Abstract
Purpose
To identify the imaging manifestations of splenic involvement in babesiosis, a potentially fatal tick-borne zoonosis with multi-organ involvement.
Methods
In our single center HIPAA compliant IRB-approved study, we performed a retrospective search of the electronic medical record at our institution to identify all patients with known or suspected acute babesiosis from 2000 to 2017. We then reviewed all abdominal imaging of patients with confirmed disease to identify incidence and characteristics of splenic involvement. Splenomegaly was determined using a height- and gender-adjusted reference.
Results
After exclusions, 63 patients with a confirmed diagnosis of babesiosis and contemporaneous imaging of the spleen were included in the final cohort. Within this cohort, 56 (89%) had splenomegaly at a minimum and 13 had splenic infarcts. Splenic rupture was present in eight patients with three having a pseudoaneurysm. In 14 patients with follow-up imaging, the spleen subsequently diminished in size. One additional patient with ruptured spleen underwent emergency splenectomy prior to imaging.
Conclusion
Although the literature suggests splenic involvement is a rare finding, acute parasitemia with babesiosis commonly affects the spleen. Recognition of this association can aid radiologists diagnosing splenic involvement in babesiosis and can lead to appropriate intervention in the minority with splenic hemorrhage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Babesiosis is a tick-borne parasitic infectious disease caused by protozoa of the genus Babesia. Once known only on Cape Cod and New England coastal islands, since the 1980s the primary pathogen Babesia microti has been spreading throughout the northeastern USA and Midwest [1]. The usual tick vector, Ixodes scapularis, also carries Lyme disease, which is a frequent co-infection [1, 2]. Related babesia species have been recognized as human pathogens in warmer parts of the Americas, south and eastern Asia, and Europe.
The clinical manifestations of human babesiosis vary from asymptomatic infection to multi-organ failure and death. Severe disease requiring hospitalization is found most often in older patients (> 50 years of age), asplenic individuals, or the immunocompromised. However, the majority of cases produce only a mild viral-like febrile illness that frequently goes unrecognized. The non-specific nature of early symptoms often contributes to inaccuracies and delay in diagnosis. Typical laboratory abnormalities are in keeping with a hemolytic anemia (low hemoglobin/hematocrit and elevated lactate dehydrogenase), as well as thrombocytopenia and elevated liver enzymes [3,4,5]. The diagnosis is confirmed by identification of babesia organisms within circulating erythrocytes on Giemsa or Wright-stained thin blood smears or with polymerase-chain-reaction (PCR) detection of babesia DNA [6, 7]. Serology is inaccurate, as seropositivity can linger for months after infection has cleared [7].
Babesia are malaria-like blood parasites. It is reasonable that, like malaria [8], babesiosis might affect the spleen. From case reports and systematic reviews, splenic rupture is a known complication of severe babesiosis [9, 10]. Publications to date have focused on the clinical features of babesiosis. In our series, we evaluated the spectrum of splenic findings on imaging in babesiosis.
Methods
This is a HIPAA compliant IRB-approved retrospective cohort study of patients with acute babesiosis at an urban tertiary care center in the New England region of the USA. We performed a retrospective search of the electronic medical record at our institution to identify all patients with known or suspected acute babesiosis between 2000 and 2017. Our initial search yielded 2112 patients with an associated diagnosis of babesiosis. Patients were excluded if their diagnosis was not confirmed by thin smear or PCR (n = 1247). Any patient who did not undergo imaging within 30 days of the diagnosis of babesiosis being established (n = 711) was also excluded. In our initial filtration, we included all patients with any imaging in which the spleen could conceivably be seen. This included radiographs, ultrasound, CT, and MRI. We then reviewed all imaging of the 154 patients in our final cohort to identify incidence and characteristics of splenic involvement. Figure 1 provides a flow chart describing the process used to arrive at the final cohort. Splenomegaly was determined using a height- and gender-adjusted reference for adults [11] and an age-adjusted reference for children [12].
Statistical analysis
For patients with spleen in situ and cross-sectional imaging, descriptive statistics included median and range of the longitudinal dimension. Chi-square statistic was computed to test the influence of Lyme co-infection on splenomegaly.
Results
From 2000 to 2017, 154 patients had an established diagnosis of babesiosis and had imaging within 30 days. The cohort was predominantly male (119 males, 35 females) with age ranging from less than 1 year to 89 years (median 65 years).
The spleen was not imaged in the entire cohort as 23 patients had a prior splenectomy and an additional 68 patients had imaging in which the spleen was not assessable, mostly radiographs. The remaining 63 patients had imaging in which the spleen was seen in its entirety. In this sub-cohort, 57 patients (89%) had spleen findings, 56 having splenomegaly at a minimum. One patient had a pseudoaneurysm and splenic rupture without splenomegaly. Spleen size ranged from 11 to 24 cm (median 14.4 cm). Infarcts were the second most common splenic finding with 13 patients having at least one splenic infarct (Fig. 2). More rare findings included splenic rupture (n = 8) (Fig. 3) and pseudoaneurysm (n = 3) (Fig. 4). The median age of those with splenic rupture was 62 (range 28–83 years), and all eight were male. A breakdown of the imaging modalities and splenic findings is provided in Table 1. Of the 56 patients with splenomegaly, 15 had follow-up imaging, with all demonstrating a subsequent decrease in spleen size (Fig. 5). This included two patients with cirrhosis and portal hypertension and three patients with lymphoma.
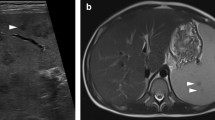
Coronal CT image in the arterial phase (a) of a 62-year-old male with acute babesiosis shows splenomegaly (16.2 cm) with splenic rupture and active contrast extravasation (white arrow). Axial-delayed post-contrast image (b) shows peri-splenic hemorrhage (black arrow) and pooling of extravasated contrast (white arrows)
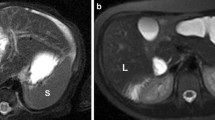
Coronal SSFSE image (a) of a 33-year-old male with a history of primary sclerosing cholangitis shows a normal size spleen (11.5 cm long axis) 1 month prior to the episode of acute babesiosis. Coronal CT image (b) during the episode of acute babesiosis shows interval enlargement of the spleen, now 14 cm long axis. Coronal SSFSE MR image (C) 10 months after the episode of acute babesiosis shows normalization of spleen size
Most patients were admitted, with only eight (13%) of the final 63 patient cohort not requiring admission. All but one of the 23 patients who had a prior splenectomy were admitted. Babesia parasitemia levels ranged from less than 1 to 23%.
Of the final 63 patient cohort, 15 patients were transferred from another hospital, with all having splenomegaly at a minimum. In addition, six others with a prior splenectomy were also transferred from another hospital.
Co-infection with Lyme disease was seen in 16 of the 63 patients (25%), and splenomegaly was present in 15 of those 16 patients (94%). Chi-square analysis demonstrated no significant difference in rates of splenomegaly between those solely with babesiosis compared with those with concomitant babesiosis and Lyme disease (p = .488).
Discussion
Our series shows that splenic abnormalities are a common manifestation of acute babesiosis, with splenomegaly being the most common finding. However, the pathophysiology of the splenic involvement in babesiosis is unclear.
Historically babesiosis was thought to be limited geographically to the endemic areas of New England and the upper Midwest; however, recent surveillance data from the Centers for Disease Control show that it is spreading beyond the traditional endemic areas [13]. Lyme disease has a similar geographic distribution and is also on the rise, relating to the common tick vector—Ixodes scapularis. The role of concomitant Lyme disease and acute babesiosis is not yet fully understood, but there is evidence that combined infection with B. burgdorferi and B. microti results in a synergistic suppression of the immune response in mice and thus increased disease severity [14, 15]. Although co-infection with Lyme disease is common, this is unlikely to be the link to the spleen as Lyme disease alone is rarely associated with splenomegaly [16, 17]. Furthermore, the presence of splenomegaly was not significantly different between the patients who had concomitant babesiosis and Lyme disease versus those with babesiosis alone. More likely, the splenic abnormalities relate to the spleen’s role in sequestering the infected erythrocytes so they can be cleared by macrophages [1, 18,19,20,21].
Although splenomegaly was the most common finding, more severe splenic involvement including infarcts and rupture were also identified. In total, we encountered nine patients with splenic rupture, eight with imaging, and one taken directly to the operating room prior to imaging. Kuwayama and Brones were the first to describe the association between acute babesiosis and splenic rupture. In their case report, the spleen was normal in size and they theorized that the rupture was related to degradation and friability of the parenchyma rather than enlargement and increased intra-capsular pressure [22]. The most recent series and systematic review each showed that babesiosis related splenic rupture is more common in middle-aged healthy men (median ages of 48 and 55 years, respectively) who mount a more robust immune response [9, 23]. The sub-cohort of patients with splenic rupture in our series is also entirely male, but slightly older (median age 62 years). Patel et al theorize this robust immune response leads to greater mechanical strain [23]. However, in this series, the authors did not describe the presence or absence of splenomegaly.
Some insights may be gleaned from similarities to malaria, another intracellular protozoan parasite that affects erythrocytes and is well known to cause splenic rupture. The immune response in malaria results in the adhesion of infected erythrocytes (cytoadherence), which become sequestered in the spleen to be phagocytosed leading to congestion of the red pulp [24, 25]. This can lead to vascular occlusion and infarcts and the splenic capsule may be easily torn [26]. However, this theory based on cytoadherence has yet to be proven in human babesiosis [27, 28].
Our series included eight babesiosis patients whose imaging showed splenic hemorrhage, which to our knowledge is the most of any series to date. Hemorrhage in at least three of these patients was due to pseudoaneurysm formation. Whether other patients with hemorrhage had pseudoaneurysms too small for detection by routine cross-sectional imaging is unknown. Any tendency for babesiosis to produce splenic pseudoaneurysms will be understood only from additional research into the pathogenesis of babesiosis.
As our series was based on imaging findings, the cohort was likely skewed towards more severe cases of acute babesiosis, as these sicker patients would more likely need diagnostic imaging. Our cohort shared many of the common risk factors for severe acute babesiosis, as they were generally older patients (median age 66 years) and many had a prior splenectomy. In addition, the patients transferred in to our tertiary care center from outlying hospitals would likely have disease of greater severity.
Three children were included in our cohort with their main risk factor being tick exposure and blood transfusions. However, in the case of one infant, the mode of transmission was suspected to be vertical as the mother had a tick exposure in the 3rd trimester. Vertical transmission of babesiosis is a known but uncommon mode of transmission, with acquired babesiosis (through a tick bite or blood transfusion) being much more common [29].
Limitations
As we note, a small percentage of patients had underlying medical conditions that would predispose to splenomegaly (portal hypertension and lymphomas), but all patients in this sub-cohort had follow-up imaging demonstrating a subsequent decrease in spleen size. Through this, we can infer that the acute worsening of splenomegaly was likely related to babesiosis.
The majority of patients had a CT during their episode of acute babesiosis, and this was preferentially used for our assessment. However, some only had an ultrasound or MRI. While ultrasound and MRI are adequate for the assessment of splenomegaly, the use of different modalities introduces some nonuniformity in our data due to differences in measurement technique. Furthermore, ultrasound is not a sensitive modality for detecting infarcts or other complications, and the rate of these more severe complications may be under represented.
Also, some patients were transferred from regional medical centers where they may have had imaging which was not reported or accessible.
Conclusion
Our series shows that splenic abnormalities on imaging are a common manifestation of acute babesiosis. Increasing radiologists’ awareness of this common association may improve diagnosis and expedite care, particularly in endemic areas.
References
E. Vannier, P.J. Krause, Human Babesiosis, N. Engl. J. Med. 366 (2012) 2397–2407. https://doi.org/10.1056/NEJMra1202018.
Spielman, M.L. Wilson, J.F. Levine, J. Piesman, Ecology of Ixodes Dammini-Borne Human Babesiosis and Lyme Disease, Annu. Rev. Entomol. (1985). https://doi.org/10.1146/annurev.en.30.010185.002255.
J.T. Joseph, S.S. Roy, N. Shams, P. Visintainer, R.B. Nadelman, S. Hosur, J. Nelson, G.P. Wormser, Babesiosis in lower Hudson valley, New York, USA, Emerg. Infect. Dis. (2011). https://doi.org/10.3201/eid1705.101334.
D.J. White, J. Talarico, H.G. Chang, G.S. Birkhead, T. Heimberger, D.L. Morse, Human babesiosis in New York State: Review of 139 hospitalized cases and analysis of prognostic factors, Arch. Intern. Med. (1998). https://doi.org/10.1001/archinte.158.19.2149.
J.C. Hatcher, P.D. Greenberg, J. Antique, V.E. Jimenez-Lucho, Severe Babesiosis in Long Island: Review of 34 Cases and Their Complications, Clin. Infect. Dis. (2001). https://doi.org/10.1086/319742.
G.R. Healy, T.K. Ruebush, Morphology of Babesia microti in human blood smears, Am. J. Clin. Pathol. (1980). https://doi.org/10.1093/ajcp/73.1.107.
P.J. Krause, S. Telford, A. Spielman, R. Ryan, J. Magera, T. V. Rajan, D. Christianson, T. V. Alberghini, L. Bow, D. Persing, Comparison of PCR with blood smear and inoculation of small animals for diagnosis of Babesia microti parasitemia, J. Clin. Microbiol. (1996).
H.A. Del Portillo, M. Ferrer, T. Brugat, L. Martin-Jaular, J. Langhorne, M.V.G. Lacerda, The role of the spleen in malaria., Cell. Microbiol. 14 (2012) 343–55. https://doi.org/10.1111/j.1462-5822.2011.01741.x.
S. Li, B. Goyal, J.D. Cooper, A. Abdelbaki, N. Gupta, Y. Kumar, Splenic rupture from babesiosis, an emerging concern? A systematic review of current literature, Ticks Tick. Borne. Dis. (2018). https://doi.org/10.1016/j.ttbdis.2018.06.004.
Dumic, J. Patel, M. Hart, E.R. Niendorf, S. Martin, P. Ramanan, Splenic Rupture as the First Manifestation of Babesia Microti Infection: Report of a Case and Review of Literature., Am. J. Case Rep. 19 (2018) 335–341. https://doi.org/10.12659/ajcr.908453.
K.U. Chow, B. Luxembourg, E. Seifried, H. Bonig, Spleen Size Is Significantly Influenced by Body Height and Sex: Establishment of Normal Values for Spleen Size at US with a Cohort of 1200 Healthy Individuals, Radiology. (2016). https://doi.org/10.1148/radiol.2015150887.
H.K. Rosenberg, R.I. Markowitz, H. Kolberg, C. Park, A. Hubbard, R.D. Bellah, Normal splenic size in infants and children: Sonographic measurements, Am. J. Roentgenol. 157 (1991) 119–121. https://doi.org/10.2214/ajr.157.1.2048509.
E.B. Gray, B.L. Herwaldt, Babesiosis surveillance - United States, 2011-2015, MMWR Surveill. Summ. 68 (2019) 1–16. https://doi.org/10.15585/mmwr.ss6806a1.
Vinasco, W. Braga, O. Zegarra-Moro, M.H. Moro, Cellular Immune Responses in a Murine Model of Borrelia burgdorferi and Babesia microti coinfection (43.55), J. Immunol. 178 (2007).
K.L. Knapp, N.A. Rice, Human coinfection with Borrelia burgdorferi and Babesia microti in the United States, J. Parasitol. Res. (2015). https://doi.org/10.1155/2015/587131.
P.J. Krause, S.R. Telford, A. Spielman, V. Sikand, R. Ryan, D. Christianson, G. Burke, P. Brassard, R. Pollack, J. Peck, D.H. Persing, Concurrent Lyme disease and babesiosis: Evidence for increased severity and duration of illness, J. Am. Med. Assoc. (1996). https://doi.org/10.1001/jama.275.21.1657.
A.C. Steere, N.H. Bartenhagen, J.E. Craft, G.J. Hutchinson, J.H. Newman, D.W. Rahn, L.H. Sigal, P.N. Spieler, K.S. Stenn, S.E. Malawista, The early clinical manifestations of Lyme disease, Ann. Intern. Med. (1983). https://doi.org/10.7326/0003-4819-99-1-76.
F. Rosner, M.H. Zarrabi, J.L. Benach, G.S. Habicht, Babesiosis in splenectomized adults, Am. J. Med. 76 (1984) 696–701. https://doi.org/10.1016/0002-9343(84)90298-5.
J.M. Cullen, J.F. Levine, Pathology of experimental Babesia microti infection in the Syrian hamster., Lab. Anim. Sci. 37 (1987) 640–3. http://www.ncbi.nlm.nih.gov/pubmed/3695401 (accessed August 25, 2019).
I.G. Wright, B.V. Goodger, G.D. Buffington, I.A. Clark, F. Parrodi, D.J. Waltisbuhl, Immunopathophysiology of babesial infections, Trans. R. Soc. Trop. Med. Hyg. 83 (1989) 11–13. https://doi.org/10.1016/0035-9203(89)90596-8.
R. Siderits, N. Mikhail, C. Ricart, M.V. Abello-Poblete, C. Wilcox, J.J. Godyn, Babesiosis, Significance of Spleen Function Illustrated by Postsplenectomy Course in 3 Cases, Infect. Dis. Clin. Pract. 16 (2008) 182–186. https://doi.org/10.1097/IPC.0b013e31809fe523.
D.P. Kuwayama, R.J. Briones, Spontaneous Splenic Rupture Caused by Babesia microti Infection, Clin. Infect. Dis. 46 (2008) e92–e95. https://doi.org/10.1086/587175.
K.M. Patel, J.E. Johnson, R. Reece, L.A. Mermel, Babesiosis-associated Splenic Rupture: Case Series From a Hyperendemic Region, Clin. Infect. Dis. (2018). https://doi.org/10.1093/cid/ciy1060.
B.C. Urban, D.J.P. Ferguson, A. Pain, N. Willcox, M. Plebanski, J.M. Austyn, D.J. Roberts, Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells, Nature. 400 (1999) 73–77. https://doi.org/10.1038/21900.
Oo MM, Aikawa M, Than T, Aye TM, Myint PT, Igarashi I, Schoene WC, Human Cerebral Malaria, J. Neuropathol. Exp. Neurol. 46 (1987) 223–231. https://doi.org/10.1097/00005072-198703000-00009.
T. Akel, N. Mobarakai, Hematologic manifestations of babesiosis., Ann. Clin. Microbiol. Antimicrob. 16 (2017) 6. https://doi.org/10.1186/s12941-017-0179-z.
P.J. Krause, J. Daily, S.R. Telford, E. Vannier, P. Lantos, A. Spielman, Shared features in the pathobiology of babesiosis and malaria., Trends Parasitol. 23 (2007) 605–10. https://doi.org/10.1016/j.pt.2007.09.005.
C.L. Hutchings, A. Li, K.M. Fernandez, T. Fletcher, L.A. Jackson, J.B. Molloy, W.K. Jorgensen, C.T. Lim, B.M. Cooke, New insights into the altered adhesive and mechanical properties of red blood cells parasitized by Babesia bovis, Mol. Microbiol. 65 (2007) 1092–1105. https://doi.org/10.1111/j.1365-2958.2007.05850.x.
J.T. Joseph, K. Purtill, S.J. Wong, J. Munoz, A. Teal, S. Madison-Antenucci, H.W. Horowitz, M.E. Aguero-Rosenfeld, J.M. Moore, C. Abramowsky, G.P. Wormser, Vertical transmission of Babesia microti, United States, Emerg. Infect. Dis. (2012). https://doi.org/10.3201/eid1808.110988.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mojtahed, A., Bates, D.D.B. & Hahn, P.F. Splenic findings in patients with acute babesiosis. Abdom Radiol 45, 710–715 (2020). https://doi.org/10.1007/s00261-019-02362-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02362-z