso-hypointense signal intensity (SI) on T1 weighted (W) imaging and hypointense signal on T2 W imaging compared with liver. By 8 months of age, the white pulp begins to develop and the spleen becomes more T2 hyperintense than the liver; this T2 hyperintensity persists into adulthood (Fig. 1). After contrast, the spleen typically demonstrates a mottled, striped appearance in the arterial phase and a more homogenous enhancement on the delayed phases due to blood flow differences between red and white pulp (Fig. 2).
Normal Spleen. a Axial T2 W fat-saturated MR image of a normal spleen in a 9-day-old infant b Axial T2 W fat-saturated MR image of a normal spleen in a 12-year-old girl. Note that the spleen (S) is isointense to the liver (L) in the infant and demonstrates higher SI compared to the liver due to the increased amount of white pulp in the older child
Normal spleen enhancement. a Axial T1 W fat-saturated pre-contrast MR image of normal spleen in a 19-year-old male demonstrates a uniform appearance of the spleen (S). b Early arterial phase T1 W fat-saturated post-contrast MRI image in the same patient shows a mottled, striped appearance (black arrows) of the splenic parenchyma due to blood flow differences between red and white pulp. c Portal venous phase T1 W fat-saturated post-contrast MRI image from the same examination shows more homogeneous of the spleen (S) in this delayed phase
Imaging Techniques
Many previous publications have emphasized the multimodality approach to the evaluation of abnormalities of the spleen [2•, 3]. US is typically the first imaging study used to evaluate the spleen because it is cost effective, readily accessible and quick to perform. US has several advantages including discrimination of cystic versus solid lesions and evaluation of lesion vascularity. While CT may be more readily available and more sensitive to detect calcifications, more recently, the focus has been on utilizing MRI. With its multi-planar capability and high tissue contrast, MRI is superior to CT without the use of ionizing radiation [4]. Multiphase post-contrast enhanced MRI can be helpful to look at enhancement patterns of a lesion. Diffusion-weighted MR imaging (DWI) is being increasingly employed by many institutions as part of the routine MRI protocol to provide complementary information to standard T1 and T2 W sequences [5•, 6•]. Appearance of a splenic lesion on DWI sequences will vary based on lesion cellularity, e.g., restricted diffusion has been described in both pyogenic and tuberculous abscesses secondary to the thick, cellular center of an abscess containing purulent material, granulation tissue and debris that impairs movement of water molecules. In the author’s experience DWI sequences can clearly depict a greater number of very small infectious lesions in the spleen compared with conventional MRI T2 W sequences and can better estimate the overall burden of infection in a child.
Splenomegaly
An enlarged spleen is a common but non-specific manifestation of infection in a child. On radiography, splenomegaly is suggested when the spleen shadow extends below the left kidney into the pelvis below the level of the iliac crest. The enlarged spleen often displaces the stomach medially, the bowel inferomedially, and the diaphragm superiorly (Fig. 3). In most cases, ultrasound alone can confirm splenomegaly and can help determine the cause of the splenic enlargement. However, in isolated cases, further characterization with CT or MR may be useful.
Viral Infections
Viral infections that commonly affect the spleen include Epstein Barr Virus (EBV) and Cytomegalovirus (CMV), and usually present with splenomegaly. Epstein Barr Virus (EBV), which causes infectious mononucleosis in young children, can be due to virus shedded by infected parents or siblings. In adolescents and young adults, transmission has been associated with kissing. Other causes of primary EBV include blood transfusion, solid organ transplantation, and hematopoietic cell transplantation. Many young adults go on to develop infectious mononucleosis after primary EBV infection. Clinical symptoms include headache, fatigue, fevers, sore throat, and swollen glands, with anorexia and abdominal pain being less common. On physical examination, lymphadenopathy is the most common manifestation; however, patients may have hepatomegaly and splenomegaly [7]. Splenomegaly is seen in approximately 50 % of the patients with infectious mononucleosis [8]. Physical examination alone may underestimate the degree of splenomegaly in these patients; therefore, imaging can play an important role in evaluation. US can not only help determine the size of the organ, it can also evaluate for potential complications such as rupture or infarction (Fig. 4) [9]. Splenic rupture occurs in 0.1–0.5 % of such cases [10]. Minor trauma has been noted as a cause of rupture in these patients; however, the majority of cases of splenic rupture are spontaneous. In conjunction with US, contrast enhanced CT can be utilized for evaluation of splenic rupture, specifically to evaluate the splenic parenchyma and the vasculature (Fig. 5).
Infectious mononucleosis. a Coronal grayscale ultrasound image in a 16-year-old girl with low-grade fever and left upper quadrant pain shows a peripheral, wedge-shaped region of hypoechogenicity (white arrows) consistent with an infarct. b Coronal CECT image in the same patient demonstrates several peripheral wedge-shaped perfusion defects within the spleen (white arrows) consistent with infarcts. The liver and spleen were mildly enlarged and numerous small mesenteric lymph nodes present (not shown), which suggested EBV. Mono-spot was positive
Viral-related post-transplant lymphoproliferative disorder (PTLD) can also cause pathological abnormalities of the spleen in children, particularly in patients who have undergone various types of solid organ transplants. PTLD in post-transplant patients is often associated with either reactivation or primary infection of EBV and CMV [11]. In addition to the spleen, other organ systems can be affected, including the central nervous system, gastrointestinal tract, as well as the lungs and liver. PTLD in the spleen can manifest as splenomegaly and/or small hypoechoic or hypoattenuating lesions in the liver [12]. Similar to the imaging findings for EBV and CMV, US is useful in evaluating solid organ involvement and to assess for lymphadenopathy. However, contrast-enhanced CT or MRI are commonly used as an adjunct to assess lymph nodes, intestinal tract involvement, as well as to assess the solid organs more effectively.
Bacterial Infections
Abscess
In most cases, a splenic abscess results from hematogenous spread of infection. Other causes include penetrating trauma (15 %) and prior splenic infarction (10 %) [13]. Abscesses can be solitary, multiple or multilocular [14]. In general, the prevalence of a pyogenic abscess in the spleen is low and usually restricted to immunocompromised patients. Fever, chills, and left upper quadrant tenderness are found in less than half of all patients with splenic abscesses [11], thus rendering imaging very useful in the diagnosis.
On US, a bacterial abscess is classically seen as a large (>1 cm), cystic lesion with a defined wall and peripheral but not central vascularity with color Doppler interrogation (Fig. 6) [4]. CT often demonstrates findings seen on US as a well-defined lesion with central low attenuation, typically ranging from 20 to 40 HU, depending on degree of proteinaceous material [4]. CT is often more sensitive for the depiction of intra-abscess gas formation. MR imaging characteristics of splenic abscesses include a lesion of fluid intensity, with low SI on T1 W images and high SI on T2 W sequences with peripheral and perilesional enhancement on post-contrast T1 weighted fat saturation imaging [4] (Fig. 6).
Splenic abscess. Sagittal grayscale ultrasound image in an 11-year-old girl status-post partial splenectomy for resection of a cyst with subsequent fevers demonstrates a heterogeneous intrasplenic collection (white arrows) with a peripheral rind (arrowheads) with increased through transmission, compatible with a large solitary abscess. The abscess was percutaneously drained; however, the patient ultimately underwent splenectomy for persistent fevers. On histopathology, findings were consistent with disrupted spleen with a splenic abscess containing bacterial organisms with associated lymphoid hyperplasia and perisplenitis
Fungal Infection
Fungal abscesses are usually small, measuring less than 1 cm in diameter and variable peripheral enhancement on post-contrast T1 W fat saturation imaging depending on the immune status of the host. When present, the degree of peripheral enhancement is often less than that observed in hepatic abscesses [13]. In immunocompromised patients, Candida albicans is the most common cause of fungal infection involving the spleen [3]. Typically, systemic candidiasis presents with fever and gastrointestinal symptoms that increase with recovering neutrophil counts. Spleen and liver involvement is commonly seen with disseminated fungal infection, with the kidney being third most common viscus involved [4, 5•]. With profound neutropenia, the number of Candida colonies increases and Candida species can invade the intestinal mucosa and infect the liver via the portal circulation and ultimately the spleen [5•]. Since blood cultures are usually negative, histopathologic findings are invaluable and biopsy can be performed with image guidance to reduce sampling error.
CT and MR imaging can demonstrate hepatosplenic candidiasis in 90 % of patients, whereas US can depict lesions in 70–75 % of patients [15]. Fungal splenic abscesses consist of purulent material, necrotic tissue, and fungus surrounded by layers of chronic inflammatory cells and adjacent fibrosis. This layered phenomenon gives rise the “wheel-within-a-wheel” or “target” appearance of fungal microabscesses on US with a peripheral hypoechoic zone (fibrosis), an enclosed hyperechoic zone (inflammatory portion) and hyperechoic center (purulent material, necrotic debris and fungal elements) [16]. With healing, these lesions become uniformly hyperechoic due to fibrosis with or without calcifications. On CT, the abscesses are small, well-defined low attenuating lesions that do not enhance (Fig. 7). Occasionally, there is central hyperattenuation which is thought to be reflective of pseudohyphae. Some reports have suggested that MR is more sensitive than CT for depicting visceral Candida infection [6•, 17•]. On MR, fungal microabscesses appear as small (<1 cm) hyperintense SI lesions on T2 W imaging [18]. In the acute phase, these microabscesses appear as multiple hypointense SI lesions on T1 W imaging with or without ring enhancement [4, 14]. In chronic phases, the lesions generally do not demonstrate peripheral enhancement due to fibrotic changes. On DWI, the fungal lesions exhibit restricted diffusion due to viscosity, depicted as hyperintense SI on DWI and hypointense SI on apparent diffusion coefficient (ADC) maps (Fig. 8). An important caveat is that abscesses may not be detectable in severely neutropenic patients, even in the presence of disseminated disease, because of inadequate host immune response. The lesions will become more apparent with the return of neutrophil function [16].
Disseminated fungal infection. a CECT image in a 7-year-old boy with relapsed acute lymphoid leukemia, prolonged neutropenia and fevers, negative blood cultures demonstrates multiple hypoattenuating lesions within the spleen (white arrows) and kidneys. b Grayscale ultrasound of the spleen shows two rounded hypoechoic ‘target’ lesions (white arrows). An ultrasound guided biopsy was performed depicted hyphal elements
Invasive Aspergillosis. a Axial T1 W fat-saturated post-contrast MRI image in a 4-year-old girl with history of medulloblastoma, on chemotherapy with fevers and resolving neutropenia demonstrates innumerable small, rounded enhancing lesions within the spleen (circle). There are also multiple lesions within the kidneys (black arrows) and liver (white arrow). b Diffusion-weighted images and c corresponding ADC map shows restricted diffusion as demonstrated by increased SI on diffusion-weighted images and decreased SI on ADC images (circle). Biopsy of a splenic lesion yielded Aspergillus galactomannan
Histoplasmosis is an infection caused by breathing in spores of a fungus (Histoplasma) often found in bird and bat droppings. Histoplasma mainly lives in the central and eastern United States, especially in the Ohio and Mississippi River valleys. Histoplasmosis can be seen in both immunocompetent and immunocompromised patients; however, the prevalence is higher in the immunocompromised population. On MR, histoplasmosis lesions can be seen in the acute and subacute phases as scattered hypointense lesions both on T1 and T2 W images. Chronic granulomas can become calcified and demonstrate blooming artifact, best appreciated on GRE T1 W images (with long echo time) [14].
Parasitic Infections
Echinococcal Disease
Echinococcosis (hydatid disease) is a parasitic infection produced by the larval stage of the Echinococcus tapeworm. Echinococcus granulosus is the most frequently encountered type of hydatid disease in humans [19]. Primary splenic hydatidosis accounts for less 2 % of all hydatid disease [20]. Involvement of the spleen occurs either by hematogenous spread or intraperitoneal dissemination from a ruptured liver cyst. Clinically, patients with splenic hydatid disease present with abdominal pain, splenomegaly, and fever when the eggs of the mature tapeworm are ingested.
Once the embryo reaches an organ, it develops into a small cyst. The cyst generally grows to 1 cm within the first 6 months and 2–3 cm each year thereafter, depending on the host tissue resistance [19, 21]. Typical “daughter-cysts” appear as detached floating loculations within the original “mother” cyst and are an important distinguishing imaging feature. Infrequently, these daughter vesicles, which contain protoscolices, can rupture within the mother cyst, creating white sediment known as “hydatid sand.” The majority of splenic hydatid cysts are solitary. On US, the cyst wall usually manifests as double echogenic lines separated by a hypoechogenic layer. Simple cysts do not contain internal structures; however, “hydatid sand” may be seen as mobile echogenic foci. This sand will fall to the most dependent portion of the cavity with patient repositioning and is referred to as the “snowstorm sign” [21]. Daughter cysts appear as well-defined fluid collection with floating membranes inside the mother cyst cavity (Fig. 9). US is the most sensitive modality for depiction of the membranes, septa, and hydatid sand [21] and is also an important modality to monitor the efficacy of medical therapy [19].
Echinococcal disease. a Axial contrast-enhanced CT image of a 7-year-old girl from Morocco with a history of echinococcal disease shows multiple large cysts within the liver and a single large cyst in the spleen. An enhancing septation is noted within the splenic cyst (black arrow). b Transverse grayscale ultrasound image of the spleen better demonstrates several thin echogenic internal membranes within the cyst, typical of echinococcus infection
On CT, the cyst fluid demonstrates low attenuating fluid and the wall typically appears hyper-attenuating, even without contrast or calcification (Fig. 9). Daughter vesicles have been described as containing fluid which is higher in attenuation compared with the fluid in the mother cyst [21]. Overall these calcifications are better depicted on CT than MRI.
On MR, the cyst fluid demonstrates hyperintense SI on T2 W and hypointense SI on T1 W imaging with a hypointense SI rim on T2 W imaging due to dense collagen in the outer layer, which has been proposed to be a characteristic sign of hydatid disease. Daughter cysts appear as cystic structures that are hypointense relative to the main cyst fluid on both T1 and T2 W images [21]. Calcification can occur within the cyst as well as within the cyst matrix [4]. The differential diagnosis of splenic hydatid disease includes epidermoid cysts, pseudocysts, abscesses, hematoma, and neoplasms [20].
Splenic Granulomas
Granulomas may be caused by a number of infectious agents, most commonly Bartonella henselae, a Gram-negative bacillus that is usually introduced by the scratch of a kitten causing Cat-scratch disease, and Tularemia caused by the organism Franciella tularenis [22]. Typically, Cat-scratch presents with painful, regional lymphadenopathy proximal to the site of inoculation, with a single node or nodal group and most commonly involves the upper extremity, axilla, or neck. In 5 to 10 % of Cat-scratch cases, disseminated infection is seen with multiple granulomata forming in the liver and spleen. This can be seen with or without hepatosplenomegaly.
Cat-scratch splenic lesions can range in size from 3 to 30 mm in diameter. On US, splenic granulomata range in appearance from well-defined and homogeneous to indistinct and heterogeneous (Fig. 10) [22]. On unenhanced CT, the lesions are hypointense to the normal spleen. On contrast-enhanced CT (CECT) imaging, three different appearances have been described: persistent low attenuation, iso-attenuating to the adjacent splenic tissue, and demonstrating marginal enhancement. As imaging findings may mimic malignancy, it is imperative that they be recognized early in order to avoid unnecessary diagnostic and therapeutic measures [22].
Cat-scratch disease. Sagittal grayscale ultrasound image of the spleen in a 4-year-old girl with fever of unknown origin demonstrates innumerable hypoechoic foci relative to the normal splenic tissue compatible with multiple abscesses. Blood titers were positive for Bartonella henselae. Liver lesions were also present (not shown). History revealed exposure to a kitten
Tularemia, caused by the organism Franciella tularenis, is transmitted via infected ticks and is endemic in several areas of North America. This infection clinically presents with fever, malaise, pharyngitis, cervical lymphadenopathy, and hepatosplenomegaly mimicking mononucleosis and Cat-scratch disease both in symptomatology and imaging features [23]. Similar to Cat-scratch, Tularemia manifests as small round hypoechoic or low attenuation lesions of the liver and/or spleen on US and CT, respectively (Fig. 11).
Tuleremia. Axial CECT image of a 4-year-old male who was bit by a tick three weeks prior and subsequently developed a left groin ulcer and fevers. Multiple splenic abscesses (black arrows) were present along with a left pleural effusion, liver and renal abscesses and a pelvic abscess (not shown). Subsequent laboratory tests were positive for Tularemia
Mimickers of Splenic Infection
Hemophagocytic Lymphohistiocytosis (HLH)
This pediatric disease of immune dysregulation is caused by the overproduction of cytokines and diminished immune surveillance. Clinical presentation includes nonspecific symptoms such as fever, lymphadenopathy, and cytopenia. Multiple organ systems can be involved, including the central nervous system. Common presentation of HLH in the abdomen is hepatosplenomegaly; however, other nonspecific manifestations can occur in the spleen, such as multiple infarcts or abscesses (Fig. 12) [24, 25]. In the author’s experience, splenic abscess can be the first presentation of HLH.
Hemophagocytic lymphohistiocytosis. a Coronal grayscale ultrasound image of a 13-month-old girl with a 2-month history of fever and lymphadenopathy. Multiple complex hypoechoic lesions were seen within the spleen (white arrows). Skin lesion biopsy confirmed HLH. b Coronal T2 W fat-saturated MRI image obtained two weeks later, demonstrated increased size of the multiple masses (white arrows)
Splenic Cysts
Cystic splenic masses may be congenital, inflammatory, vascular, posttraumatic, or neoplastic [13]. Generally, congenital splenic cysts are asymptomatic but may become symptomatic if they enlarge due to trauma, hemorrhage, superinfection or rupture. On US, a splenic cyst appears as a rounded, homogeneous, anechoic lesion with a smooth thin wall, in contrast to an abscess that usually demonstrates poorly defined borders. Complexity of a congenital cyst can also be delineated by the presence of internal septae, irregularity of the wall, internal debris from prior hemorrhage or peripheral calcification. On CT, splenic cysts are round low attenuation (similar to water) lesions with an imperceptible wall that generally does not enhance after contrast administration, as opposed to the peripheral enhancement seen with splenic abscess (Fig. 13). On MR, cysts demonstrate hyperintense SI on T2 W and hypointense SI on T1 W imaging with no enhancement following contrast administration [2•]. In cases with prior hemorrhage, the cystic fluid may contain hyperintense T1 W SI.
Congenital splenic cyst. a Axial CECT image in a 9-year-old boy with right upper quadrant abdominal pain demonstrates a large cyst within the spleen, an incidental finding. b Transverse Doppler ultrasound image obtained three years later shows unchanged size of the spleen and several small internal septations. No internal blood flow was depicted within the cyst
Lymphatic Malformations
Primary splenic lymphatic malformations are rare and may occur in isolation or in the context of lymphangiomatosis. Splenic lymphatic malformations tend to occur in subcapsular locations, which reflect the distribution of the splenic lymphatic system, helping differentiate this entity. The appearance can vary, ranging from small thin-walled subcapsular or parenchymal cysts to global splenic enlargement by an infiltrative lesion. On US, lymphatic malformations are most often multilocular cystic masses that are anechoic or contain low level echoes or debris. On CT, the lesion is overall low attenuation, similar to water, and post-contrast imaging may demonstrate enhancement of the lining or septae. Occasionally, negative HU may be present due to the presence of chylous fluid. On MRI, the fluid within the malformation demonstrates hyperintense SI on T2W and hypointense SI on T1W imaging (Fig. 14). Mural or septal calcifications may be present [26]. The presence of hemorrhage or infection may alter the lesion characteristics on CT or MRI [13].
Lymphangiomatosis. a Sagittal T2 W fat-saturated MRI image of an 8-month-old boy with a large left axillary mass representing a lymphatic malformation (white arrows). b Axial T2W fat-saturated MRI image in the same patient shows innumerable, unexpected cystic lesions throughout the spleen and osseous structures (block black arrows)
Malignancy
Leukemia and lymphoma are the most common malignancies of the spleen in children. Imaging evaluation of the spleen is indicated in the diagnosis and management of leukemia. Patients can present with splenomegaly, either isolated or with other organomegaly, secondary to massive leukemic infiltration. Leukemic infiltration of the spleen on ultrasound appears as heterogeneous echogenicity of the spleen or focal hypoehoic lesions that appear hypoattenuating on CT [27, 28, 29].
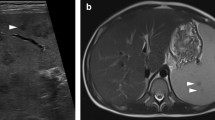
Hodgkin and non-Hodgkin lymphoma can both involve the spleen, with Hodgkin being more common. Lymphomatous involvement of the spleen can manifest as diffuse infiltration without a focal lesion, multiple miliary lesions (less than 5 mm), multiple focal masses, or a single focal discrete lesion. Focal lesions on US are hypoechoic without acoustic enhancement. On CT, the lesions are hypoattenuating with lack of contrast enhancement (Fig. 15). Diffuse lymphomatous involvement can manifest as splenomegaly alone with normal US and CT appearance secondary to diffuse organ infiltration.
Lymphoma. a Axial unenhanced CT image of the neck in a 14-year-old boy shows a large left submandibular mass (white arrows) b Axial FDG PET image shows that the lesion is metabolically active. Subsequent biopsy revealed Hodgkin’s Lymphoma. c Axial CECT image demonstrates several non-enhancing hypoattenuating lesions within the spleen (black arrows). d Coronal whole body maximum intensity projection (MIP) from FDG PET-CT demonstrates multiple foci of increased avidity within the spleen (circle) consistent with lymphomatous involvement
Conclusion
Infections of the spleen are important and often under recognized. There are multiple modalities that can be used to evaluate splenic infections, usually beginning with US. With the increasing use of MRI, one needs to recognize the imaging features of splenic infections and the role of DWI in early recognition of multifocal fungal infection. In addition, it is important to recognize entities that can be mistaken for infection so that proper timely treatment may be implemented.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Cirocchi R, Boselli C, Corsi A, Farinella E, Listorti C, Trastulli S, Renzi C, Desiderio J, Santoro A, Cagini L, Parisi A, Redler A, Noya G, Fingerhut A. Is non-operative management safe and effective for all splenic blunt trauma? A systematic review. Crit Care. 2013;17(5):R185. doi:10.1186/cc12868.
• Thipphavong S, Duigenan S, Schindera ST, Gee MS, Philips S (2014) Nonneoplastic, benign, and malignant splenic diseases: cross-sectional imaging findings and rare disease entities. AJR Am J Roentgenol. 203(2):315–22. doi:10.2214/AJR.13.11777. In this review the authors emphasize using a multi-modality approach to recognizing and evaluating non-neoplastic splenic diseases covering a wide range of ages, both children and adults.
Gaetke-Udager K, Wasnik AP, Kaza RK, Al-Hawary MM, Maturen KE, Udager AM, Azar SF, Francis IR. Multimodality imaging of splenic lesions and the role of non-vascular, image-guided intervention. Abdom Imaging. 2014;39(3):570–87. doi:10.1007/s00261-014-0080-6.
Luna A, Ribes R, Caro P, Luna L, Aumente E, Ros PR. MRI of focal splenic lesions without and with dynamic gadolinium enhancement. AJR Am J Roentgenol. 2006;186(6):1533–47. doi:10.2214/AJR.04.1249.
• Neubauer H, Platzer I, Mueller VR, Meyer T, Liese J, Koestler H, Hahn D, Beer M (2012) Diffusion-weighted MRI of abscess formations in children and young adults. World J Pediatrics. 8(3):229–34. doi:10.1007/s12519-012-0362-4. This original work describes the performance of DWI in the evaluation of abdominal and soft-tissue abscesses in 17 patients. The authors found that DWI had a high sensitivity and specificity for the detection of an abscess and complemented T1 weighted contrast enhanced sequences.
• Lim J, Yu JS, Hong SW, Chung JJ, Kim JH, Kim KW (2011) A case of mass-forming splenic tuberculosis: MRI findings with emphasis of diffusion-weighted imaging characteristics. J Korean Med Sci. 26(3):457–60. doi:10.3346/jkms.2011.26.3.457. In this case report the authors discuss that there is marked restricted diffusion in a rare case of extra-pulmonary tuberculosis in the spleen. The normal spleen parenchyma has a low ADC value, whereas the thick rind of granulation tissue in the tuberculoma will exhibit higher ADC values and the central areas of necrosis have a lower ADC.
Kinderknecht JJ. Infectious mononucleosis and the spleen. Curr Sports Med Rep. 2002;1(2):116–20.
Sumaya CV, Ench Y. Epstein-Barr virus infectious mononucleosis in children. I. Clinical and general laboratory findings. Pediatrics. 1985;75(6):1003–10.
Li Y, Pattan V, Syed B, Islam M, Yousif A. Splenic infarction caused by a rare coinfection of Epstein-Barr virus, cytomegalovirus, and Mycoplasma pneumoniae. Pediatr Emerg Care. 2014;30(9):636–7. doi:10.1097/PEC.0000000000000211.
Maki DG, Reich RM. Infectious mononucleosis in the athlete. Diagnosis, complications, and management. Am J Sports Med. 1982;10(3):162–73.
Spasojevic-Dimitrijeva B, Peco-Antic A, Paripovic D, Kruscic D, Krstic Z, Cupic M, Cvetkovic M, Milosevski-Lomic G, Kostic M. Post-transplant lymphoproliferative disorder–case reports of three children with kidney transplant. Srp Arh Celok Lek. 2014;142(1–2):83–8.
Pickhardt PJ, Siegel MJ, Hayashi RJ, Kelly M. Posttransplantation lymphoproliferative disorder in children: clinical, histopathologic, and imaging features. Radiology. 2000;217(1):16–25. doi:10.1148/radiology.217.1.r00oc3816.
Urrutia M, Mergo PJ, Ros LH, Torres GM, Ros PR. Cystic masses of the spleen: radiologic-pathologic correlation. Radiographics. 1996;16(1):107–29. doi:10.1148/radiographics.16.1.107.
Elsayes KM, Narra VR, Mukundan G, Lewis JS Jr, Menias CO, Heiken JP. MR imaging of the spleen: spectrum of abnormalities. Radiographics. 2005;25(4):967–82. doi:10.1148/rg.254045154.
Moore NJ, Leef JL 3rd, Pang Y. Systemic candidiasis. Radiographics. 2003;23(5):1287–90. doi:10.1148/rg.235025162.
Chew FS, Smith PL, Barboriak D. Candidal splenic abscesses. AJR Am J Roentgenol. 1991;156(3):474. doi:10.2214/ajr.156.3.1899741.
• Fasih N, Gulati A, Ryan J, Ramanathan S, Prasad Shanbhogue AK, McInnes M, Macdonald DB, Fraser-Hill MA, Walsh C, Kielar AZ, Bhagat K (2014) The mysterious organ. Spectrum of focal lesions within the splenic parenchyma: cross-sectional imaging with emphasis on magnetic resonance imaging. Can Assoc Radiol J Journal l’Association canadienne des radiologistes 65(1):19–28. doi:10.1016/j.carj.2012.03.004. In this review the authors use a cross-sectional approach to imaging the spleen. A spectrum of diseases have been discussed in this review including in brief about splenic infections. Although CT and US are very useful in the initial phase to identify a splenic lesion, dynamic contrast enhanced MRI is helpful to further characterize, and classify, based on signal characteristics, into a benign or malignant group.
Brown ED, Semelka RC. Magnetic resonance imaging of the spleen and pancreas. Top Magn Reson Imaging. 1995;7(2):82–9.
Czermak BV, Unsinn KM, Gotwald T, Niehoff AA, Freund MC, Waldenberger P, Vogel W, Jaschke WR. Echinococcus granulosus revisited: radiologic patterns seen in pediatric and adult patients. AJR Am J Roentgenol. 2001;177(5):1051–6. doi:10.2214/ajr.177.5.1771051.
Ilica AT, Kocaoglu M, Zeybek N, Guven S, Adaletli I, Basgul A, Coban H, Bilici A, Bukte Y. Extrahepatic abdominal hydatid disease caused by Echinococcus granulosus: imaging findings. AJR Am J Roentgenol. 2007;189(2):337–43. doi:10.2214/AJR.07.2255.
Pedrosa I, Saiz A, Arrazola J, Ferreiros J, Pedrosa CS. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000;20(3):795–817. doi:10.1148/radiographics.20.3.g00ma06795.
Hopkins KL, Simoneaux SF, Patrick LE, Wyly JB, Dalton MJ, Snitzer JA. Imaging manifestations of cat-scratch disease. AJR Am J Roentgenol. 1996;166(2):435–8. doi:10.2214/ajr.166.2.8553962.
Garver MK, St Geme JW 3rd, Siegel MJ. Tularemia presenting with splenic nodules. Pediatr Infect Dis J. 1994;13(9):830–2.
Fitzgerald NE, MacClain KL. Imaging characteristics of hemophagocytic lymphohistiocytosis. Pediatr Radiol. 2003;33(6):392–401. doi:10.1007/s00247-003-0894-9.
Schmidt MH, Sung L, Shuckett BM. Hemophagocytic lymphohistiocytosis in children: abdominal US findings within 1 week of presentation. Radiology. 2004;230(3):685–9. doi:10.1148/radiol.2303030223.
Levy AD, Cantisani V, Miettinen M. Abdominal lymphangiomas: imaging features with pathologic correlation. AJR Am J Roentgenol. 2004;182(6):1485–91. doi:10.2214/ajr.182.6.1821485.
Hilmes MA, Strouse PJ. The pediatric spleen. Semin Ultrasound CT MR. 2007;28(1):3–11.
Paterson A, Frush DP, Donnelly LF, Foss JN, O’Hara SM, Bisset GS 3rd. A pattern-oriented approach to splenic imaging in infants and children. Radiographics. 1999;19(6):1465–85. doi:10.1148/radiographics.19.6.g99no231465.
Giovagnoni A, Giorgi C, Goteri G. Tumours of the spleen. Cancer Imaging. 2005;5:73–7. doi:10.1102/1470-7330.2005.0002.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pediatrics.
Rights and permissions
About this article
Cite this article
Ayyala, R.S., Anupindi, S.A., Taylor, G.A. et al. Imaging of Splenic Infections (and Their Mimickers) in Children. Curr Radiol Rep 4, 2 (2016). https://doi.org/10.1007/s40134-015-0129-5
Published:
DOI: https://doi.org/10.1007/s40134-015-0129-5